1 Introduction
Protection of functional groups plays an important role in multi-step synthesis of natural products. However, selective protection and deprotection of carbonyl groups are substantial steps in synthetic organic chemistry [1]. Acylals (1,1-diacetates) are appropriate candidates to this aim due to their stability in basic and neutral reaction media as well as in aqueous acids [2]. Meanwhile, gem-diacetates derived from α,β-unsaturated aldehydes are useful as dienes for Diels–Alder cycloaddition reactions. Moreover, acylals are used as cross linking reagents for cellulose in cotton [3].
Several methods have been used for the synthesis of 1,1-diacetates from strong acids including sulfuric acid [4], methane sulfuric acid [5], sulfamic acid [6], Lewis acids as lithium bromide [7], aluminum chloride [8], anhydrous ferrous sulfate [9], PCl3 [10], FeCl3 [11], NBS [12], Nafion-H [13], sulfated zirconia [14], montmorillonite clay [15], expansive graphite [16], aluminum dodecatungstophosphate [17], Well–Dawson acid (H6P2W18O62 .24H2O) [18] zeolite HSZ-360 [19], Cu(OTf)2 [20], Sc(OTf)3 [21], Bi(OTf)3 [22], Zn(BF4)2 [23], Bi(NO3)3.5H2O [24] and ZrCl4 [25] which are also efficient for this conversion. However, many of these methodologies have drawbacks, and involve strongly acidic conditions, corrosive reagents, long reaction times, high temperature and high toxicity. Recently, solvent-free reactions were developed because of their ecological and low-cost advantages. Some of these catalysts are P2O5/Al2O3 [26], dodecamolybdophoshporic acid [27], SO42−/SnO2 [28], bromodimethylsulfonium bromide [29], solid lithium perchlorate [30], [bmim]BF4 [31], [Hmim]HSO4 [32] zirconium sulfophenyl phosphonate [19] and Zeolite Y [33].
In this article, we report a new and efficient method for solvent-free conversion of aromatic aldehydes to their corresponding 1,1-diacetates with acetic anhydride and their deprotection in acetonitrile using encapsulated molybdophosphoric acid in dealuminated Y zeolite (MPA-DAZY) as a reusable catalyst (Scheme 1).
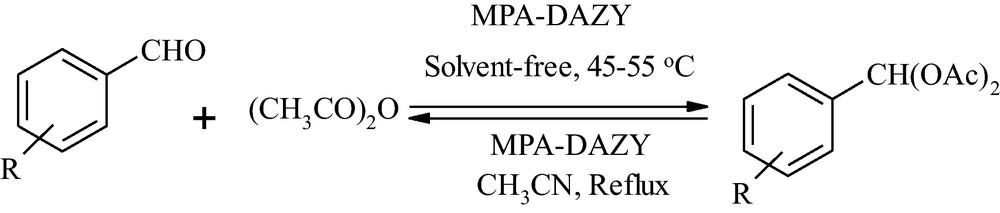
Synthesis of 1,1-diacetates under solvent-free conditions and their deprotection in acetonitrile using encapsulated molybdophosphoric acid in dealuminated Y zeolite (MPA-DAZY).
2 Experimental
All solvents and reagents were of the commercial reagent grade and obtained from Merck or Fluka. All of reaction mixtures were monitored by TLC. Melting points were recorded on a Barnstead Electrothermal 9200 apparatus, and are uncorrected. The NaY-zeolite was purchased from Sigma-Aldrich Chemical Company. The framework of NaY-zeolite contains aluminum atoms are basic and therefore, increase the decomposition of MPAs, or disturb the formation of MPA into the supercages of zeolite. Therefore, the modified DAZY (dealuminated zeolite Y) as support, was prepared by hydrothermal treatment [34]. Dealumination of NaY zeolite not only improves the zeolite stability but also yields a secondary pore system in zeolite matrix for deposition of the large MPA species [35]. Therefore, zeolite Y was dealuminated. The method reported by Mukai was used for the synthesis of H3PMo12O40 encapsulated into dealuminated zeolite (MPA-DAZY) [36,37]. All products were known compounds and identified by comparing their physical data by their authentic samples.
