1 Introduction
Rosemary (Rosmarinus officinalis L.) is a perennial shrub, which is originated in the Mediterranean area. The plant is also cultivated in Spain, Morocco, Tunisia, and the south-east of Europe. Leaves of rosemary have an intense aromatic flavor and bitter, slightly spicy taste. Rosemary is widely used for seasoning and flavoring foods, as a preservative agent and an antioxidant. Also pharmaceutical applications are known [1].
The essential oil from rosemary is commonly gained by hydro (HD) or steam distillation (SD) with a maximum extraction yield of 1.0–2.5%. The colorless or slightly yellow oil contains 1,8-cineole (15–30%), camphor (10–25%), α-pinene (10–25%), and borneol (3–20%). The chemical structures of these substances are presented in Fig. 1. Other compounds are bornyl acetate (1–5%), camphene (5–10%), α-/β-terpineol, myrcene, limonene, and caryophyllene. Essential oils from Spain or Tunisia can additionally contain a relatively high amount of verbenone. The ratio of these terpenes varies depending on the origin and chemo type of the rosemary plant [1–3]. The essential oil is located in glandular trichomes on the surface of the rosemary leaves [4]. Rosemary oil is used as an antibacterial, antifungal, and anticancer agent [5]. Hydro and steam distillations are easy methods to extract the essential oil from rosemary leaves. For hydro distillation, rosemary leaves and water are put together into a flask. The suspension is heated until boiling. This procedure is in contrast to steam distillation, where the steam is generated in a separate flask and guided through the plant material. The steam takes the essential oil along and the water/oil mixture and is condensed. A two phase system with water and the essential oil is produced, where the oil can be decanted and recovered [6]. The distilled and condensed water phase is called hydrosol. If this hydrosol is recycled and taken to carry out another steam or hydro distillation the process is called cohobation [7]. Hydro and steam distillations merely work because of the coexistence of two immiscible liquids (water and essential oil). The vapor pressure of the system is equal to the sum of the vapor pressures of the pure compounds. The boiling point of the mixture is lower than the boiling points of water and the essential oil. Thus, the essential oil can be extracted without reaching the boiling point of the single compounds. A limitation of this method is that low volatile substances can only be recovered in small quantities [2,8]. Alternative methods to extract the essential oil from rosemary are supercritical carbon dioxide extraction [5,9] and subcritical water extraction [10].

Chemical structures of the main compounds present in rosemary leaves, subdivided into antioxidants (rosmarinic acid, carnosic acid, and carnosol) and essential oils (camphor, 1,8-cineol, α-pinene, borneol, and α-terpineol).
Antioxidants (AO) are compounds which can inhibit or retard the oxidation of lipids and other biomolecules. They prohibit the start of an oxidizing chain reaction by radicals or quench the propagation. These reactions can cause functional damage to the human body, like cancer or cardiovascular diseases. Antioxidants can prevent this process due to their redox properties like reductive behavior, the donation of hydrogen or quenching of singlet oxygen [11,12]. Rosemary is one of the major resources for natural antioxidants. The most important compounds are the phenolic diterpene carnosic acid (CAc) and the phenolic rosmarinic acid (RAc). Carnosol (CA) and rosmanol are formed by oxidative degradation of carnosic acid and are not contained initially in the leaves. Thus, these compounds are artifacts of the extraction process. The chemical structures of these antioxidants are shown in Fig. 1 [3,13]. The content of these antioxidants in the leaves varies in a large range due to seasonal variations, environmental influences, species, and growing origin. Also large fluctuations in the individuals of the same population have been reported. In the literature the content of carnosic acid varies from 4 to 30 mg per 1 g of rosemary. The mass concentration of rosmarinic acid in the leaves is in the range between 2 and 25 mg/g [14–16]. However, these compounds do not only show antioxidant activity. Rosmarinic acid is also known for its antiviral, antibacterial, anti-inflammatory, and chemo-protective properties. Moreover, rosmarinic acid is a potent HIV-1 integrase inhibitor [17,18]. Furthermore, carnosol and carnosic acid have anti-carcinogenic and anti-inflammatory properties [19,20]. Common methods to extract antioxidants from rosemary leaves are solvent extraction (methanol, acetone, hexane, etc.) sometimes assisted by sonication [21,22], supercritical carbon dioxide extraction [23,24], and subcritical water extraction [25]. Since 2010, rosemary extracts are classified as food additives by the European Commission and assigned the number E392 [26]. They can be an alternative for artificial antioxidants, which have been partially restricted for food additives [27]. Synthetic antioxidants like butylated hydroxyanisole (BHA; E320) and butylated hydroxytoluene (BHT; E321) are harmful and potential carcinogens [28–30]. For this reason, the application of natural plant extracts as natural antioxidants in the food industry gains more and more interest and importance.
There are several procedures to determine the total antioxidant activity of substances. For example spectrophotometric methods like DPPH assays [31], Folin-Ciolteau assays [32], and many others. Also (bio-)amperometry and cyclic voltammetry can be used [33]. In this study 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays were chosen, because of the fast and easy method to investigate the total antioxidant scavenging activity. Also the experimental procedure can be easily and fast adjusted and customized to the analysis experiments. It was first developed by Blois to determine the total antioxidant activity of compounds [34]. DPPH is a dark colored powder of stable free-radical molecules, which is soluble in methanol or ethanol. It has a strong absorption maximum at a wavelength around 515 nm due to the presence of an unpaired electron. As this electron becomes paired off in the presence of an antioxidant (hydrogen donor), the absorption strength decreases. A color change from violet to yellow can be observed (see Fig. 2). This change of color due to change in absorbance can be used to determine the antioxidant activity of compounds [35,36]. To obtain reproducible results it is important to investigate the reaction kinetics of DPPH and the antioxidant. The time until the reaction reaches a steady state depends on the antioxidant compound [31].

Reaction between the DPPH radical (violet) and an antioxidant yielding the neutralized DPPH molecule (orange). The corresponding UV/VIS spectra are also shown. A significant decrease of the absorbance at 518 nm appears during the reaction and can be used to follow the reaction.
2 Materials and methods
2.1 Plant materials and characterization
Dried rosemary (R. officinalis L.) leaves were obtained from Phytotagante/France. The plants were cultivated in the region of Oujda, in the north of Morocco and dried there in the sun after harvesting.
The residual moisture was determined in a compartment drier at 40 °C. Higher temperatures should be avoided to prevent the loss of flavor through volatilization of essential oil [37]. For this purpose, the mass loss of five samples (each 1 g) with finely ground rosemary leaves was determined every hour until a constant weight was reached. A constant value was achieved after approximately 48 h.
