1 Introduction
Stephania cambodica Gagnep. (Menispermaceae) is a woody climber found in mountainous regions in Cambodia and Vietnam [1,2]. The main characteristic of this species is the absence of leaves during the blooming period [1]. This medicinal plant is widely used in traditional Cambodian medicine in the form of an aqueous decoction or alcoholic maceration to treat a number of diseases and symptoms particularly malaria, fever, joint pains, wounds, psychological disorders, fatigue, and male sexual dysfunction [3]. In Vietnam, the tuber of S. cambodica is used in combination with other plants for the treatment of various diseases such as depression, asthma, and hypertension [4].
Previously, we have reported three main alkaloids from the tuber of S. cambodica, namely, tetrahydropalmatine (THP), palmatine (PAL), and roemerine (ROE) (Fig. 1) [5]. THP exhibits a wide number of pharmacological actions particularly on the central nervous system, including analgesic, sedative effects, and hypnotic actions. This compound is approved in China and Vietnam for a number of clinical indications and frequently used as anxiolytic and hypnotic drugs. It is also a promising drug candidate for the treatment of cocaine addiction [6]. PAL has been reported as an in vitro antimalarial, antitumor, and anti-inflammatory agent [7]. This protoberberine alkaloid has also shown noteworthy memory enhancing activity in mice and has inhibited action of the neurotransmitter responsible for the pathogenesis of dementia [7,8]. ROE, an aporphine alkaloid, is a remarkable molecule for the treatment of schizophrenia and insomnia [9]. Against multidrug-resistant cancer cells, ROE has increased the efficacy of vinblastine [10]. These pharmacological findings may hence contribute to the validation of traditional uses of this medicinal plant.
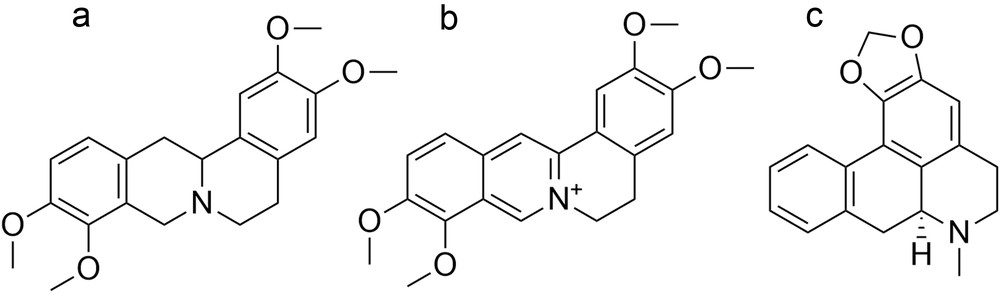
Chemical structures of (a) THP, (b) PAL, and (c) ROE.
In literature, a number of extractive techniques such as maceration, percolation, and heat reflux extraction have been conducted to extract alkaloidal constituents from species of Stephania [11–13]. It has frequently been reported that these conventional solid–liquid extractions are arduous time-consuming processes, requiring high consumption of solvent and in some cases, providing low recovery [14]. Recently, innovative extractions such as microwave-assisted extraction (MAE) and ultrasound-assisted extraction (UAE) have been shown to be a viable alternative to conventional procedures for the extraction of bioactive alkaloids from Stephania rotunda Lour. and Stephania sinica Diels, as they are more efficient, faster, ecologically friendly, and use less toxic solvents [15,16].
To the best of our knowledge, optimization for simultaneous extraction of the three biologically active alkaloids by innovative procedures such as MAE or UAE from the tuber of S. cambodica has not to date been documented. In this particular work, we compared the efficiency of UAE and MAE of THP, PAL, and ROE from the tuber of S. cambodica with that of percolation. The aim of our work was primarily to implement an efficient, green extractive technique optimized by response surface methodology (RSM) that could replace the conventional extraction of alkaloids from S. cambodica. We examined then the structural change of the plant powders treated by conventional extraction and the optimized procedure. The selected green extraction would not only be applied to the quality control of S. cambodica tuber but it would also contribute to the pharmaceutical, biomedical, and clinical sciences related to the three alkaloids because they are being used in some Asian countries as a drug.
2 Materials and methods
2.1 Plant material
S. cambodica tuber was collected in the Northern Cambodian province of Preah Vihear in 2014. It was authenticated by Dr. S. Hul; the voucher (Dary 18) was then deposited at the Paris Herbarium, France. The fresh tuber of the plant was cut into pieces and air-dried at room temperature over a period of 2 weeks. Samples were grinded into a homogenous powder and protected from light and humidity until the time of desired use.
2.2 Chemicals
The ultra-high performance liquid chromatography (UHPLC) grade acetonitrile, ethanol, and formic acid were purchased from Carlo Erba (Val de Reuil, France). The potassium phosphate monobasic was obtained from Fluka (Saint Quentin Fallavier, France). The ultrapure water (18.2 MΩ) for chromatographic analysis was obtained from a Milli-Q Reference A+ system (Millipore, Co., Bedford, MA, USA). PAL, ROE, and THP (Fig. 1) were purchased from Sigma–Aldrich (#361615; Saint Quentin Fallavier), Ambinter (#Amb4417140; Orléans, France), and Phytolab (#89807, Vestenbergsgreuth; Germany), respectively.
