1 Introduction
One of the most interesting and studied reductive processes is the one of cinnamaldehyde. From a mechanistic point of view, reduction of cinnamaldehyde can be a regioselective process, since this compound presents three distinct reducible sites: the >C O double bond, the >C C< double bond and the aromatic nuclei [1,2]. On the other hand, the reduction products, along with the starting material, are important raw materials in the pharmaceutical, fragrance and aroma industries [3] (Scheme 1).

Partial/total reduction of cinnamaldehyde.
Most of the catalysts used in hydrogenation produce a mixture of hydrogenated compounds requiring an expensive separation, the number of selective catalysts being rather scarce [4–6]. The selective hydrogenation of cinnamaldehyde is affected by many factors, such as the type of catalyst, the reaction conditions, the solvents and the electronic effects of the support [7–15].
We have previously reported, for several types of organic compounds, some mild reduction methods that use noble metal catalysts in only pure water as solvent, under atmospheric pressure or in sealed tube [16–18]. For example, the carbonyl moiety of acetophenones or benzophenones is effectively reduced to the corresponding methylene group by either Raney Ni–Al alloy [17] or a mixture of a noble metal catalyst and Al powder [18].
Two relatively and another one extremely recent communications guided us toward a connection with our studies: first, a report about the selective hydrogenation of cinnamaldehyde in the presence of a bicatalytic system formed of palladium black and Raney Ni, [19] yielding a mixture of phenylpropanal 3 and phenylpropanol 4, the ratio between them depending on the use of ultrasonication (with ultrasonication activation, the major compound was phenylpropanal 3), and another report of an efficient method for the NaBH4 reduction of carbonyl compounds (including cinnamaldehyde 1) in solvent-free conditions, but using wet SiO2 as support [20]. Moreover, a Japanese team reported recently a highly efficient and selective hydrogenation of unsaturated carbonyl compounds using Ni–Sn alloy catalysts, working at high temperatures (453 K) [21]. If Raney Ni or Raney alloys need no introduction as hydrogenation/reduction catalysts, the use of water (even as “wet support”) in the reduction of an α,β-unsaturated carbonyl compound is surprising. Moreover, it proved to be definitely beneficial, improving dramatically the result of the process: indeed, a dry support offered after 3 hours a 5% yield of alcohol, while the wet support permitted a complete transformation of the aldehyde in the corresponding alcohol in less than 1 minute.
Therefore, we decided to combine these aspects along with our previous investigations and study the reductive process of cinnamaldehyde in completely aqueous media, in the presence of a Ni–Al Raney alloy.
2 Experimental
All reagents were used as purchased, with no prior purification step. Distilled water was used in all experiments. Reactions were carried out in usual reflux installation (50 mL round-bottom flask, reflux condenser) or in sealed and pressure-resistant tube of 35 mL i.v. In the reaction flask (open flask or sealed tube, as specified for each case in the “Results and discussion” part), cinnamaldehyde (0.01 moles) was introduced along with the reduction catalyst (the amount is specified for each case in the “Results and discussion” part) and 5-18 mL of water. Powerful magnetic stirring (400 rpm) is applied for the specified reaction time (Tables 1–5), at room temperature. After completion of the process, the reaction mixture is extracted with three portions of ethyl ether. The organic layers are dried over MgSO4, filtrated, evaporated and submitted to GC–MS analysis. All experiments were performed in triplicate, giving similar results.
Cinnamaldehyde reduction experiments in aqueous media.
| Entry | Reduction catalyst | Additive |
| 1 | Raney alloy: Ni–Al | Al |
| 2 | Raney metals: Ni, Cu, Fe, Co | Al, Zn |
| 3 | Salts: NiCl2, CuCl2, CoCl2 | Al |
Reduction of cinnamaldehyde with Raney alloy.