2.2 Soxhlet extraction
To determine the total content of rosmarinic acid and carnosic acid in the leaves, various Soxhlet extractions were carried out. For this purpose, about 6 g of ground rosemary leaves were extracted for 4 h with approximately 50 mL of solvent. Three different solvents were investigated: water (millipore), methanol (HPLC-grade, Merck), and acetone (p.a., Merck). After extraction, the volume of the extract was readjusted at room temperature to 50 mL with the corresponding solvent. Then, 0.5 mL of the extract solution was mixed with 0.5 mL of methanol (90%) and afterwards 1 mL of the internal standard solution (see Section 2.5.2) was added. The solution was filtrated through a 0.2 μm PTFE-syringe filter and then analyzed by HPLC/UV. All extractions were carried out three times.
2.3 Steam distillation
Steam distillations of rosemary leaves were performed in an industrial scale. For this purpose, 800 kg of dried rosemary leaves were loaded on a perforated grid at the bottom of a stainless-steel preheated alembic and compacted to ensure the spreading of steam over the entire load. The alembic top lid was closed and the water at the bottom was heated until boiling. The pressure regulation valve was fully opened until the first drops of the distillate appeared. The valve was then slightly closed so that the distillate could be rightly cooled in the condenser which prevented the oil from being evaporated. The essential oil and the hydrosol were simultaneously collected in essence containers. The effect of cohobation was investigated for three experiments. In this method, the water phase from the distillate is poured back into the alembic to avoid the loss of essential oil in the hydrosol. Every 30 min the yield of the essential was examined and a sample was taken for GC analysis.
2.4 Hydro distillation
Hydro distillations of rosemary leaves were performed in a lab scale. For this purpose, 25 g of dried rosemary leaves were added in a 500 mL round bottom flask and 400 mL of water (Millipore) were added. A condenser was put on the top of the flask. The essential oil/water mixture was collected in a 100 mL graduated cylinder filled with 10 mL methyl tert-butyl ether (≥99%, Merck) as the receiver for the essential oil. The suspension was heated with a heating mantle while stirring. Extractions were carried out for 0.5, 1.5, 2.5, and 4 h each three times.
The hot rosemary/water mixture was filtrated immediately after the experiment in a 500 mL volumetric flask through a filter paper. The brown solution, called “tea”, was filled up to the end volume at room temperature and stored at −20 °C until analyses. For HPLC analyses 1 mL of the aqueous solution was mixed with 1 mL of the internal standard solution, filtrated through a 0.2 μm PTFE-syringe filter and then analyzed by HPLC/UV (see Section 2.5.2). For DPPH assays the crude product or dilutions were directly used (see Section 2.5.3).
For the preparation of the distillate, the ether phase with the dissolved essential oil was collected in a vial. The volatile solvent was evaporated under a nitrogen stream and the mass of the pure essential oil was examined. For GC analyses a 10 mg/mL solution of the essential oil in ethanol (≥99.8%, Sigma-Aldrich) was prepared, filtrated through a 0.2 μm PTFE-syringe filter and then analyzed by GC/FID (see Section 2.5.1).
The residual leaves were dried in a compartment drier over night at 40 °C. To determine the remaining amount of antioxidants, Soxhlet extractions with methanol (see Section 2.2) were carried out.
2.5 Analysis methods
2.5.1 Gas chromatography (GC)
GC analyses of the essential oil samples of hydro distillation were carried out on a Hewlett Packard HP 6890 Series GC system equipped with a flame ionization detector (FID). A nonpolar HP-5 (5% phenyl- and 95% methyl-siloxane) capillary column (30 m × 0.32 mm i.d., 0.25 μm film thickness) was used for separation. Helium was applied as carrier gas at a flow of 1 mL/min. The GC was equipped with a split/split less injector which was held at 275 °C. An HP 6890 Autosampler was employed to inject 1 μL of the sample in the split mode using a split ratio of 1:10. The FID was maintained at 275 °C. The temperature of the oven was initially held at 70 °C for 3 min and then increased to 220 °C at 6 °C/min. Finally the temperature was raised to 250 °C at 10 °C/min and was then remained at 250 °C for 10 min. Analysis of each sample was carried out three times.
The content of the camphor in the essential oil was determined quantitatively by external calibration. For this purpose, a stock solution (1 mg/mL) of camphor (96.5%, Alfa Aesar) in ethanol (≥99.8%, Sigma-Aldrich) was prepared. This primary stock solution was diluted to concentrations of 5.0, 2.5, and 0.5 mg/mL. The solutions were filtrated through a 0.2 μm PTFE-syringe filter and then measured by GC/FID.
GC analyses of the essential oil samples of steam distillation were carried out on a Hewlett Packard HP 6850 Series GC system equipped with a flame ionization detector (FID). A polar DB-WAX (polyethylene glycol) capillary column (20 m × 0.1 mm i.d., 0.2 μm film thickness) was used for separation. Hydrogen was applied as the carrier gas at a flow of 0.7 mL/min. The temperature of the injector was held at 250 °C and 0.2 μL of the pure essential oil was injected. The FID was maintained at 275 °C. The temperature of the oven was initially held at 60 °C for 2 min, increased to 248 °C at 12 °C/min and remained for 5 min. Analysis of each sample was carried out three times. The quantification of camphor was performed by external calibration.
2.5.2 High performance liquid chromatography (HPLC)
The contents of rosmarinic acid (RAc) and carnosic acid (CAc) in the extracts were determined by HPLC/UV. The analyses were performed on a “Waters HPLC System” with two Waters 515 HPLC Pumps, a Waters 717plus Autosampler and a Waters 2487 UV/VIS-Detector. Separation was achieved on a Knauer Eurosphere C18-column (100 Å, 250 × 4.6 mm). The injection volume was each 10 μL. The compounds were eluted at a flow rate of 1.0 mL/min and a temperature of 30 °C. The solvents for gradient HPLC consisted of 0.1% formic acid (A) and acetonitrile (B) (HPLC-grade, Merck). The composition of the mobile phase started at 10% B, it was increased to 40% B within 40 min, further increased to 100% B within 20 min and then hold for 20 min. The detection wavelength was 204 nm. Analysis of each sample was carried out three times.