2.1 General procedure for the preparation of 1,1-diacetates
In a round bottom flask equipped with a magnetic stirrer, aldehyde (1 mmol), acetic anhydride (3 mmol) and catalyst (250 mg, 0.01 mmol) were mixed and stirred at 45–55 °C for an appropriate time. The progress of the reaction was monitored by TLC (ethyl acetate/n-hexane 7:1). After the reaction was completed, saturated NaHCO3 (5 ml) was added and the catalyst was filtered. The product was extracted with diethyl ether (2 × 15 ml) and the etherates were dried over Na2SO4. The solvent was evaporated to give the corresponding 1,1-diacetate.
2.2 Catalyst recovery and reuse
The reusability of catalyst also was investigated in the multiple sequential reaction of 3-nitrobenzaldehyde as a model substrate. At the end of each reaction, the catalyst was filtered, activated by washing with ethyl acetate and drying at 120 °C for 3 h, and reused with fresh aldehyde and acetic anhydride.
2.3 General procedure for the deprotection of 1,1-diacetates
In a typical procedure, 1,1-diacetates (1 mmol) in acetonitrile (3 ml) and MPA-DAZY (500 mg, 0.02 mmol) were mixed and refluxed for an appropriate time. The progress of reaction was monitored by TLC (ethyl acetate/n-hexane 7:1). After completion of the reaction, the reaction mixture was diluted with ethyl acetate and filtered. The organic layer was washed with NaHCO3 (10%, 2 × 5 ml) and dried over Na2SO4. The organic solvent was evaporated to produce the crude aldehyde. The residue was purified by plate chromatography (n-hexane/ether 4:1) to give the corresponding aldehyde.
3 Results and discussion
3.1 Catalytic performance in the protection of aldehydes
The MPA-DAZY catalyst was synthesized and characterized by infrared spectroscopy (FT-IR), X-ray diffraction (XRD), differential thermal gravimetry (DTG) and atomic absorption spectroscopic (AAS) techniques. The content of MPA in the synthesized sample, obtained by dissolving a small amount of the washed catalyst in hydrofluoric acid and hot concentrated hydrochloric acid, was determined by atomic absorption spectroscopy (AAS) [37].
The results showed that the catalyst loading was about 0.043 mmol/g of encapsulated catalyst. The reaction of aldehydes with acetic anhydride in the presence of MPA-DAZY produces the correponding 1,1-diacetates (Scheme 1). First, the amount of catalyst was optimized in the reaction of 3-nitrobenzaldehyde with acetic anhydride. The results showed that highest yield was obtained with 250 mg (0.01 mmol) of catalyst (Table 1). For optimization of the reaction media, the same reaction was performed in different solvents and also under solvent-free conditions. When the reaction was performed in solvent, the progress of the reaction was lower in comparison with solvent-free conditions. Therefore, the solvent-free conditions was used for the conversion of aldehydes to their corresponding 1,1-diacetates (Table 2). The amount of acetic anhydride was also optimized and the best result was obtained with a 3:1 molar ratio of acetic anhydride: aldehyde.
Optimization of the catalyst amount in the reaction of 3-nitrobenzaldehyde with acetic anhydride under solvent-free conditions after 1 h.a
| Entry | Catalyst amount (mg) | Yieldb (%) |
| 1 | 100 | 40 |
| 2 | 150 | 65 |
| 3 | 200 | 84 |
| 4 | 250 (0.01 mmol of MPA) | 95 |
a Reaction conditions: 3-nitrobenzaldehyde (1 mmol), acetic anhydride (3 mmol).
b Isolated yield.
The effect of solvent on the protection of 3-nitrobenzaldehyde with acetic anhydride catalyzed by MPA-DAZY after 1 h.a
| Entry | Solvent | Yieldb (%) |
| 1 | CH3CN | 44 |
| 2 | CH3COCH3 | 35 |
| 3 | CH3OH | 40 |
| 4 | CH2Cl2 | 25 |
| 5 | Solvent-free | 95 |
a Reaction conditions: 3-nitrobenzaldehyde (1 mmol), acetic anhydride (3 mmol), catalyst (250 mg, 0.01 mmol).
b Isolated yield.
