1 Introduction
Iron is the most abundant transition metal on the Earth with the content of 5.1% after oxygen, silicon and aluminum, and one of the most inexpensive and environmentally friendly metals. In ancient Greek, ‘star’ and ‘iron’ are the same word because iron was firstly discovered in the aerolite. The first discovered bronze sword made in Shang Dynasty of China about 3300 years ago was unearthed in 1973 and proved the edge of the sword was made of the iron meteorite. Nowadays, iron is extensively utilized to produce steel, and chemists are kept in the dark regarding the influence and importance of using iron. Indeed, iron-promoted catalytic reactions have played important roles such as the ammonia synthesis [1–3] and cross couplings [4–8]. Considering additional coupling reactions, products of tremendous industrial importance result from the likes of olefin oligomerization and polymerization. In 1998, the emergence of iron pro-catalysts for ethylene polymerization spurred extensive investigations, and the number of papers mushroomed on iron complexes as pro-catalysts [9–13]. The iron pro-catalysts in ethylene reactivity was proclaimed as the “Iron Age”, however, it appears more likely “a flash in the pan” due to recently, there being only a few publications dealing with iron pro-catalysts. That is caused by more researchers focusing on understanding nature of iron pro-catalysts and few models of iron pro-catalysts have been extensively explored. Herein, the development of iron pro-catalysts in ethylene reactivity is discussed and their promising future in industrial applications is illustrated.
2 Bis(imino)pyridyliron derivatives
As a typical model in ethylene reactivity, the bis(imino)pyridyliron pro-catalysts have attracted much attention from both the academic and industrial areas [9–13]. The steric and electronic influences of the substituents in the ligands could affect the catalytic activity of their iron pro-catalysts, and the products could be verified; therefore many researches are concerned with the variation of imino aryl of 2,6-ortho substituents [14–18]. In general, higher catalytic activities were achieved by the iron complexes bearing bulky alkyl-substituents at the ortho-position or additional methyl group at the para-position of imino-aryl groups due to the positive steric effects and better solubility brought about by the substituents. The iron complexes ligated by bis(ketimino)pyridines are favorable for ethylene polymerization; however, iron complexes bearing bis(aldimino)pyridines prefer to produce lower molecular weight polyethylene waxes or oligomers. Meanwhile, investigations on the influence of electronic properties of the aryl group of bis(imino)pyridyliron complexes have been carried out, and the relationships between the complex structure and the activity have also been identified [19–22] (Fig. 1).

Bis(imino)pyridyliron pro-catalysts.
To get first hand information about the catalytic behaviour of bis(imino)pyridyliron dichlorides, the modified nonsymmetrical and non-conjugated ligands were synthesized and used to form the iron derivatives (Fig. 2) [23]. Interestingly, three iron complexes with the same coordination framework showed different characteristic features in ethylene reactivity under the same reaction conditions: the catalytic performance by A was similar to observations by the more common bis(imino)pyridyliron pro-catalysts [14–16]; catalysis by B showed oligomerization only, but no activity was observed by C in Fig. 2. The observations indicated the significant effects of ligands on the catalytic behaviour. The causes come from the natures of the three ligands relying on the steric and electronic influences and electron-conjugation within the molecular system. The different catalytic behaviour of their iron complexes in ethylene reactivity indicated the power of homogeneous catalysis by complexes with many variations and interesting aspects.