The content of the antioxidants (AO) was determined quantitatively by internal standard (IS) calibration. For this purpose, stock solutions (1 mg/mL) of rosmarinic acid (99%, Sigma-Aldrich) and carnosic acid (99%, Phytolab) in methanol (90%) were prepared. These primary stock solutions were diluted to concentrations of 1.0, 0.8, 0.6, 0.4, 0.2 mg/mL. To 1 mL of each sample, 1 mL of a 1 mg/mL solution of the internal standard gemfibrozil (98%, Cayman) was added [38]. The solutions were filtrated through 0.2 μm PTFE-syringe filters and then measured by HPLC/UV. All samples were analyzed three times. Afterwards the response factor K was calculated, which is K(CAc) = 1.36 for carnosic acid and K(RAc) = 0.84 for rosmarinic acid. For the analysis of the extracts, a gemfibrozil solution (1 mg/mL) was added to every sample and the concentration of the antioxidants was estimated with the response factors and according to Eq. (1).
| (1) |
2.5.3 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assays
The free radical scavenging activity was determined using the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical (Sigma-Aldrich). The experiments were performed according to methods proposed by Popovici et al. [39] and Roby et al. [40], which were modified for the present assays. For calibration, a stock solution (0.1 mg/mL) of DPPH in methanol (90%) was prepared. Solubilization of the compound was enhanced in an ultrasonic bath. The solution was diluted to concentrations of 0.075, 0.05, 0.025, and 0.0125 mg/mL. Due to photosensitivity the DPPH samples are protected from light until analyses. All samples were transferred in disposable polystyrene cuvettes (10 × 10 × 45 mm, Sarstedt). The UV/VIS spectra from 350 to 700 nm of the solutions were acquired using a Varian Cary 3E UV/VIS spectrometer.
The antioxidant activity was measured for different pure compounds. For this purpose, solutions of rosmarinic acid, carnosic acid, butylated hydroxyanisole (BHA) (96%, Acros organics), ascorbic acid (reagent grade, Sigma-Aldrich), and α-tocopherol (≥95.5%, Sigma-Aldrich) with a concentration of 1 mg/mL in methanol (90%) were prepared. Each solution was diluted to concentrations of 0.75, 0.5, 0.25, and 0.125 mg/mL. Dilutions of the hydro distillation water residues were prepared with the following proportions: 1/2, 1/5, 1/10, 1/20. To classify the results of the antioxidant activity, DPPH assays were also carried out for the Soxhlet extracts. For this purpose, dilution of the Soxhlet extract solutions with proportions of 1/2, 1/5, 1/10, 1/20, and 1/40 were prepared.
0.05 mL of every sample was mixed with 3.95 mL of DPPH-solution (0.1 mg/mL = 250 μM) in a lockable glass envelope, transferred in a disposable polystyrene cuvette and the UV/VIS spectrum was measured after exactly 60 min of reaction time. A blank sample was prepared in the same way, but only with 0.05 mL methanol and 3.95 mL DPPH-solution. Every sample was prepared and measured three times.
The inhibition I of the DPPH radical was calculated with Formula 2. Inhibition is the ratio between the decrease of the absorbance in the sample and the initial absorbance of the blank DPPH solution at 518 nm.
| (2) |
3 Results and discussion
3.1 Yield of essential oil
First, the differences between steam and hydro distillations for the recovery of the essential oil were examined. These processes have been already extensively studied for rosemary leaves from different origins [6,41,42]. In this study, the results refer to rosemary which was cultivated in Morocco. The maximum extraction yield of essential oil obtained by steam distillation is 2.5% (w/w), whereas hydro distillation only provides 1.8% (w/w) of the initial leave weight. These values are higher than the one of the Algerian and Tunisian rosemary, where the maximum yield of essential oil by steam distillation was approximately 1.2% and 0.44% by hydro distillation [6]. Fig. 3 shows the differential and total extraction yields of essential oil as a function of distillation time. A similar behavior of hydro and steam distillations can be observed. At the beginning the yield increases quickly and then becomes slower with time until a plateau is reached. The trend of the curves is similar to the ones reported in the literature [43]. After 4 h of extraction the complete amount of essential oil is recovered. Moreover, it is shown that after 30 min of distillation 67% of the essential oil are recovered by steam distillation, but only 22% by hydro distillation. The yield after 0.5 h of extraction is indeed 1.7% (w/w) for steam distillation and 0.54% (w/w) for hydro distillation.

(A) Differential and (B) total extraction yield of essential oil gained by hydro distillation and steam distillations at different times. The yield is given in weight percent of the initial mass of rosemary leaves.
In addition, the influence of cohobation during steam distillation has been investigated. Normally, the rosemary hydrosol is cohobated separately in an empty alembic after reaching a certain volume. In this case, a fixed volume of hydrosol was added to the alembic filled with rosemary before the distillation. In view of the results, the addition of hydrosol negligibly decreases the extraction yield of rosemary oil from 2.5% to 2.3% by slowing down the distillation.
3.2 Content of camphor in essential oil
The appearance of crystallized camphor was once noticed during the steam distillation of rosemary after 1.5 h. The crystals generated significant attention with regard to the rosemary essential oil quality. Actually, a high dose of camphor is toxic when ingested and may cause convulsions and vomiting [44]. Therefore, the composition of rosemary essential oil for commercial use is regulated in the NF ISO 1342:2001 [45]. The content of camphor in the essential oil gained by hydro and steam distillations is thus investigated over time for Moroccan rosemary.
Fig. 4 presents the camphor content in the essential oil as a function of distillation time. Again, a similar behavior of hydro and steam distillations can be observed. At the beginning, the camphor content increases quickly and then decreases again. Only the distillation time with the maximum content differs between hydro and steam distillations. Hydro distillation reaches a maximum value of camphor after 1 h of distillation, whereas the maximum is reached only after 2 h. The differences can be explained by the varying order of compounds depending on the distillation method. In the literature it is suggested that the volatile compounds are recovered in the ascending order of their boiling points by steam distillation. By contrast, in the case of hydro distillation the order of compound recovery depends on their polarity [6]. Due to the high boiling point (209 °C) of camphor and the slightly polar structure, the extraction time is smaller in hydro than in steam distillation.