| Run | Flask type | Reaction time (h) | Added Al (g) | Final reaction mixture (%)a | |||||
| 1 | 2 | 3 | 4 | Others | |||||
| C6H5–C3H7 | C6H5–C2H5 | ||||||||
| 1 | Open | 0.5 | – | 39.6 | 0.5 | 40.0 | 18.0 | 1.3 | 0.6 |
| 2 | Open | 1 | – | 3.8 | 0.8 | 55.0 | 37.1 | 1.7 | 1.6 |
| 3 | Open | 1 | 2 | 1.5 | – | 20.9 | 74.3 | 1.0 | 2.4 |
| 4b | Open | 1 | 4 | 86.0 | 0.1 | 12.0 | 1.0 | 0.4 | 0.1 |
| 5 | Open | 1 | 4 | – | – | 9.5 | 88.0 | 0.5 | 2.0 |
| 6 | Open | 3 | – | 3.4 | – | 49.4 | 40.4 | 3.8 | 3.1 |
| 7 | Open | 3 | 1 | 4.0 | – | 47.0 | 45.0 | 2.5 | 1.5 |
| 8 | Open | 3 | 2 | – | – | 3.0 | 90.0 | 3.0 | 4.0 |
| 9 | Open | 5 | 1 | – | – | 8.5 | 83.5 | 0.8 | 5.5 |
| 10 | Open | 12 | 1 | – | – | – | 95.0 | 1.0 | 4.0 |
| 11 | Sealed | 1 | – | 70.0 | – | 28.4 | 0.1 | 1.4 | – |
| 12 | Sealed | 2 | – | 5.8 | – | 56.4 | 31.3 | 3.3 | 3.2 |
| 13 | Sealed | 3 | – | – | – | 13.2 | 76.3 | 2.0 | 8.3 |
| 14 | Sealed | 1 | 0.4 | 58.5 | 0.2 | 39.4 | – | 1.8 | – |
| 15 | Sealed | 1 | 1 | 20.3 | – | 44.2 | 32.1 | 0.4 | 3.0 |
| 16 | Sealed | 2 | 1 | – | – | 9.0 | 81.7 | 3.7 | 5.5 |
a Determined through GC–MS.
b Raney Ni–Al alloy: 5 g.
Reduction of cinnamaldehyde with Raney metals and saltsa.
| Run | Raney metal or salt | Added Al (g) | Final reaction mixture (%)b | |||||
| 1 | 2 | 3 | 4 | Others | ||||
| C6H5–C3H7 | C6H5–C2H5 | |||||||
| 1 | Ni | – | 3.4 | – | 49.4 | 40.4 | 3.8 | 3.1 |
| 2 | Ni | 2 | – | – | 3.0 | 90.0 | 3.0 | 4.0 |
| 3 | Cu | – | 91.6 | 1.1 | 7.0 | 0.3 | – | – |
| 4 | Cu | 2 | 91.9 | 2.8 | 5.1 | 0.2 | – | – |
| 5 | Fe | – | 45.8 | 21.0 | 28.0 | 5.2 | – | – |
| 6 | Fe | 2 | 0.8 | 14.6 | 37.8 | 46.8 | – | – |
| 7 | Coc | – | 11.9 | 37.3 | 14.2 | 36.4 | 0.2 | – |
| 8 | Coc | 1 | 0.6 | 44.0 | 3.8 | 51.3 | 0.3 | – |
| 9d | NiCl2 (4.45)g | 1.25 | 47.5 | 12.3 | 24.6 | 15.0 | 0.6 | – |
| 10 | NiCl2 (4.45)g | 1.25 | – | – | 12.2 | 80.3 | 7.5 | – |
| 11d | NiCl2(4.45)g | 2.50 | 5.4 | 0.6 | 7.0 | 81.6 | 5.3 | 0.1 |
| 12d,e | CuCl2(4.74)g | 1.13 | – | – | – | 50.3 | 49.7 | – |
| 13f | CoCl2(4.74)g | 1.25 | 5.7 | – | 0.9 | 93.1 | 0.3 | – |
a Cinnamaldehyde 0.01 moles, metal/salt 10 g, water 18 mL, stirring, reflux 3 h.
b Determined through GC–MS.
c Raney cobalt 5 g.
d Reaction time 1 h.
e 1.13 g of Zn was also added.
f Reaction time 0.5 h.
g The quantity of metal is given in brackets.