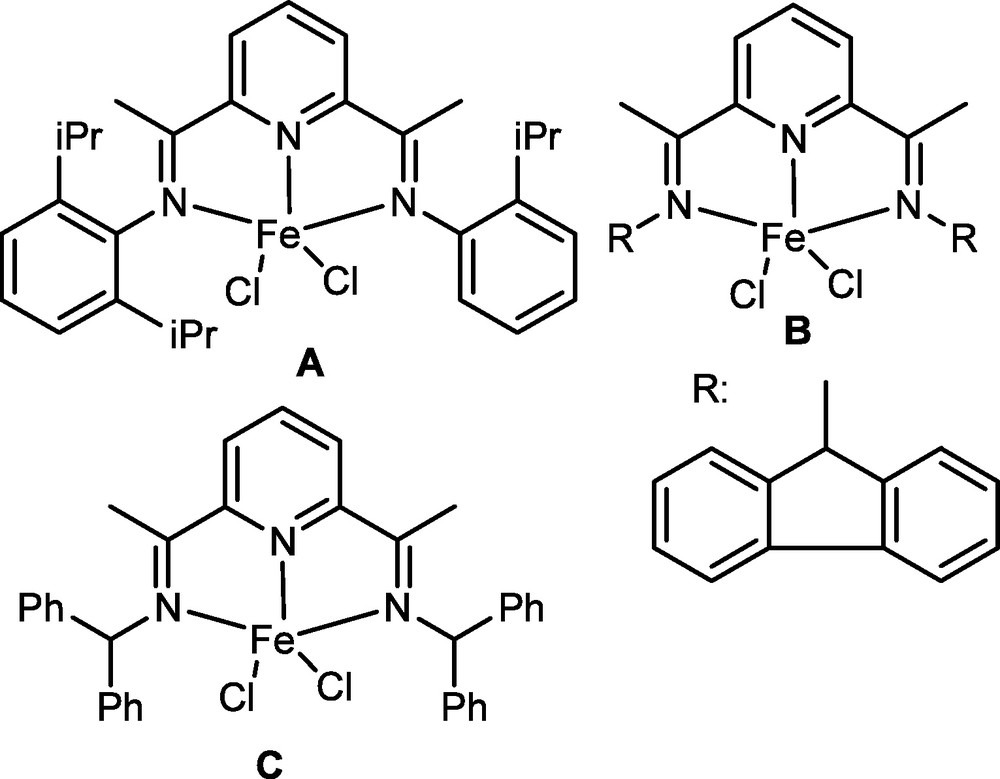
Our variation of bis(imino)pyridyliron dichlorides.
Further investigations have been divided into two targets: academically understanding the active species and catalytic mechanism, and some potential scale-up processes towards ethylene oligomerization and polymerization. Research has been conducted in investigating the actual oxidation state of the iron in the active species and the structure of the active species as well as the reaction mechanism of the polymerization process [24–44]; however, there were many debates on the actual oxidation state of iron in the catalytically active species [24–32]. Gibson suggested a trivalent Fe species formed by oxidation with methylalumiuminoxane [MAO] on the basis of Mössbauer and EPR studies [25]. This conclusion was further testified by the calculation results from Bruin's group and Cruz's group based on DFT calculation [41,42]. But the different view about active species was proposed by Talsi et al. [24,26,27], suggesting a paramagnetic Fe(II) alkyl complex bridging the aluminum co-catalyst as the active species illustrated by spectroscopic analysis. The commonly acceptable precursors are alkylated cationic Fe(II) alkyl derivative with a 14e intermediate, which was demonstrated by the recent achievement in Chirik's group [28,29] with isolating the dialkyliron intermediates. Moreover, Fe complexes could provide active species even upon treatment with Al or Zn alkyls [30], and the UV-vis spectra of the Fe pro-catalyst/TMA adducts show no significant changes for different Fe/Al ratios, suggesting similar species formed [33]. Though the iron hydride species could be identified at high Fe/Al ratio, a cationic monochloro-iron species could be identified at low Fe/Al ratio [34].
The study of the catalytic mechanism has been approached with experimental and theoretical techniques, focusing on the catalytic system of bis(imino)pyridyliron complexes. The computation simulations have been extensively done [38–44], including the initial full ab initio study [38] and the DFT and a combined DFT/MM (QM/MM) method [39,40]. The higher catalytic activities correlate with higher stabilities of the iron pro-catalysts at elevated temperatures. These calculation results were consistent with the experiment results. Recently, combined methods of structural, spectroscopic, and computational study were evaluated to understand the redox activity of the bis(imino)pyridyliron complexes by Chirik and coworkers [43,44]. The study indicated that the ferrous oxidation state was not transformed during treatment with MAO or other co-catalyst, and redox reactions are confined at the bis(imino)pyridine ligand.
The metal net charge correlation (MANCC) was employed in our study of the activities of bis(imino)pyridyl iron(II) pro-catalysts in ethylene oligomerization/polymerization [45]. The catalytic activity greatly relied on the electronic configuration of the ligands used, especially the net charge on the central metal atom, however, the catalytic activity did not monotonously vary with the net charge on the central metal.