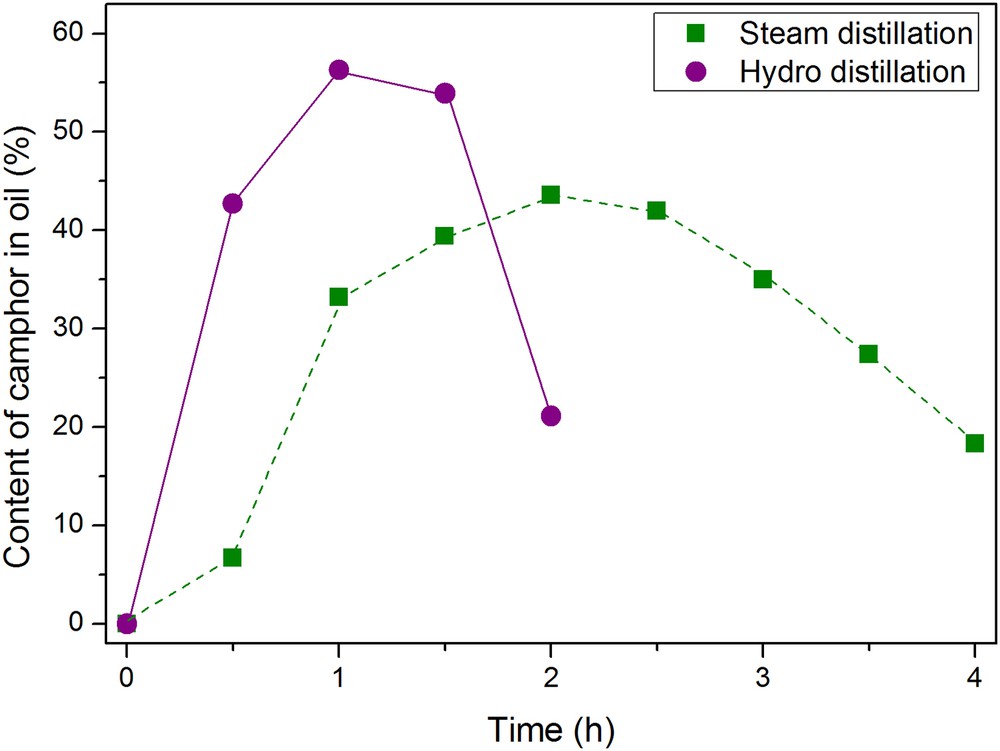
Time-dependent content of camphor in the rosemary essential oil gained by hydro distillation and steam distillation. The yield is given in weight percent of the mass of essential oil collected after the given time.
In addition, the content of camphor in the essential oil is relatively high and the analyses of the different oil fractions show that only the sample of a 0.5 h lasting steam distillation meets the ISO 1342 international standard. Here, the content of camphor is limited to a value from 5 to 15% in the essential oil of Moroccan rosemary. Cohobation of the hydrosol even increases the content of camphor in the essential. This can be a result of the solubility of camphor in water. After the first extraction the hydrosol is saturated with camphor. If this residue is cohobated, no more camphor is soluble due to the saturated water. As a result, more camphor can be extracted.
3.3 Total antioxidant content and residual moisture of leaves
The residual moisture of the rosemary leaves was determined to be 3.5 ± 0.1%. Soxhlet extractions were carried out to determine the total amount of rosmarinic acid and carnosic acid. The challenge to extract these compounds is that rosmarinic acid is soluble in water, whereas carnosic acid is not. Fig. 5 shows the three different investigated solvents for Soxhlet extractions. As expected, water, a polar protic solvent, can extract the highest amount of rosmarinic acid with 8.90 mg per 1 g rosemary, but only a negligible amount of carnosic acid. The extraction behavior of the aprotic polar solvent acetone is opposite and the highest amount of carnosic acid with 23.62 mg/g can be extracted. Methanol is also a protic solvent, but less polar than water. It combines the extraction efficiency of both, water and acetone.

Mass concentration of rosmarinic acid and carnosic acid yields obtained by Soxhlet extraction lasting for 4 h with different solvents: water, methanol, and acetone. Concentrations are given in mg of antioxidant per 1 g of rosemary leaves.
The differences in extraction selectivity can also be seen in the chromatograms of the different solvent extracts (see Fig. 6). Water extracts compounds with small retention times (r.t.), which are polar substances, whereas acetone extracts less polar substances with higher retention times. The chromatogram of the methanol extract is nearly a combination of both. Also the main degradation product of carnosic acid, which is carnosol, can be extracted with each solvent. Other antioxidants which are present in the extracts are rosmanol (r.t. = 28.2 min) and methyl carnosate (r.t. = 57.9 min). As a result, methanol is a suitable solvent for Soxhlet extraction to determine the total amount of both antioxidants, rosmarinic acid and carnosic acid, from rosemary leaves.

HPLC-chromatograms of Soxhlet extracts from rosemary leaves obtained by different solvents: water, methanol, and acetone. Peak identification: (1) rosmarinic acid; (2) carnosol; (3) gemfibrozil (IS); (4) carnosic acid.
3.4 Content of antioxidants in hydro distillation water residues
After the hydro distillation of rosemary leaves the remaining water is brown in color. Normally this residue is waste, because the main focus in this method is the essential oil. But the plant material always contains some hydrophilic water-soluble compounds which can be dissolved in the water residue during hydro distillation. For this reason it is worthy to analyze this residual water for the antioxidant content.
Fig. 7 presents the influence of distillation time on the concentration of rosmarinic acid and carnosic acid in the water residue. It is found that the content of rosmarinic acid in the water residue increases over extraction time. After 2.5 h of distillation a maximum content of 8.5 mg rosmarinic acid per 1 g of dry rosemary leaves is reached. This value is close to the maximum rosmarinic acid amount of 8.9 mg/g in the leaves, which was investigated by Soxhlet extractions. The large standard deviations of the single measurements, especially at longer extraction times, can be explained by the significant variation of some extraction parameters, for example irregularities in the stirring rate of the suspension and other inevitable inhomogeneities in the process. As expected, no carnosic acid is determined in the water residue. Fig. 9 shows the chromatogram of the water residue from 2.5 h lasting hydro distillation. It looks quite similar to the chromatogram of the water Soxhlet extract (see Fig. 6). In general, compounds with small retention times are extracted. It is apparent that only small amounts of carnosol and rosmanol are contained in the water residue.
