1 Introduction
The study of inorganic–organic hybrid materials has showed considerable attraction due to their potential for the development of new material with designed properties [1,2]. Hybrid materials are in the edge of two different chemical worlds, since they not only combine the advantages of both organic components (flexibility, low dielectric constant, low density and process ability) and inorganic ones (rigidity, strength, durability, and heat resistance), but also often exhibit exceptional properties that exceed what would be expected for a simple mixture of the components [3–5].
According to the nature of the links and interactions between the organic and inorganic parts, the hybrids can be divided into two main different classes [6]: the first class would be composed of the molecule-based composite materials, in which the organic and inorganic components are linked together through strong chemical interactions (covalent, ion-covalent, or coordination bonds), and the second one belongs to the hybrid systems, in which one of the components (usually the organic) is entrapped within a network of the other. For the latter, only weak interactions, such as hydrogen bonding, Van der Waals forces or weak static effects, operate [7,8]. These materials with tailored properties are adequate for use as adsorbent synthesis [9], gas separation techniques [10], catalysis [11–14], molecular recognition [15,16], nanoreactors [17], and biological uses [18,19].
Silica is a widely involved inorganic material in the synthesis of inorganic–organic hybrid materials. On the other hand, poly(ethylene glycol) is the most useful polymer in terms of its water solubility, body reservation, and biological safety. It has been used as a catalyst or (and) solvent in many organic transformations [20–22]. The main purposes of the present work are sol–gel synthesis, structural characterization and potential applications of PEG–silica hybrids as new phase transfer catalysts for rapid, efficient and regioselective formation of β-azido alcohols and β-cyanohydrins.
2 Experimental
2.1 General
Epoxides and other chemical materials were purchased from Fluka and Merck in high purity. Products were characterized by comparison of their physical data (IR, 1H NMR, and 13C NMR spectra) with those of known samples. NMR spectra were recorded in CDCl3 on a Bruker Advance DPX 400 MHz instrument spectrometer using TMS as the internal standard. FT–IR spectra were recorded on a BOMEM MB-Series 1998 FT–IR spectrometer.
The morphology was examined by SEM using a Philips XL30 scanning electron microscope. The DTA curve of the PEG–silica hybrid was recorded on a BAHR, SPA 503 at heating rates of 10 °C min−1.
2.2 Preparation of PEG–silica hybrid particle
To a solution of PEG-300 (3 g, 10 mmol) in toluene (20 mL), NaH (1.44 g, 60 mmol) suspended in toluene (10 mL) was added at 0 °C under nitrogen atmosphere. Then, the system was stirred at 60 °C for 1 h to complete the terminal alkoxides formation of PEG and cooled again to 0 °C. To the mixture, 3-chloropropyltrimethoxysilane (5.97 g, 30 mmol) was added and stirred at 60 °C for 90 h. After removing of the solvent under reduced pressure, 7.5 g of deionized water and 33.75 g of 2 M HCl solution were added, then the mixture is stirred at 40 °C for 24 h under an argon atmosphere. The resulting mixture was then transferred into a Teflon-lined autoclave and heated at 100 °C for 72 h under static conditions. The obtained mixture was first thoroughly washed with deionized water/ethanol solvent and then dried at room temperature.
2.3 Typical procedure for the preparation of β-azido alcohols and β-cyanohydrins in water catalyzed by PEG–silica hybrid.
To a magnetically stirred mixture of the epoxide (1.0 mmol) and nucleophilic reagents (NaN3 or NaCN) (2 mmol) in H2O (5 mL), PEG–silica hybrid (0.2 g) was added. The resulting mixture was stirred at 90 °C for the appropriate time (0.5–2.5 h). After completion of the reaction as indicated by TLC [using n-hexane/ethyl acetate (5:1)], the insoluble catalyst was filtered off and the filtrate extracted with diethyl ether (2 × 10 mL). The organic phase was dried over calcium chloride, and evaporated in vacuo to give the product in 78–86% isolated yields.