Reduction of α-substituted cinnamaldehydes.
| Run | X | Reaction time (h) | Final reaction mixture (%)a | |||||||
| 1 | 2 | 3 | 4 | Others | ||||||
| 5 | 6 | 7 | 8 | |||||||
| 1 | -CH3 | 1 | 0.5 | – | – | 84.5 | – | – | 6.0 | 9.0 |
| 2 | 2 | – | – | – | 94.0 | – | – | 5.6 | 0.4 | |
| 3 | 3 | – | – | – | 93.0 | – | – | 6.6 | 0.4 | |
| 4 | –H | 1 | 3.8 | 0.8 | 55.0 | 37.1 | 1.7 | 1.6 | – | – |
| 5 | 3 | 3.4 | – | 49.4 | 40.4 | 3.8 | 3.1 | – | – | |
| 6 | –Cl | 1 | 33.5 | – | 7.2 + [26.5]b | [32.1]b | 0.3 | 0.3 | – | – |
| 7 | 2 | 10.4 | – | 10.2 + [13.3]b | [62.6]b | 1.5 | 2.0 | – | – | |
| 8 | 3c | 8.1 | – | 12.0 + [10.9]b | [66.5]b | 0.8 | 1.4 | – | – |
a Determined through GC–MS.
b [] represents percentage of corresponding compound with the loss of the chlorine atom.
c A phenyl–chloropropene derivative (0.3%) was also detected by GC–MS.
Reduction of other cinnamyl derivativesa.
| Run | X | Raney catalyst | Added metal (mg) | Final reaction mixture (%)b | |||||||
| 2/9 | 1 | 3 | 4 | 5 | 6 | 7 | Others | ||||
| 1,ω- | |||||||||||
| 1 | –Cl | Ni–Al | – | – | – | 18 | 47 | 16 | – | 6 | 13 |
| 2 | Ni–Al | 200 (Al) | – | – | 3 | 25 | 30 | – | – | 42c | |
| 3 | Co–Al | – | – | – | 12 | 54 | 16 | 2 | – | 5 | |
| 4e | –OH | Ni–Al | – | 41 | 20 | 13 | 40 | 1 | 2 | 1 | – |
| 5f | Ni–Al | – | 54 | 12 | 9 | 35 | – | 1 | – | – | |
| 6 | Ni–Al | 200 (Zn) | 1 | 21 | – | 95 | 3 | 1 | – | – | |
| 7e | Co–Al | – | 57 | 9 | 1 | 38 | 2 | 2 | 1 | – | |
| 8 | Rh/Cd | 100 (Al) | 27 | 2 | 3 | 9 | 2 | 1 | 16 | – | |
| 9 | Pd/Cd | 100 (Al) | – | 1 | – | 75 | 10 | 3 | – | – | |
| 10 | Ru/Cd | 100 (Al) | 34 | – | 15 | 7 | 2 | 2 | 18 | – | |
| 11 | Pt/Cd | 100 (Al) | 87 | 1 | – | 1 | 1 | 1 | 1 | – |
a Substrate 200 mg, catalyst 200 mg, water 2 mL, sealed tube, 120 °C, 2 h.
b Determined through GC–MS.
c Three derivatives were obtained: with two double bonds (22%), one double bond (16%) and no double bond (4%).
d Hundred milligrams of catalyst.
e Reaction time = 3 h.
f Reaction time = 1 h.
3 Results and discussion
Cinnamaldehyde was submitted to the catalytic action of several Raney metals or alloys, in a completely aqueous media, with different results. A brief picture of all experiments is presented in Table 1.
3.1 Reduction of cinnamaldehyde with Raney catalysts
3.1.1 Reduction of cinnamaldehyde with Raney alloy
The chosen Raney alloy for this series of experiments was Ni–Al. Since in our previous experiments for reductive process in aqueous media we noticed the effect of Al powder [18], we decided to use it as an additive in order to boost the reactivity of the Raney Ni–Al alloy. We investigated the process both under reflux conditions (open vessel) or in a sealed tube. When working in open flask, the stoichiometry used was: 0.01 moles of cinnamaldehyde was mixed with 10 g of Raney Ni–Al alloy in 18 mL water. When sealed tubes were used, the stoichiometry was changed to 0.01 moles of cinnamaldehyde, 2 g Raney Ni–Al alloy in 5 mL water. The added quantities of Al powder as well as other experimental conditions are summarized in Table 2.