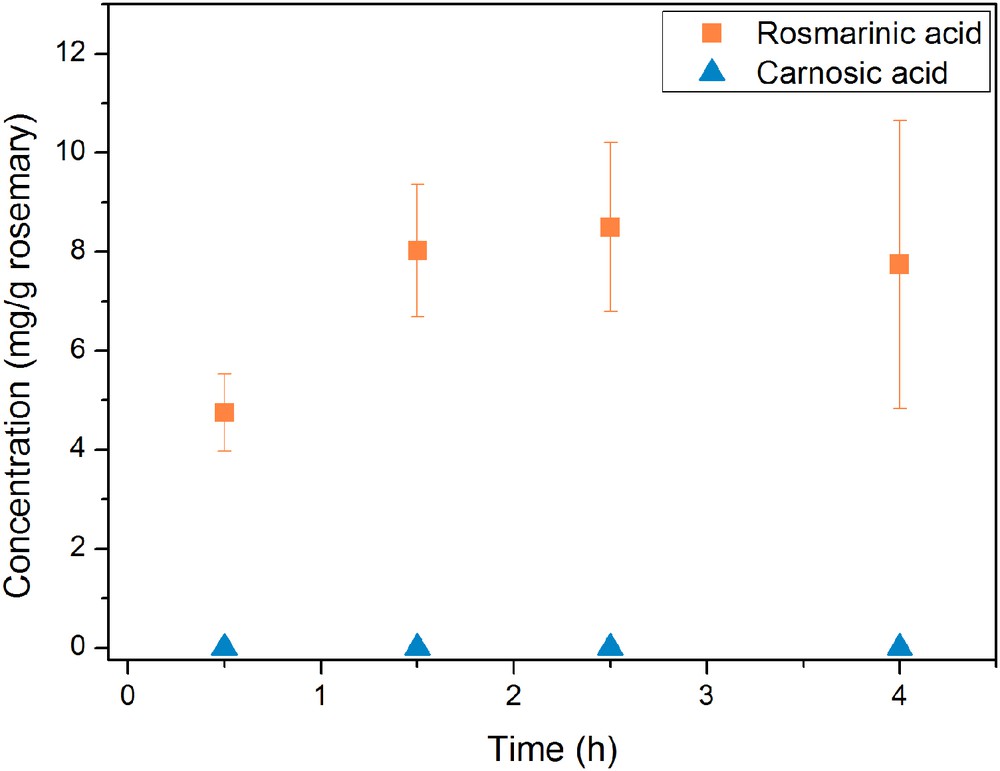
Time-dependent mass concentrations of rosmarinic acid and carnosic acid yields obtained by hydro distillation of rosemary leaves. The yield is given in weight percent of the initial mass of rosemary leaves.

HPLC-chromatograms of the hydro distillation water residue after 2.5 h and the subsequent Soxhlet extraction of the corresponding residual rosemary leaves with methanol. Peak identification: (1) rosmarinic acid; (2) carnosol; (3) gemfibrozil (IS); (4) carnosic acid.
3.5 Content of antioxidants in the residual leaves after hydro distillation
After distillation the residual rosemary leaves were dried and extracted by using Soxhlet with methanol to investigate the influence of hydro distillation on the residual content of antioxidants, especially rosmarinic acid and carnosic acid. It is shown (see Fig. 8) that there is still rosmarinic acid left in the rosemary leaves after hydro distillation but the content decreases from an initial amount of 8.9 mg/g to 2.1 mg/g after 4 h of distillation. The time of the proceeding hydro distillation also influences the content of carnosic acid residual leaves. The initial content of 23.6 mg/g decreases significantly by 16.3 mg/g after 1.5 h of distillation, followed by a small increase to 18.9 mg/g after 4 h. The chromatogram of the Soxhlet extract of the residual leaves (see Fig. 9) after 2.5 h lasting hydro distillation is similar to that of the standard methanol extract (see Fig. 6). Differences are the lower content of polar compounds, especially rosmarinic acid. Also an extension of the methyl carnosate, rosmanol and particularly the carnosol peak area can be determined. With these results the trend of the antioxidant concentrations in the rosemary leaves during hydro distillation can be explained. The decrease of rosmarinic acid is due to its water solubility. The longer the distillation, the lower is the residual amount of rosmarinic acid in the leaves. After 2.5 h a saturation of the solution due to the solubility limit of rosmarinic acid in water is reached. For this reason, there is still some rosmarinic acid left in the leaves after hydro distillation. The trend of carnosic acid is a bit different. First the concentration in the leaves decreases, but this is not based on the solubility of carnosic acid in water. This compound is water insoluble. The decrease of the concentration can be explained by the degradation of carnosic acid to rosmanol and carnosol, which were detected in the water residue and the residual leaves. The subsequent increase of the carnosic acid concentration in the leaves is more or less an artifact, because the results are given in mg of antioxidant per 1 g of rosemary. It has to be mentioned that with increasing hydro distillation time more and more compounds are extracted from the leaves. This leads to a reduction of the mass of the rosemary leaves and thus the remaining compounds get more and more concentrated in the leaves. As the content of carnosic acid is not reduced in the leaves after 1.5 h, the calculated concentration in mg per 1 g of the residual rosemary leaves is hence higher. Results from the literature also show that steam distillation influences the composition of antioxidants in the residual leaves. The degradation of carnosic acid to carnosol and the loss of rosmarinic acid were also determined [46]. In summary, it can be said that hydro and steam distillation have a strong influence on the antioxidant content of rosemary leaves. During distillation, antioxidants are lost by solubilization or degradation.

Time-dependent mass concentrations of rosmarinic acid and carnosic acid in the residual rosemary leaves after hydro distillation. The content of antioxidants was investigated by Soxhlet extractions with methanol. The yield is given in weight percent of the acids relative to the initial mass of rosemary leaves.
Nevertheless, the residual leaves can be re-processed to extract the containing antioxidants after hydro distillation. This would be in line with the concept of biorefinery. This concept is defined as “the sustainable processing of biomass into a spectrum of marketable products and energy”. However in future it would be better to focus on alternative extraction methods for essential oils especially in order to minimize the decomposition of antioxidants in the plant material. Another drawback of hydro and steam distillations is the high energy consumption to generate the steam and also to condense the essential oil/steam mixture. In general, it is known that an industrial extraction cycle needs at least 50% of the energy of the whole industrial process [47,48]. An appropriate option could be microwave-assisted extraction techniques like microwave hydro diffusion and gravity (MHG). With this method, the rosemary essential oil can be extracted within 10 min instead of 240 min for hydro distillation. Another advantage of MHG compared to hydro distillation is the saving in solvent, because no additional water (fresh plants) or only a small amount (dry plants) is needed. The impacts on antioxidants during extraction can probably be decreased with this method. There is also a saving in energy, solvent, waste and time, which would decrease the environmental impact of the extraction process. Minor disadvantages of microwave-assisted distillation are the higher acquisition costs and higher level of safety and attention compared to steam or hydro distillations. Also up-scaling of the microwave-assisted extraction process is not arbitrarily possible. Large-scale microwave reactors are suitable to extract up to 100 kg of fresh plant material per batch [43,47]. This utilizes less plant material compared to steam distillation where 800 kg of rosemary leaves were extracted per batch.