3 Results and discussion
The synthetic strategy for the PEG–silica hybrid by sol–gel method is presented in Scheme 1. To introduce PEG into the network structure of the silica matrix, first, the bis(3-trimethoxysilylpropyl)-polyethylene glycol precursor was synthesized by the reaction of 3-chloropropyltrimethoxysilane with the alkoxides formed on the PEG terminals. The organic–inorganic hybrid silica, PEG–silica, was then synthesized by hydrolysis and polycondensation of the precursor under mild acidic conditions (Scheme 1).

Synthesis of the PEG–silica hybrid. Color available online.
The chemical structure of PEG–silica hybrid was characterized using FT–IR, XRD, SEM, and DTA analyses.
The FT–IR spectra of the prepared PEG–silica hybrid (Fig. 1) showed bands corresponding to the structural units of the solid network. The broad band near 3400 cm−1 is assigned to the SiO–H stretching of surface silanols. In addition, the peak at 1635 cm−1 is also assigned to the adsorption of moisture on the material surface [23]. The intensities of these peaks in PEG–silica hybrid were reduced compared to silica, which indicated the enhanced hydrophobicity of the PEG–silica hybrid surface. The intense and broad band at 1100–1050 cm−1 and the shoulder at around 1200 cm−1 are attributed to the Si–O–Si asymmetric stretching vibrations of the dense silica network. In addition, the Si–O–Si symmetric stretching vibrations and its bending mode appeared at 800 and 460–465 cm−1, respectively. The peaks at 2928 and 2881 cm−1 presented the CH2 groups in the PEG backbone. The peaks at 1350 and 1440 cm−1 are shown in those of PEG with slight shifts. The spectral shifts occurring by a strong interaction between the two components indicate the hybrid nature. Through the FT–IR spectra, the successful introduction of PEG onto the silica surface was verified.
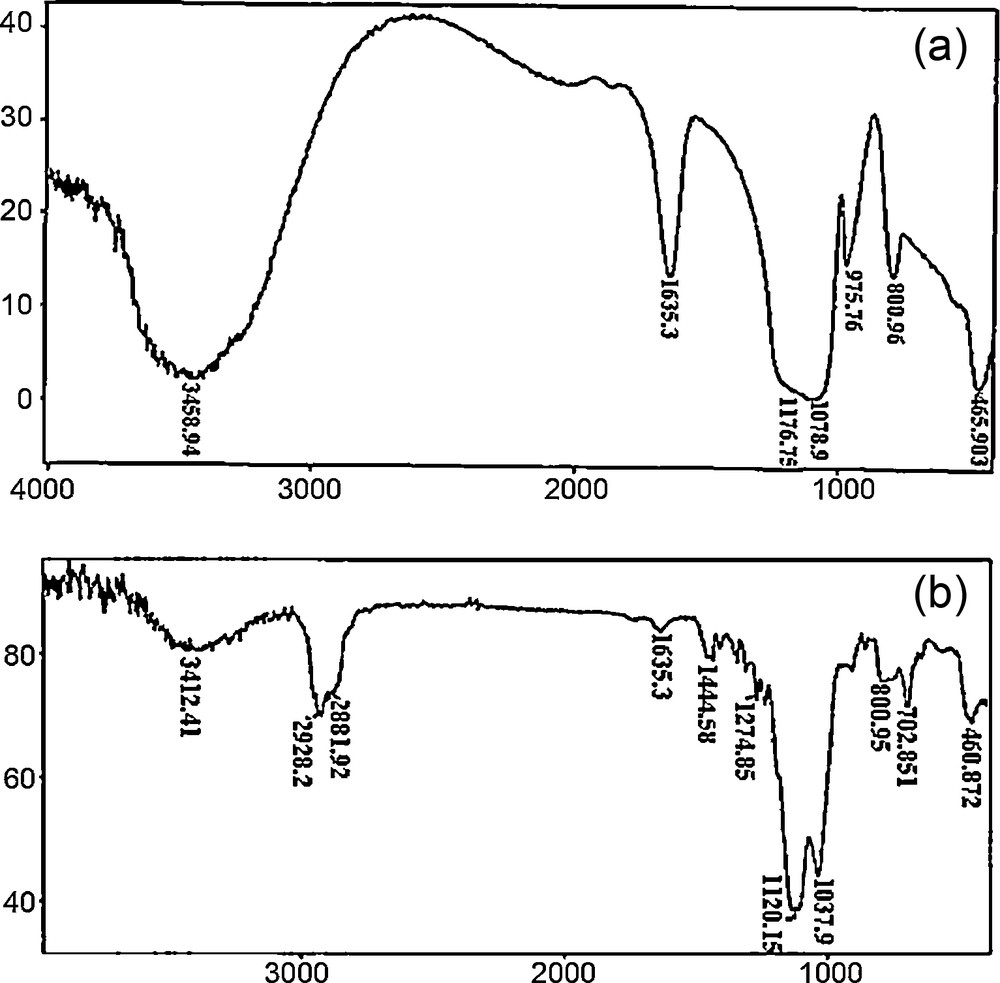
FT–IR spectra (a) of silica, and (b) of the PEG–silica hybrid.
