1 Introduction
Little attention has been paid to schistosomiasis (also known as bilharzia, bilharziosis or snail fever) over the last 30 years, for many different reasons including the fact that tourists traveling in endemic zones can easily escape from this parasitic disease by avoiding contact with infected waters. This neglected disease is a major parasitic disease with more than 200 million infected people in more than 74 countries in the tropical and subtropical zones, and continues to spread [1,2], even in areas where it was previously under control [3].
Schistosomiasis is caused by the trematode worms Schistosoma spp. (five species infect humans) which reside mainly in the abdominal veins of the infected vertebrates [4]. Infection occurs by penetration, through the skin of humans, of cercariae, the larval schistosome stages freely swimming in contaminated water. Then, parasite larvae invade capillaries and lymphatic vessels and accumulate in the liver for a rapid growth with portal vein blood. Four to 6 weeks after the infection, young adult worms migrate to the mesenteric veins (S. mansoni and S. japonicum) or the vesical plexus (S. haematobium) for a sexual maturation and mating period. For S. mansoni, egg production (200 to 2000 eggs/female per day) begins 6 weeks after the initial infection and continues for the all life of the worms. The lifespan of an adult schistosome averages 3 to 5 years, but can be as long as 30 years. So, the theoretical reproduction potential of one schistosome pair can reach 600 billion eggs [4]. The immune response of the host to schistosome eggs induces chronic inflammation, fibrosis and ulceration of the tissues at the sites of egg accumulation: intestine and liver for S. mansoni and S. japonicum or the genito-urinary tract for S. haematobium. So, the late and life-threatening consequences of chronic schistosomiasis include bladder cancer or serious kidney dysfunction and severe damage of the liver and spleen.
It should be noted that despite the high level of prevalence of this tropical disease, few therapies are accessible. Several vaccine strategies have been explored without success over the last 30 years including IgE antibodies [5] or genomic approaches [6,7]. Few drugs have been developed for the treatment of schistosomiasis over the last century. In vivo trematode models for chemotherapy have been recently reviewed [8]. Among the few drugs that have been used, one can mention antimony derivatives, metrifonate (1a) and oxamniquine (1b) (see Fig. 1 for structures) [9]. Most of these molecules have been withdrawn from the market because of toxicity and/or lack of efficacy. Oxamniquine is only efficient on S. mansoni, but not on S. japonicum which is endemic in Asia. In addition, it is too expensive, and its commercial availability is uncertain [10]. Consequently, praziquantel 2 (PZQ), a pyrazinoisoquinoline marketed as a racemate, is the only current drug of choice for the treatment of all schistosome species infecting humans. Since only one enantiomer is active, the (–)-(R) one [11], it is reasonable to consider that the drug target is chiral (possibly a calcium carrier protein) [12–14]. Few years after its discovery by researchers of two German companies, E. Merck (Darmstadt) and Bayer (Leverkusen), in the mid-1970s [15], PZQ has been widely used due to its qualities: efficacy by oral administration, safety and affordability (5 to 7 USD cents for a 600 mg-tablet). This drug is orally administered at a single dose of 40 mg/kg of body weight, curing 60 to 90% of the patients, or substantially decreasing the worm burden and egg production when complete curing is not achieved.
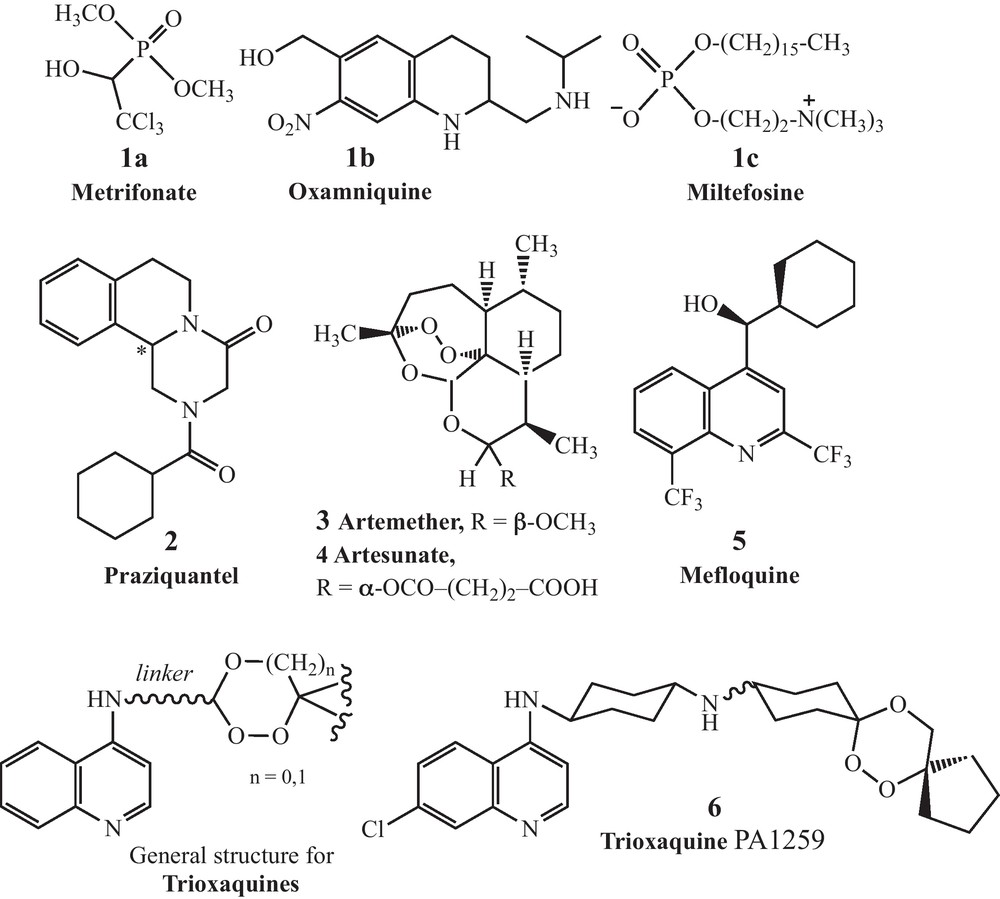
Structures of metrifonate, oxamniquine, miltefosine, mefloquine, praziquantel, and artemisinin and trioxaquine derivatives.