Under the optimized reaction conditions, a wide range of aromatic aldehydes bearing electron-donating and electron-withdrawing groups were reacted with acetic anhydride in the presence of MPA-DAZY under solvent-free conditions at 45–55 °C and the corresponding 1,1-diacetates were obtained in good to excellent yields (80-98%) in 60-110 min (Table 3). The results showed that the nature of substituent (electron-withdrawing or electron-donating) has no significant effect on the catalyst activity. In the case of 2-hydroxybenzaldehyde (Table 3, entry 3), only the aldehyde group was reacted with acetic anhydride and no actate was detected in the reaction mixture.
Synthesis of 1,1-diacetates with anhydride acetic catalyzed by MPA-DAZY under solvent-free conditions and their deprotection in acetonitrile.a
| Entry | Substrate | Product | Protection | Deprotection | |||
| Yieldb (%) | Time (min) | Reported | Yieldb (%) | Time (h) | |||
| 1 | 80 | 90 | 52–53 [19] | 45 | 4 | ||
| 2 | 98 | 70 | 64–65 [26] | 75 | 4 | ||
| 3 | 98 | 75 | 103 [38] | 75 | 4 | ||
| 4 | 95 | 60 | 65–66 [39] | 90 | 4 | ||
| 5 | 95 | 85 | 127 [26] | 60 | 4 | ||
| 6 | 92 | 110 | 81–82 [26] | 76 | 4 | ||
| 7 | 90 | 70 | 82–83 [39] | 40 | 4 | ||
| 8 | 98 | 75 | 70–71 [40] | 70 | 4 | ||
| 9 | 85 | 85 | 82–83 [41] | 80 | 4 |
a Reaction conditions: aldehyde (1 mmol), Ac2O (3 mmol) catalyst (250 mg, 0.01 mmol).
b Isolated yield.
The ability of NaY zeolite (250 mg), DAZY (250 mg) and molybdophosphoric acid (1 mol%) as catalyst was also investigated in the protection of 3-nitrobenzaldehyde with Ac2O. The results showed that the amount of the corresponding 1,1-diacetate was 30%, 42% and 65%, repectively. These observations clearly showed that upon encapsulation of molybdophosphoric acid in the zeolite cavitiy, its catalytic activity increases.
Moreover, the ability of this catalyst was investigated in the deprotection of 1,1-diacetates in refluxing acetonitrile (Table 3). The results showed that this catalytic system is efficient for deprotection of 1,1-diacetates to their corresponding aldehydes in good yields in the presence of 2 mol% of the catalyst. The lower yield for 1,1-diacetates bearing substituent in the ortho position of the aromatic ring can be attributed to the difficulty in the entering in the zeolite cavity.
3.2 Catalyst reusability
The reusability of MPA-DAZY catalyst was investigated in the multiple sequential reaction of 3-nitrobenzaldehyde with acetic anhydride. At the end of the each reaction, the catalyst was separated by simple filtration, washed with ethyl acetate and Et2O and dried carefully before using it in the next run. After using of catalyst for four consecutive times, the yields were 75% (Table 4). The filtrates were collected for determination of Mo leaching (measured by AAS). The results showed that Mo catalyst is leached from zeolite and therefore, the yield decreased (Table 4).
The results obtained in the reusability of MPA-DAZY and the amount of molybdenum leached in the reaction of 3-nitrobenzaldehyde with Ac2O under solvent-free conditions after 1 h.a
| Run | Yieldb (%) | Mo leachedc (%) |
| 1 | 95 | 3 |
| 2 | 87 | 4 |
| 3 | 80 | 5 |
| 4 | 76 | 1 |
a Reaction condition: 3-nitrobenzaldehyde (1 mmol), acetic anhydride (3 mmol).
b Isolated yield.
c Determined by atomic absorption spectroscopy.
4 Conclusion
In summery, in this paper we reported a mild and efficient protocol for the protection of aldehydes with acetic anhydride under solvent-free conditions and 1,1-diacetates were obtained in good to excellent yield. This catalytic system was also used for deprotection of 1,1-diacetates refluxing acetonitrile. The advantages of this catalytic system are high catalytic activity, environmentally benign nature of the reaction and reusability of the catalyst.