3.6 Antioxidant activity of hydro distillation water residues
3.6.1 Calibration
First of all, different concentrations of a DPPH solution were measured for calibration. It was determined that the absorbance maximum is at a wavelength of 518 nm. When the concentration is plotted as a function of the absorbance, a linear trend can be observed. The exact DPPH concentration of the blank samples can be calculated with the following Eq. (3).
| (3) |
3.6.2 Reference substances
The antioxidant activity of the following pure compounds was investigated: rosmarinic acid, carnosic acid, butylated hydroxyanisole, ascorbic acid, and α-tocopherol. First, the kinetic of the reaction was determined. It is necessary to assume a complete reaction of DPPH with the antioxidant. Rosmarinic acid shows a slow reaction kinetic. After approximately 1 h of reaction the absorbance of the sample at 518 nm stays constant. In contrast, ascorbic acid reacts very fast with the DPPH radical. Here, a complete reaction of the antioxidant is reached after approximately 1 min. To make all the results comparable and dismiss some disturbing factors, the absorbance of the samples was measured after exactly 1 h of reaction time.
Fig. 10 shows the influence of mass concentration on the inhibition for different antioxidants. A linear trend between the inhibition and the concentration of the antioxidant solutions can be observed. The larger the slope of the trend line, the more efficient is the antioxidant. At higher antioxidant concentrations the curve reaches a maximum value due to the lack of DPPH. The order of the analyzed antioxidant in ascending antioxidant power with regard to their mass concentration is: α-tocopherol, ascorbic acid, carnosic acid, rosmarinic acid, and butylated hydroxyanisole. But to compare the antioxidant activity of a single molecule these results need to be converted into values which are depended on the amount of substance.
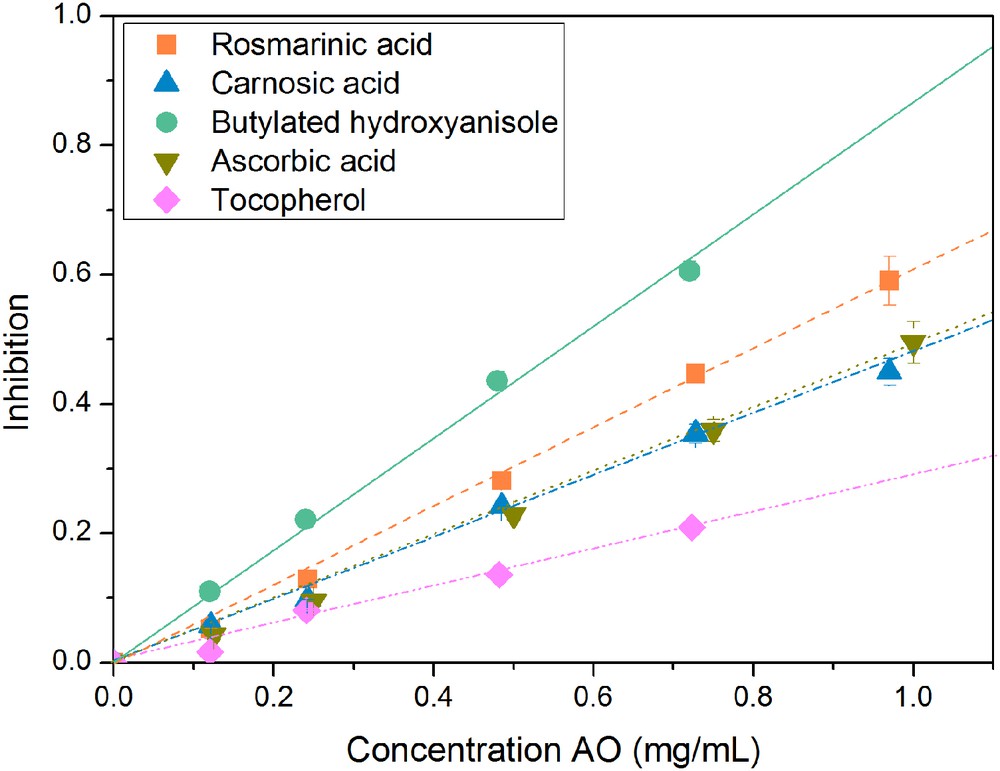
Influence of concentration on the inhibition of different compounds: rosmarinic acid, carnosic acid, butylated hydroxyanisole, ascorbic acid, and α-tocopherol.
Fig. 11 shows the influence of the ratio n(antioxidant)/n(DPPH) on the inhibition of different antioxidants. Again, a linear trend of the values can be observed. The larger the slope of the trend line, the higher is the antioxidant activity of the molecule. The value of the slope gives the number of reduced DPPH radical molecules per one antioxidant molecule, whereas the linear extrapolation of the measured point to an inhibition value of 1 provides the stoichiometric value of the reaction. An inhibition of 1 means that all of the DPPH has reacted with the antioxidant. The antioxidant power of the compounds in ascending order is as follows: rosmarinic acid, carnosic acid, butylated hydroxyanisole, α-tocopherol, and ascorbic acid. The absolute values of the results are summarized in Table 1. The experimentally determined values in this work are in agreement with the literature data [31]. Also a new value for the reaction of carnosic acid with DPPH is described, which has not been specified in the literature before. In this reaction, 2.57 DPPH molecules react with one molecule of carnosic acid. The corresponding stoichiometric value was determined to be 0.39.

Influence of the molar ratio of the compound to DPPH on the inhibition of different compounds: rosmarinic acid, carnosic acid, butylated hydroxyanisole, ascorbic acid, and α-tocopherol.
Stoichiometric value of the reaction DPPH-AO and the number of reduced DPPH molecules per molecule antioxidant of different compounds: rosmarinic acid, carnosic acid, butylated hydroxyanisole, ascorbic acid, and α-tocopherol.
| Compound | Stoichiometric value | Number of reduced DPPH | |
| Experimental | Literature | ||
| Rosmarinic acid | 0.24 | 4.25 | 3.33 [31] |
| Carnosic acid | 0.39 | 2.57 | – |
| Butylated hydroxyanisole | 0.40 | 2.49 | 2.63 [31] |
| Ascorbic acid | 0.63 | 1.60 | 1.85 [31] |
| α-Tocopherol | 0.45 | 2.21 | – |
3.6.3 Soxhlet extracts
The choice of the solvent also influences the antioxidant activity of the Soxhlet extracts, as can be seen in Fig. 12. First, it can be noted that the water Soxhlet extracts show the highest antioxidant activity of the samples, followed by the methanol extract. Soxhlet extracts of rosemary leaves with acetone show the lowest antioxidant power. At first sight, these results are not in agreement with the determined total amount of rosmarinic acid and carnosic acid by HPLC-UV (see Section 3.3). There, methanol extracted the highest mass concentration of both antioxidants. As a result, it can be said that methanol extracts the highest amount of antioxidants, speaking of rosmarinic acid and carnosic acid, but water delivers the extract with the highest total antioxidant activity. This may be explained by the assumption that water extracts antioxidants, which react better in a smaller stoichiometric value (see Table 1) with the DPPH radical.