3 2-(2-benzimidazolyl)-6-(1-(arylimino)ethyl)pyridyliron derivatives
Besides the bis(imino)pyridine derivatives, much research on the 2,6-substituted-pyridines has attracted great attentions, and some successful derivatives of iron pro-catalysts are collected as the following in Fig. 3.
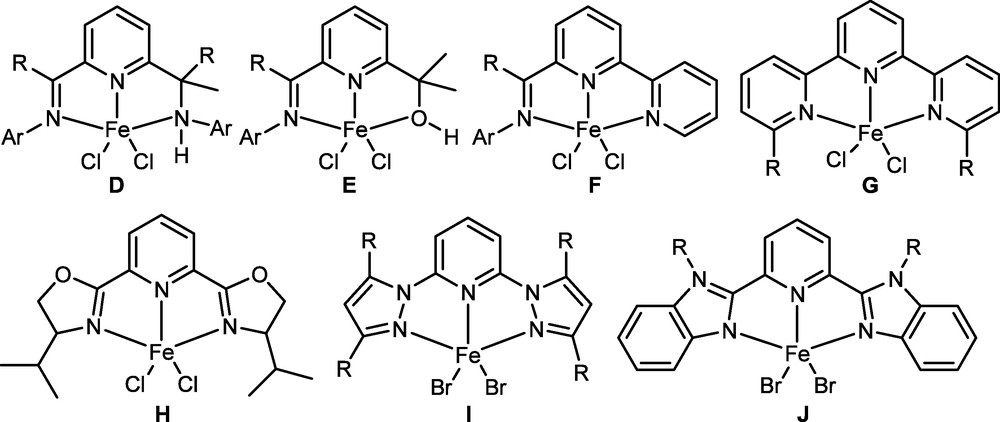
Iron pro-catalysts based on 2,6-substituted-pyridines.
Upon activation with MAO, both pro-catalysts D and E displayed moderate activities in ethylene polymerization, suggesting weaker interactions of the ligands, resulting in dissociation between iron and the ligands [46,47]. Pro-catalyst F performed high activity towards ethylene oligomerization with major products of 1-butene and 1-hexene as [48]. However, low activities towards ethylene polymerization was only observed by pro-catalyst G [49] due to the weak coordination. Meanwhile, pro-catalyst H showed low activity for ethylene polymerization and no activity for the copolymerization of ethylene with 1-hexene [50]. Moreover, pro-catalysts I and J initiated the ethylene oligomerization with high selectivity of α-olefins; however, the productivities were quite low [51].
Our attempts to use 2,6-bis(2-benzimidazole)pyridines [52] failed in the isolation of their iron complexes. And so a series of 2-(2-benzimidazolyl)-6-(1-(arylimino)ethyl) pyridines were synthesized and used in forming their iron complexes (Fig. 4) [53–55]. The pro-catalysts K showed high activity towards ethylene oligomerization with some amounts of polyethylene waxes, and indicated that their activities were in the order with R = H [55] > R = Me [53] > R = i-Pr [54] due to the electronic influence of the substituents.
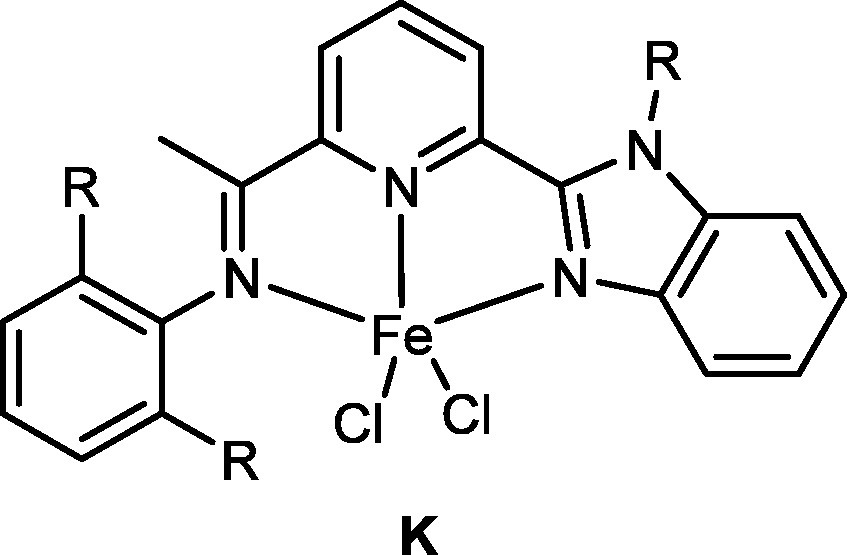
2-(2-benzimidazolyl)-6-(1-(arylimino)ethyl)pyridyliron pro-catalysts.