During the last 30 years, PZQ has been widely used as the only drug in order to reduce the morbidity of schistosomiasis. The main risk in monotherapies to treat pathogen-induced diseases is the risk of development of drug-resistant strains, as observed in malaria with chloroquine-resistant strains of Plasmodium falciparum [16]. Possible resistance of schistosomes to PZQ has been reported during the recent years [17–21]. Up to now, the level of PZQ resistance in schistosome species isolated from treated but uncured patients is relatively low, with ED50 values that do not exceed five to six times those of drug-susceptible isolates (ED stand for “effective dose”, and ED50 is the amount of drug that produces a therapeutic response in 50% of the subjects taking it). However, a decrease of the observed drug-susceptibility creates a real concern in having a single drug to control a disease affecting millions of people in endemic countries.
To our knowledge, there is currently no new antischistosomal drug in clinical development: the only proposal is to produce the active enantiomer of PZQ for less than the cost of the racemic drug [9]. Antiandrogens (Ro 13-3978) have been investigated in the early 1980s [22,23]. Oxadiazoles have recently been identified as new drug leads [24]. A recent report suggested that thioredoxin gluthatione reductase, a parasitic enzyme with several functions, might be a target for antischistosomal therapy [25]. Miltefosine, an alkylphosphocholine analogue registered in India to treat visceral leishmaniasis [26], has recently been considered for antischistosomal activity [27]. However, it is associated with severe gastrointestinal side-effects and a high level of teratogenicity and fetotoxicity until several months after the end of treatment [28]. New PZQ analogs have also been recently considered [29].
Schistosomes are hematophagous parasites which ingest and lyze in their oesophagus the red blood cells of their vertebrate hosts [30]. The proteolytic digestion of hemoglobin occurs in the schistosome intestine, generating amino acids that are directly used by the parasite, and free heme, which is an out-of-control redox active entity when not inserted within its apoproteins (hemoglobin or heme-enzymes). To avoid the toxicity of iron(II)-heme, the parasite is able to aggregate it as a dark pigment very similar to hemozoin, the pigment produced by Plasmodium in malaria [31,32]. Due to this similitude in hemoglobin catabolism between Schistosoma spp. and Plasmodium spp., several antimalarial drugs targeting heme metabolism have been evaluated as potential antischistosomals: artemisinin and its semi-synthetic derivatives (artemether 3 and artesunate 4) [33–36], synthetic peroxides containing a trioxolane entity (instead of a 1,2,4-trioxane as in artemisinin) [37], or mefloquine 5 [38]. With the different peroxidic molecules, the dose to reduce by 50% the worm number in infected mice (ED50) can be as high as 200 to 400 mg/kg, whereas the ED50 value of PZQ is usually between 50 and 110 mg/kg for susceptible parasite strains [39]. So, the curative dose of peroxide drugs should be far above the dose that will be acceptable for an efficient monotherapy. However, it should be noticed that artemisinin derivatives are active on the schistosomules during the first 21 days of the infection [35], while PZQ has a limited activity on this parasite stage, but has a more potent activity on adult worms (6 to 7 weeks post-infection for S. mansoni). This stage-dependent activity creates desirable complementary effects between these two types of drugs [40,41]. So, we have evaluated in vitro several trioxaquines (see Fig. 1 for the general structure) initially designed as antimalarials [42–47]. In a first study carried out on cultured S. mansoni, the activity of the trioxaquine PA1259 (6) (Fig. 1) was significantly higher than that of ARTM or MFQ, and close to that of PZQ, both on larval- and adult-parasite stages [48].