Fig. 2 shows powder X-ray diffraction (XRD) patterns of the hybrid material prepared through a covalent self-assembly process via a sol–gel technology. The broad peak around 22° in the XRD patterns is ascribed to amorphous silica [24,25].
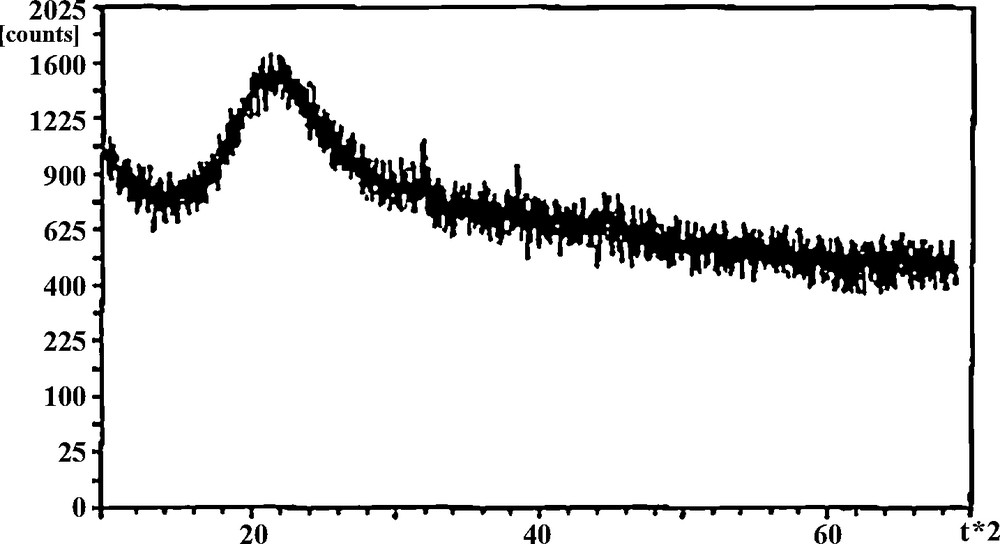
XRD pattern of the PEG–silica hybrid.
The introduction of PEG onto the silica network was also confirmed through thermogravimetric analysis (TGA) and differential thermal analysis (DTA). The weight loss by changing temperature was measured to estimate the rate of thermal degradation. Fig. 3a shows TGA the thermogram of the PEG–silica hybrid particles. The TGA curves of the hybrid particles show a weight loss of about 55% at around 610 °C. The PEG–silica hybrid material loses most of the weakly bonded water molecules below 300 °C. At higher temperatures, around 300–400 °C, the unreacted PEG monomers are released and the PEG polymer near the surface of hybrid particles is also decomposed. Finally, the PEG polymer is lost in the core of the particles at around 600–700 °C. Considering that the free PEG decomposes completely at 610 °C, its temperature of complete decomposition is delayed by 300 °C compared to that of the free PEG polymer.

TGA (a) and DTA (b) curves of the PEG–silica hybrid.