4 2-iminophenathrolyliron derivatives
Inspired by the bis(imino)pyridyliron pro-catalysts, it was not difficult to image a model pro-catalyst as the 2-iminophenathrolyliron dichlorides (L, Fig. 5). However, there were no available substances used for the 2-acetylphenathrolyline. Instead, the 2,9-dimethyl-1,10-phenanthroline was commercially available; therefore a series of 2,9-bis(imino)-1,10-phenanthrolines and their iron complexes (M, Fig. 6) [56] were synthesized. Surprisingly, the iron complexes showed quite low activity towards ethylene polymerization, and it was believed that the additional imino group can coordinate with the iron center and occupy the space required for the ethylene. So there was no other choice, but to synthesize the iron pro-catalyst with the due model (L, Fig. 5).
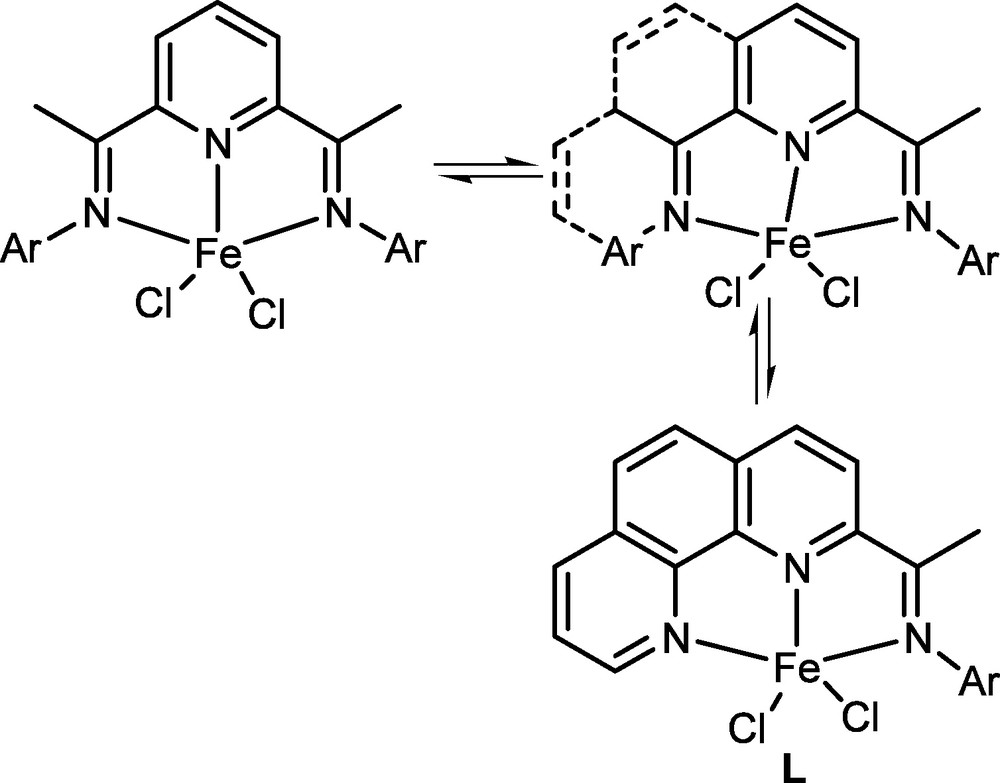
Designing 2-iminophenathrolyliron complexes.