Although no precise stereoselectivity is recorded, reduction of cinnamaldehyde in aqueous media seems to proceed according to a step-by-step reduction: first the more sensitive >C C< double bond and next the >C O double bond. However, the final distribution of products is clearly dependent on the reaction time and on the amount of Al powder. Thus, when considering the experiments carried out in open flask, under reflux conditions, it can be seen that when only Raney Ni–Al alloy is used, phenylpropanal 3 is the major compound, while when Al powder is added the reductive power of the system is enhanced and phenylpropanol 4 is formed. When the reaction time is prolonged, the reduction is completing the formation of the final phenylpropanol 4 in almost quantitative yields. In most of cases, two hydrocarbons are present in the final reaction mixture: n-propylbenzene 5 and ethylbenzene 6. If n-propylbenzene 5 is probably formed through a Clemmensen-type reduction of the carbonyl moiety, ethylbenzene 6 in its turn is formed either through decarboxylation of cinnamic acid, eventually formed in situ as an intermediate, either (more probably) by direct decarbonalytion of cinnamaldehyde. Indeed, it has been known for a while that aldehydes, in the presence of some metallic catalysts, can undergo a decarbonylation process, yielding the corresponding alkene [22,23]. Even recently, the role of palladium catalyst in such decarbonylation of aldehydes has been revisited [24]. However, in most of these processes, the main reaction product was styrene. Nevertheless, Keresszegi and coworkers have recorded the presence of both compounds (ethylbenzene and styrene) during the catalytic treatment of cinnamyl alcohol over an alumina-supported palladium catalyst [25], along with cinnamaldehyde and dihydrocinnamaldehyde as intermediates. These previous results support our assumption that the treatment of cinnamaldehyde with metal catalyst is in fact an oxido-reduction process. It is interesting to mention that in our processes, although we have recorded the presence of both n-propylbenzene 5 and 1-propenyl-benzene 7, but only ethylbenzene 6, we did not obtain any styrene.
Similar results are obtained when switching from open to closed systems.
3.1.2 Reduction of cinnamaldehyde with Raney metals and salts
The results registered in the reaction of cinnamaldehyde in water with various Raney metals (with or without any additives) are displayed in Table 3. Simultaneously with these processes, we carried out similar reactions, but in the presence of different salts of the same metals. All reactions were performed in classic reflux apparatus. The stoichiometry of these reactions is as follows: for 0.01 moles of cinnamaldehyde, 10 g of metal or salt and 18 mL of water were used. In several cases, Al powder was used as additive.
With the exception of Raney Cu and partly NiCl2 at lower reaction time, all other reagents afforded complex mixture of reduced compounds. The addition of metallic Al powder seemed to enhance the reductive capacity of the aqueous system. No special selectivity is observed, though in some cases the yield in phenylpropanol 4 is over 90% (runs 2 and 13). Higher amounts of cinnamyl alcohol 2 are obtained when Raney Co is used, which confirms the selectivity of this metal in the reduction of the carbonyl moiety of cinnamaldehyde 1 [26]. Paradoxically, cobalt chloride yielded the highest amount of phenylpropanol 4. Iron permitted the formation of somehow higher quantities of phenylpropanal 3, but in a mixture with the other reduced compounds. The addition of Zn powder to the reaction mixture seemed to drive the reductive process to a new level, since n-propylbenzene 5 is formed in almost equimolecular quantities with phenylpropanol 4. In some cases, traces of ethylbenzene were also recorded.
3.2 Reduction of cinnamaldehyde derivatives with Raney catalysts
3.2.1 Reduction of α-substituted cinnamaldehydes
In order to investigate how the existence of an electron-donating or electron-withdrawing substituent on the >C C< double bond influences the reductive process, we submitted 2-methyl respectively 2-chlorocinnamaldehyde to the following experimental conditions. Thus, 0.01 moles of substrate was treated with 10 g of Raney Ni–Al alloy in 18 mL water, under reflux conditions. The results are listed in Table 4 (Scheme 2).