The DTA curve of the PEG–silica hybrid also shows that most of the degradation occurred around 620 °C (Fig. 3b), which is rather high compared to conventional PEG, which generally starts decomposing at around 400 °C [24]. It is believed that the thermal stability of PEG bounded to silica is largely improved because:
- • the interaction between PEG and silica is very strong and;
- • the silica matrix prevents the transfer of heat to the PEG polymer located inside this network [26,27].
The morphology of the samples was observed by scanning electron microscopy. Fig. 4 shows SEM images of unmodified amorphous silica [28] and of PEG–silica hybrid material. The separated and amorphous hybrid particles were observed for the PEG–silica hybrid. In addition, the SEM images show the presence of voids that can be attributed to the silica network. SEM micrographs of the synthesized nanocomposite also show that the particles have a spongy structure and a uniform distribution of the hybrid matrices.

SEM image (a) of unmodified amorphous silica and (b) of the PEG–silica hybrid.
We examined the catalytic ability of PEG–silica hybrid for nucleophilic ring opening of epoxides in H2O. Initially, ring opening of phenyl glycidyl ether was investigated with NaN3 in the presence of PEG–silica hybrid. TLC analysis of the reaction mixture interestingly showed that this catalyst acted very efficiently in H2O, and that 0.2 g of the catalyst was enough to convert 1 mmol of different epoxides, carrying electron-donating or -withdrawing groups, to their corresponding β-azido alcohols in high yields. It is noteworthy that no evidence of the formation of diols as a by-product of the reaction was observed (Scheme 2).
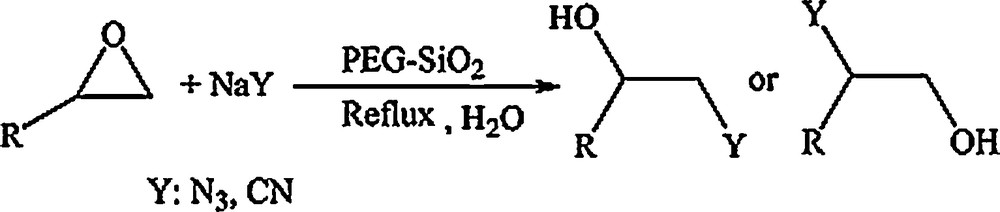
Preparation of β-azido alcohols and β-cyanohydrins catalyzed by the PEG–silica hybrid.
As shown in Table 1, using the optimized reaction conditions (3:1 molar ratio of NaN3:epoxide and 0.2 g of the catalyst), styrene oxide afforded the product of the nucleophilic attack at the benzylic position as the major product, while 2-alkyl epoxides gave the products just formed by the cleavage of the CH2–O bond. These observations demonstrated that in the former case, the product was generated through the formation of a stabilized benzylic cation during the reaction and that in the second case, they were formed by predominant attack of the azide ion on the less hindered carbon of the epoxide. The structures of the products were established from their spectroscopic (1H and 13C NMR) data. Furthermore, cyclohexene oxide as a cycloalkyl epoxide reacted smoothly in an SN2 fashion with NaN3 in the presence of PEG–silica hybrid to afford the corresponding β-azido alcohol in high yield. The configuration of the ring opening product was found to be trans from the coupling constants of the ring protons in the 1H NMR spectrum.
Nucleophilic ring opening of epoxides with azide and cyanide anions in water catalyzed by our polyethylene glycol–silica hybrid.
| Entry | Epoxides | Product(s)a | Yield (%)b |
| 1 | 86 (90:10)c | ||
| 90 | |||
| 2 | 89 | ||
| 93 | |||
| 3 | 92 | ||
| 90 | |||
| 4 | 87 | ||
| 86 | |||
| 5 | 80 | ||
| 93 | |||
| 6 | 81 | ||
| 85 | |||
| 7 | 85d | ||
| 8 | 40e |
a Products were identified by comparison of their physical and spectral data with those of authentic samples.
b Isolated yields.
c According to GC analysis.
d In the presence of pure PEG-300.
e In the presence of pure silica.
It was noted that PEG–silica hybrid did not suffer from extensive mechanical degradation and was quantitatively recovered simply by filtration and washing with H2O and MeOH. It could be reused several times.