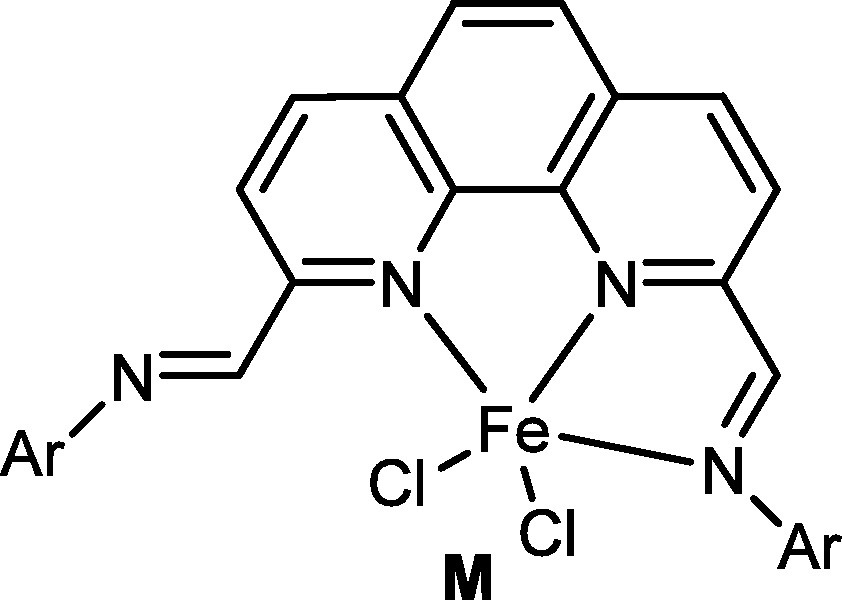
2,9-bis(imino)-1,10-phenanthrolyliron dichlorides.
Overcoming the difficulties encountered in synthesizing 2-acetyl1,10-phenanthroline, the 2-imino-1,10-phenanthrolines and their iron complexes (L, Fig. 5) were finally prepared. As expected, the pro-catalyst L showed very high activity up to 4.91 × 107 g mol−1 h−1 for ethylene oligomerization with a high selectivity for α-olefins [57]. During the reviewing of that manuscript, the referees made comments questioning about products in the case with 9-substituted 2-imino-1,10-phenanthrolines. When the paper appeared [57,58], readers kindly gave us suggestions. Subsequently the iron complexes bearing 2-imino-9-phenyl-1,10-phenanthrolines (N, Fig. 7) were synthesized; however, they exhibited lower activities (2.69 × 106 g mol−1 h−1) [59] in comparison with the model pro-catalyst L [57,58]. The presence of a phenyl group at the 9-position may hinder the coordination and insertion of ethylene.
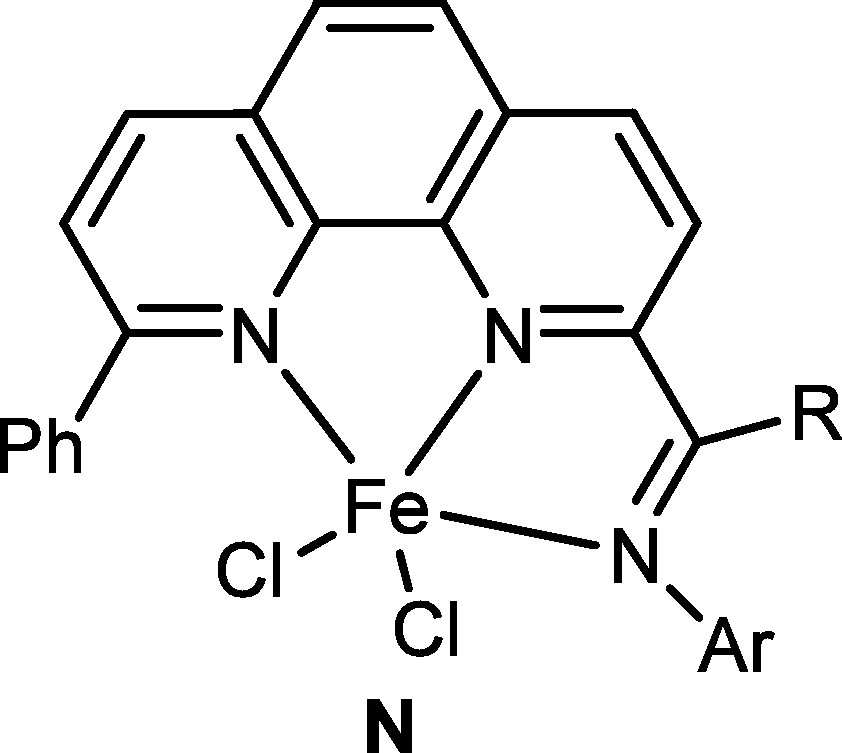
2-Imino-9-phenyl-1,10-phenanthrolyliron pro-catalysts.