(A) Influence of the dilution on the inhibition of hydro distillation water residues gained after 0.5, 1.5, 2.5, and 4.0 h and (B) Soxhlet extracts obtained with different solvents: water, acetone, and methanol.
3.6.4 Hydro distillation water residues
The previous results already showed that there is a significant amount of antioxidants contained in the residual water of hydro distillations. But only the amount of rosmarinic acid and carnosic acid could be quantitatively determined by HPLC-UV. The chromatograms already showed that there are more antioxidants in the residue. To quantify them, the water residues of hydro distillation were analyzed by DPPH assays to determine the total antioxidant power. For comparison and classification of the results, the DPPH assays were also carried out for the water, methanol, and acetone Soxhlet extracts. Preliminary to the assays of the samples, the UV/VIS spectra of the pure hydro distillation water residue and Soxhlet extracts were measured. It was found that the absorbance of the extracts does not influence the absorbance of DPPH.
As can be seen in Fig. 12, the duration of hydro distillation influences significantly the inhibition of different dilutions of the water residue. As mentioned previously, the bigger the slope of the linear trend line, the greater is the antioxidant activity of the sample. The water residue of 0.5 h lasting hydro distillation of rosemary leaves shows the smallest antioxidant activity of all samples. With increasing hydro distillation time, the antioxidant activity of the water residue increases. A maximum value is reached after a distillation time of 2.5 h. Afterwards, the antioxidant activity of the hydro distillation water residue decreases. The value of 4 h lasting hydro distillation is in the range of a distillation time of 1.5 h. This behavior can be explained by the increasing solubilization of antioxidant compounds with increasing hydro distillation time. The decay of the antioxidant power after 2.5 h of hydro distillation is probably due to the decomposition of some antioxidant compounds. These results are in accordance with the previously investigated behavior. It can also be noted that rosmarinic acid is not the only one responsible for the high antioxidant activity in the hydro distillation water residue. Other water soluble antioxidants, like rosmanol, methyl carnosate, and unknown compounds also affect the antioxidant activity.
To classify the antioxidant activity of the hydro distillation water residues, the results should be compared with the water, methanol, and acetone Soxhlet extracts. Hydro distillations were carried out with a solid/liquid ratio of 0.05. This means that 0.05 g of rosemary leaves were “extracted” with 1 mL of water. The solid/liquid ratio for Soxhlet extraction was higher with 0.1 g rosemary leaves per 1 mL of solvent. This ratio was taken into account in the results presented in Fig. 12. Thus, the results of dilutions of hydro distillation and Soxhlet extractions are directly comparable.
It can be noted that the water Soxhlet extract has a slightly higher antioxidant activity as the water residue of 2.5 h lasting hydro distillation. This is predictable and can be explained by the fact that Soxhlet is an exhaustive extraction method, whereas, in hydro distillation the water is finally saturated with the compounds after the establishment of an equilibrium. The antioxidant activity of the water residue of 1.5 and 4 h lasting hydro distillation is in the same range as Soxhlet extraction carried out with methanol. Soxhlet extracts show the lowest antioxidant activity. The value is comparable to that of the residue of 0.5 h lasting hydro distillation. It is supposed that the water insoluble antioxidants contained in rosemary leaves have a lower antioxidant activity than the water soluble ones. The main result of these DPPH assays is that almost all of the water soluble antioxidants are lost during hydro distillation and are contained in the residual water.
As already mentioned, this residual water is normally a waste product. The results of HPLC analyses and DPPH assays show clearly that a large amount of antioxidants is contained in this residue. In future, the extraction of antioxidants from natural resources will become mandatory due to the replacement of artificial antioxidants. Here, a possible source of the natural antioxidant rosmarinic acid was identified. For this reason it is necessary to see the hydro distillation water residue as a co-product of the extraction and not as a waste product. This would be in line with the concept of biorefinery and green extraction. The approach of these two concepts is to maximize the valorization of raw materials, reduce the energy consumption and use alternative solvents for economic sustainability. The goal of the industrial process to extract rosemary should be the fast and moderate recovery of the essential oil from the leaves with a minimum impact on the antioxidants. Microwave hydro diffusion and gravity could be an alternative extraction method. Furthermore, the residual water of hydro distillation which contains a large amount of antioxidants can be used for different applications. This residual water can be taken as an additive without any further purification to increase the antioxidant activity of a product or it can be re-extracted to gain the pure antioxidants. Finally, the residual leaves can be extracted with a green organic solvent to obtain antioxidants. This process will more and more approach the concept and principles of green extraction of natural products and biorefinery [47,48].
4 Conclusion
In this paper the effect of hydro distillation on the antioxidant compounds contained in Moroccan rosemary leaves was investigated. For characterization, methanol is an appropriate solvent for Soxhlet extraction to determine simultaneously the content of rosmarinic acid (water soluble) and carnosic acid (insoluble in water) in rosemary leaves. The maximum content of rosmarinic acid was determined to be 8.9 mg/g and 23.6 mg/g for carnosic acid. The time of hydro distillation affects the antioxidants in the leaves. The longer the duration of hydro distillations, the more rosmarinic acid is present in the water residue, whereas no carnosic acid is dissolved in the residue. Also the antioxidant activity of the residual water determined by DPPH assays increases with increasing hydro distillation time. The content of rosmarinic acid in the residual leaves after 4 h lasting hydro distillation is reduced by up to 76% and 36% for carnosic acid. Also, the maximum extraction yield of the essential oil was determined. It amounts to 2.5% for steam distillation and only 1.8% for hydro distillation. The recovery over time shows a common trend. In addition, the content of camphor in the recovered essential oil is relatively high and does not fulfill the ISO 1342 international standard. Cohobation in steam distillation slightly decreases the maximum extraction yield and increases the content of camphor in the essential oil.
The obtained results show that a large amount of antioxidants are lost during the hydro distillation of rosemary leaves. Normally, this residue is a waste product and is disposed. In future, the recovery of antioxidants from natural resources becomes more and more important due to the replacement of synthetic antioxidants. Here, a possible source of the natural antioxidant rosmarinic acid was identified. For this reason it is necessary to see the hydro distillation water residue as a co-product of the extraction and not as a waste product. This residue can be taken as an additive without any further purification to increase the antioxidant activity of a product or it can be re-extracted to gain the pure antioxidants. This would be in line with the concept of biorefinery and green extraction. A paper which shows a possible application of the hydro distillation water residue of rosemary leaves is in progress.