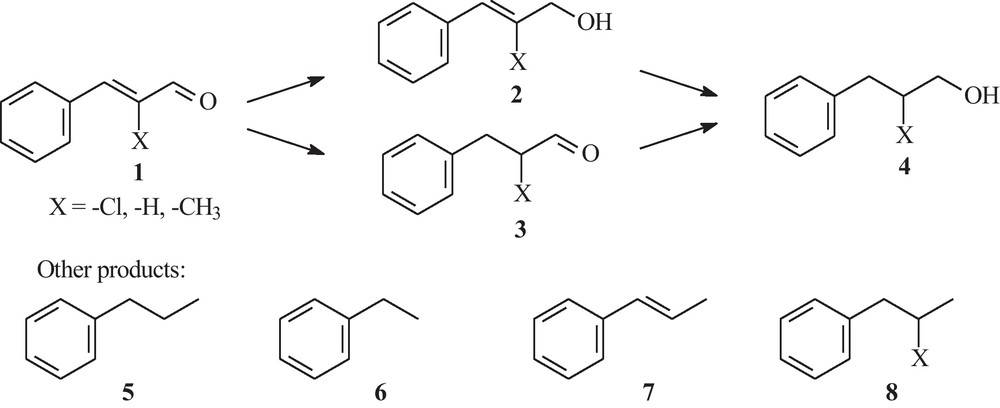
Reaction products.
The introduction of the 2-methyl group accelerated the complete reduction process toward the corresponding phenylpropanol. On the other hand, reduction of 2-chlorocinnamaldehyde proceeded poorly, high amount of starting material being recovered. Moreover, the composition of the final reaction mixture is complicated by the presence of both chlorinated and dechlorinated compounds.
3.2.2 Reduction of other cinnamyl derivatives
Two derivatives were considered for the next step: cinnamyl chloride 9 and cinnamyl alcohol 2. Because of its high reactivity, reactions with cinnamyl chloride were carried out in sealed tube. Thus, 200 mg of 9 were mixed with 200 mg of Raney catalyst in 2 mL of water and stirred for 2 hours at 120 °C (Scheme 3) (Table 5).

Reduction of other cinnamyl derivatives.
Transformation of cinnamyl chloride 9 occurred through numerous pathways:
- • reduction and hydrolysis, affording phenylpropanol 4;
- • reduction, hydrolysis and oxidation, affording phenylpropanal 3;
- • reduction and hydrodechlorination, affording n-propylbenzene 5;
- • simple hydrodechlorination, affording n-propenylbenzene 7;
- • Wurtz-type coupling, affording three 1,ω-diphenyl-C6-derivatives, with two double bonds, one double bond and no double bond.
Although catalyst poisoning due to chlorine species (especially HCl) is a possibility, [27] it has been known that when a base or a basic modifier is added in the reaction mixture, the activity and the selectivity of hydrogenation reactions conducted using catalysts such as Pt/C, Pd/C or Raney Ni, can change significantly [28,29]. In these processes, the role of the basic additive is to prevent catalyst poisoning by trapping the HCl produced by the reaction. An advantage of our process is that there is no need for a supplementary compound (base or modifier) in the reaction mixture, since Al(OH)3 is generated in situ through the necessary reaction of Al with water, reaction which generates the reductive species of hydrogen.
Nevertheless, these mixed results are an indication that the process that occurs in aqueous media is not a simple reduction, but an oxido-reduction. This assumption was confirmed by the results obtained when cinnamyl alcohol 2 was used as starting material. Indeed, in many cases, considerable amounts of cinnamaldehyde were obtained, a clear sign that an oxidative process is also occurring. This is even more obvious when treating cinnamyl alcohol with a noble metal catalyst, when only reductive processes occurred. The reductive effect of metallic Al powder is again exemplified by the result in run 2, when the yield in n-propylbenzene is doubled compared to runs 1 and 3.
Compared to some of our previous researches [16–18], the reduction in aqueous media of cinnamaldehyde is a little different. In our previous research, we have established that aromatic carbonyl compounds in similar conditions afforded hydrocarbons, a conjugation between the aromatic nuclei and the carbonyl group being necessary (e.g. benzaldehyde afforded toluene, acetophenone–ethylbenzene). In the case of cinnamaldehyde, although there is a conjugation between the carbonyl moiety and the aromatic nuclei (through the >C C< double bond), a certain number of oxygenated derivatives are obtained, especially phenylpropanol. This confirms the higher sensitivity toward reduction of the middle >C C< double bond, breaking up the conjugation and leaving thus the >CH O group as substrate for a simple and mild reduction to a–CH2-OH group, and in the same time, leaving the–CH2-OH group to suffer mild oxidation back to the >CH O group.
The evidences brought in this material show therefore that the treatment of carbonyl compounds in an aqueous media is an oxido-reductive process.