5 Iron complexes bearing quinoline derivatives
Quinoline derivatives have high potential as ligands, however, complexes are limited to in nickel pro-catalysts [60–63] without successfully isolating their iron complexes. The tridentate ligands and their iron complexes, namely O and P have been synthesized (Fig. 8). The pro-catalyst O exhibited high activities towards ethylene oligomerization with dimers and trimers as products [64]. In comparison, the pro-catalyst P showed a rather unique property towards ethylene polymerization [65]: no activity was observed at low temperatures, but high activity was achieved at higher temperatures than 80 °C (at 100 °C). To the best of our knowledge, this is the first example of iron pro-catalysts that perform at high activity for ethylene polymerization at such high reaction temperatures (above 80 °C) without noticing oligomers.
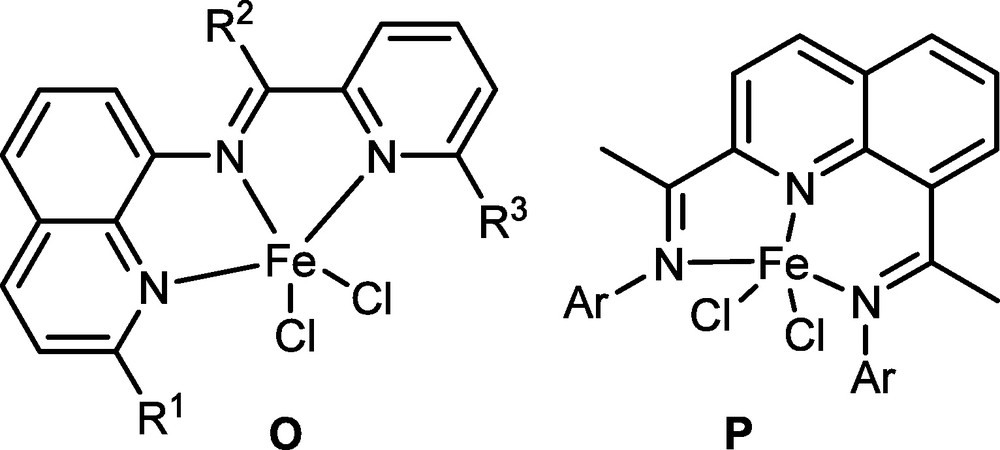
Iron pro-catalysts ligated by quinoline derivatives.
6 The critical problem and prospect
The discovery of 2,6-bis(arylimino)pyridylmetal (iron or cobalt) pro-catalysts marked a new era for late-transition metal pro-catalysts in ethylene reactivity, and the number of related papers has mushroomed. Regarding late-transition metal pro-catalysts, in general, deactivation occurs on producing polyethylenes with lower molecular weights (waxes or oligomers) on increasing reaction temperature. That is based on the concept of the same active species, which lead to more chain transfers and elimination at elevated reaction temperature, and it would be helpful to increase ethylene pressure. Though there were limited examples of 2,8-bis(imino)quinolyliron pro-catalysts, different active species would be highly likely with better activity at elevated reaction temperature [65]. A similar phenomenon was observed with cobalt pro-catalysts [66]. This provides a new strategy for targeting late-transition metal pro-catalysts, further investigation and results would not only be interesting in academic consideration but also potential applications in industry.
Acknowledgement
This work was supported by National Natural Science Foundation of China (No. 20874105) and MOST 863 program No. 2009AA033601. WHS is grateful to Prof. P. Braunstein for his kindly hosting the Trilateral Symposium between China, France and Germany.