Appendix A Supplementary data
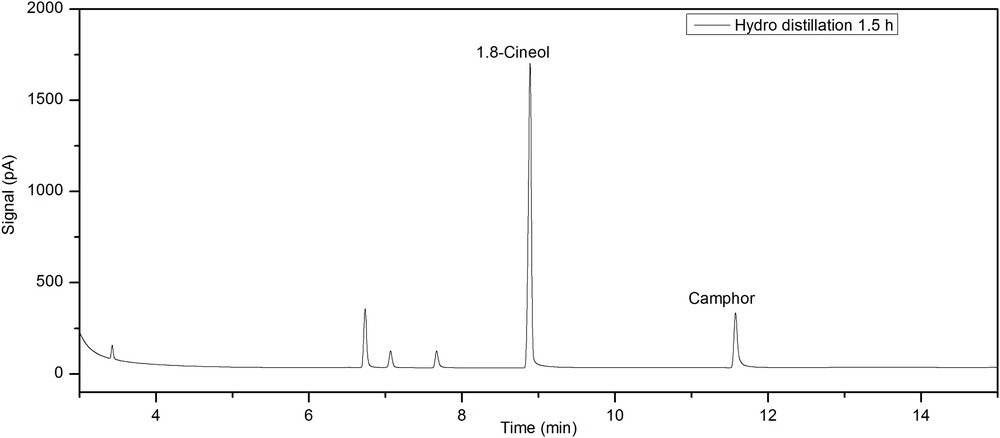
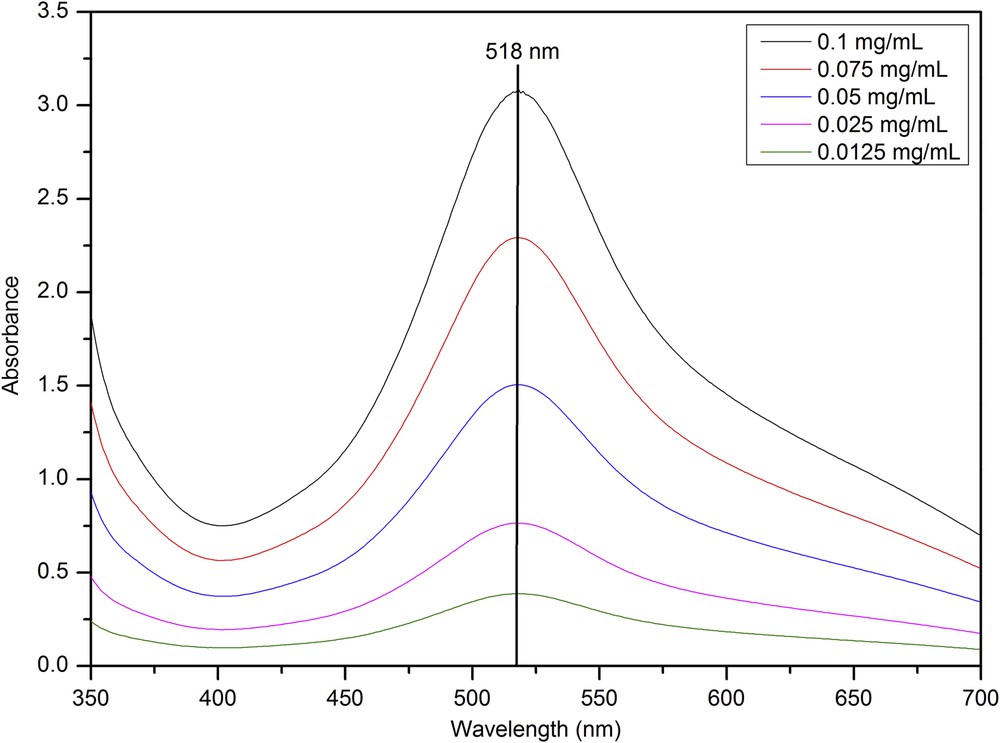
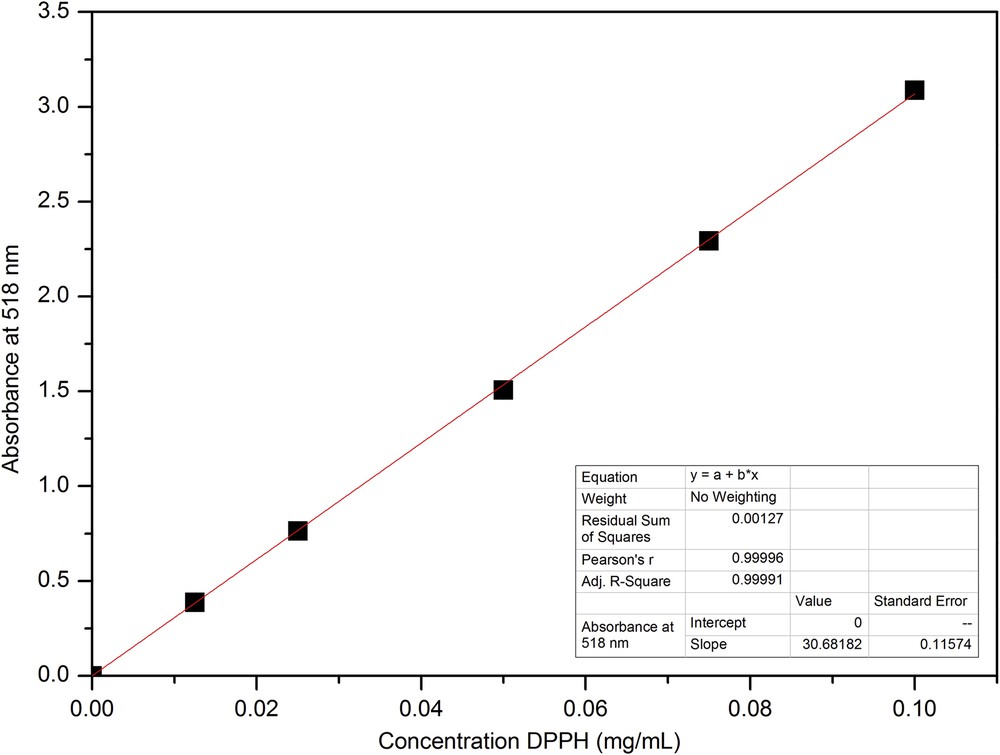
The following are the supplementary data related to this article: Example for a GC-FID chromatogram of essential oil gained by hydro distillation (1.5 h). Determination of the response factor of RAc and CAc for HPLC analyses. Gemfibrozil (Gem) was used as the internal standard. The slope of the linear fit is equitable to the response factor. UV/VIS spectra of DPPH-solutions with different mass concentrations. Calibration line of DPPH, determined at 518 nm.