With this promising results in hand and establishing the advantages of the PEG–silica hybrid as a phase transfer catalyst, we focused our attention to the ring opening of epoxides with another anion, CN– in H2O. Thus, different types of oxiranes carrying activated and deactivated groups were cleanly, easily and efficiently converted into the corresponding β-cyanohydrins in good yields under the optimized reaction condition for N3–. The scope and generality of this process is illustrated with several examples and the results are summarized in Table 1.
It should be pointed out that in the absence of the catalyst, the reaction was sluggish and a considerable amount of starting material was recovered unchanged. In addition, the reaction medium was contaminated by diol. The reaction was also performed in the presence of pure silica (Table 1, entry 7) and pure PEG-300 (Table 1, Entry 8). As shown in Table 1, although the best results were obtained in the presence of PEG–silica nanocomposite and pure PEG-300, in aqueous conditions, the PEG–silica nanocomposite is recoverable and suitable.
This organic–inorganic hybrid catalyst contains two different units linked together. It is thought that PEG can enhance the hydrophobicity as well as the hydrophilicity of silica cavities and, therefore, that it can be used as a solid–liquid phase transfer catalyst in the ring opening of epoxides in water. In addition, PEG units can probably facilitate the ring opening of the epoxide by hydrogen bonding and by forming complexes with cations, much like crown ethers, and these complexes cause the anion's activation (Scheme 3).
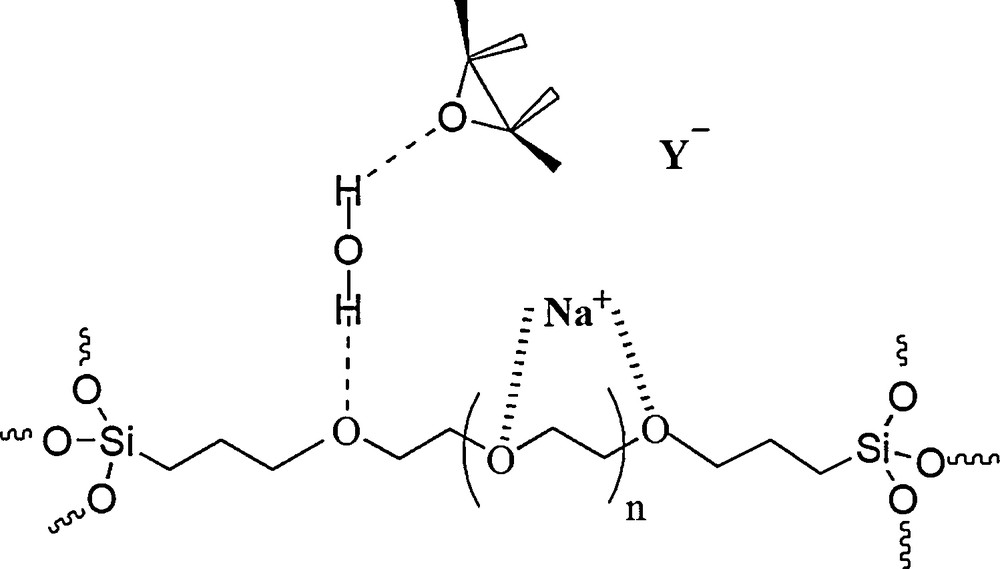
Postulated roles of the PEG–silica hybrid for the nucleophilic ring opening of epoxides.
4 Conclusion
In the present work, the PEG–silica hybrid was successfully prepared and its performance as a solid–liquid phase transfer catalyst for regioselective ring opening of epoxides in water was investigated. Compared to some previously reported results about PEG as phase transfer catalyst with major or minor drawbacks, several noteworthy features of this reagent are apparent. These are easy work-up procedure, good stability, operational simplicities, and use of inexpensive reagents. In addition, it was demonstrated to be a good alternative to the more sophisticated crown ethers as a catalyst in solid–liquid phase transfer reactions. It can be emphasized that the reaction is clean from economical and environmental points of view, since using water as the solvent is more favorable than using organic solvents.
Acknowledgements
We are grateful to the Research Council of Shahid Chamran University for financial support.