2 Results and discussion
In the present study, we report the antischistosomal activity of PA1259 in mice infected with S. mansoni. The host-parasite system used was an albino variety of Biomphalaria glabrata from Brazil and a strain of S. mansoni, also from Brazil, maintained in Swiss CD1 mice (Depré, Bourges, France). Detailed methods for mollusc and mouse infections and for parasite recovery were previously described [49]. Groups of 5 mice were percutaneously infected with 120 S. mansoni cercariae. The parasite-mouse contact lasted 45 minutes, allowing the penetration and development of 36 ± 4 cercariae. After 21 days or 49 days for evaluation on larval and adult stages, respectively, mice were orally treated by PA1259 diluted in 200 μL of excipient (an aqueous solution of methylcellulose 0.6% wt/v, containing 0.5 vol% of Tween 80). Fourteen days after treatment, mice were sacrificed and perfused by the method of Duvall et al. [50]. The blood was filtered, liver and mesenteric veins were dilacerated in order to count the worms. The worm burden reduction was calculated with respect to infected mice treated with the excipient alone. The treatment schedule consisted of four consecutive doses of 50 mg/kg each, given over a total period of 9 hours (interval between two doses was 3 hours). In mice treated by PA1259 49 days post-infection, 20 ± 5 worms were collected, corresponding to a worm burden reduction of 40% (control mice: 33 ± 5 worms). Similar treatment carried out with PZQ instead of PA1259 resulted in 86% of worm burden reduction. In mice treated 21 days post-infection, 17 ± 7 or 21 ± 6 parasites were collected after treatment by PA1259 or PZQ, respectively (control mice: 39 ± 5 parasites). These values correspond to a reduction of the worm burden of 53% and 41% with PA1259 and PZQ, respectively. So, the level of protection provided by PA1259 is slightly higher than that of PZQ for larval stage-, but lower for adult stage worms, indicating that PZQ remains the most potent on adult schistosomes up to now.
The significant activity of PA1259 on larval stage of S. mansoni prompted us to evaluate the efficacy of this drug on 21-day-old schistosomulae, when given in association with PZQ. The current objective of the WHO is indeed to associate a new drug to PZQ in order to improve efficiency of praziquantel, to clear if possible all the parasite stages at the time of treatment, and to delay the emergence of drug resistance.
For this reason, we evaluated the efficacy of PA1647, which is the diphosphate salt of PA1259, when given to mice in association with PZQ (salts of PA1259 are indeed more soluble than the base form in pharmacological excipients such as the tween/methylcellulose mixture). The overall drug dose and treatment schedule were the same as reported above for monotherapies: four equal doses of each drug (a 50/50 mixture: 25 mg/kg of PA1647/25 mg/kg of PZQ) administrated by oral route every 3 hours. This treatment resulted in a reduction of 73% of the schistosomula burden, with respect to mice treated with the excipient alone. By comparison, the worm burden reduction was 24% after 4 × 50 mg/kg of PZQ, and 18% after 4 × 50 mg/kg of PA1647. In a rough estimate, an additive effect between PZQ and PA1647 would have provided at best (24 + 18)/2 = 21% worm burden reduction. So, a reduction of 73% reveals a probable synergistic effect of PZQ and the PA1647 on 21-day-old schistosomulae.
It should be noted that, in all cases, the treatment induced no detectable toxicity in mice: all mice survived without change in behavior, and when they were perfused, 14 days post-treatment, their weight was not significantly different than that of control mice (± 5%), and there was no visible damage to organs (except usual damages due to schistosomiasis).
3 Conclusion
The trioxaquine PA1259, and its diphosphate salt PA1647, are efficacious drug candidates against S. mansoni after oral administration to infected mice. Moreover, when the association PA1647–PZQ was given orally to infected mice, it was more efficient to reduce parasitemia than PZQ alone. This synergy opens the way to a bitherapy approach that might target all the parasite human stages.
Acknowledgements
This work was supported by Palumed, CNRS, and Agence nationale pour la recherche (ANR grant No. ANR-08-MIEN-026-02). Vincent Pradines and Julien Portela are indebted to ANR for fellowships. The authors thank Sonia Kitoune and Christine Salle (both from Palumed), and Bernard Dejean (from UMR 5244) for technical assistance.