2.3 Extraction procedures
2.3.1 Ultrasonic-assisted extraction
UAE was performed with a PEX 0.5 Sonifier (R.e.u.s, Contes, France) composed of an inox jug with 150 × 137 mm internal dimensions and a maximal capacity of 500 mL, as well as a transducer, in the basis of jug, operating at a frequency of 25 kHz and with maximum input power of 150 W. The double-layered mantle enabled the temperature control of the medium by means of a cooling/heating system. The temperature in the system was stabilized by water–ethylene glycol current. Different ratios of ethanol–water (20:80, 40:60, 50:50, 60:40, 80:20, and 100:0, v/v) were tested. The liquid–solid ratios tested were 10:1, 20:1, and 30:1 in a liquid volume of 10 mL. The ultrasonic times tested were 5, 10, and 15 min. All the experiments were conducted in triplicate at 20 °C with a fixed frequency.
2.3.2 Microwave-assisted extraction
MAE was performed on a multimode microwave apparatus using a closed vessel system in CEM Mars Xpress (CEM Corporation Matthews, NC, USA). The reactor frequency was 2.45 GHz. Various percentages of ethanol, liquid–solid ratio, time, and power were tested. The tested extraction times were 5, 10, and 15 min. The optimal time determined by Xie et al. [16] for the extraction of THP and PAL from the tuber of S. sinica was also included in the study. Different ratios of ethanol–water (20:80, 40:60, 50:50, 60:40, 80:20, and 100:0, v/v) were tested. The liquid–solid ratios tested were 10:1, 20:1, and 30:1 in a solvent volume of 10 mL. The powers used were 100, 200, and 400 W. Each experiment was conducted in triplicate at 80 °C.
2.3.3 Conventional extraction
Percolation was previously reported as the conventional extraction method of THP, PAL, and ROE from S. rotunda tuber [17]. The powdered raw material (10 g) was moistened with 15 mL of water–ammoniac (99:1, v/v) for 4 h and then extracted with dichloromethane. The dichloromethane solution was collected in a volumetric flask and filled to 100 mL. The obtained solution (2 mL) was sampled and then evaporated to dryness.
2.4 Experimental design
2.4.1 Multiple-level single factor design
A single factor experimental design was conducted for the determination of each of the most efficient conditions in UAE and MAE [18]. It is noted that in UAE and MAE, solvents, extraction time, and solvent-to-material ratio are commonly studied as effective factors on extraction yields, which in this work are expressed by alkaloid content. During this step, different UAE and MAE conditions were tested (Table 1) to select the appropriate extraction method into optimization process by RSM. The quantification of alkaloids in this design step was determined by HPLC method [17].
2.4.2 Optimization of UAE conditions using Box–Behnken design
On the basis of the single factor experimental design, an extractive method was selected to be optimized using RSM. RSM consists of a collection of mathematical and statistical approaches to achieve process optimization. Among the most commonly used RSM designs, Box–Behnken design is efficient and particularly appropriate for tests of three variables of three levels [19]. It was therefore adopted in this study. The three tested independent variables (inputs) were ethanol percentage, liquid–solid ratio, and time, which each had three levels, coded −1, 0, and 1 for low, medium, and high levels, respectively. Table 2 presents the design matrix, which requires a total of 17 experimental runs. Design-Expert Version 7.0.0 software (Stat-Ease, Inc., Minneapolis, MN, USA) was used for constructing the design matrix, graph plotting, and data analysis. Results using response surface regression were fitted to a second-degree polynomial regression equation, as follows:
| (1) |
Input factors (natural and coded values) and levels defined for Box–Behnken design.
| Run | Independent variables | Responses | ||||
| Natural values (coded values) | Extraction yields: alkaloid content (%) | |||||
| X1 | X2 | X3 | THP | PAL | ROE | |
| 1 | 30 (1) | 90 (1) | 5 (0) | 1.4890 | 0.1019 | 0.6806 |
| 2 | 20 (0) | 60 (0) | 5 (0) | 2.0541 | 0.1510 | 0.9912 |
| 3 | 20 (0) | 60 (0) | 5 (0) | 2.0750 | 0.1572 | 1.0297 |
| 4 | 10 (−1) | 90 (1) | 5 (0) | 1.3177 | 0.0779 | 0.5517 |
| 5 | 20 (0) | 30 (−1) | 1 (−1) | 1.9112 | 0.1385 | 0.7913 |
| 6 | 30 (1) | 30 (−1) | 5 (0) | 2.2156 | 0.1343 | 0.9035 |
| 7 | 30 (1) | 60 (0) | 9 (1) | 2.1384 | 0.1707 | 1.0193 |
| 8 | 10 (−1) | 60 (0) | 9 (1) | 2.0628 | 0.1354 | 0.9666 |
| 9 | 20 (0) | 60 (0) | 5 (0) | 2.0517 | 0.1482 | 0.9832 |
| 10 | 20 (0) | 60 (0) | 5 (0) | 2.1264 | 0.1489 | 1.0193 |
| 11 | 10 (−1) | 30 (−1) | 5 (0) | 2.0336 | 0.1228 | 0.8059 |
| 12 | 30 (1) | 60 (0) | 1 (−1) | 2.0113 | 0.1637 | 0.9552 |
| 13 | 20 (0) | 60 (0) | 5 (0) | 2.1744 | 0.1476 | 1.0402 |
| 14 | 20 (0) | 90 (1) | 1 (−1) | 1.2128 | 0.0971 | 0.5523 |
| 15 | 20 (0) | 90 (1) | 9 (1) | 1.5157 | 0.1162 | 0.7001 |
| 16 | 20 (0) | 30 (−1) | 9 (1) | 2.1057 | 0.1416 | 0.8902 |
| 17 | 10 (−1) | 60 (0) | 1 (−1) | 1.8671 | 0.1220 | 0.7316 |
During the optimizing process by RSM, the quantitation of the content of the three alkaloids was determined by a validated UHPLC-diode array detector (DAD) method [5]. Briefly, the analyses were performed using an UHPLC Agilent Infinity 1290 Liquid chromatography system equipped with a binary pump solvent delivery system G4220A and UV photodiode array detector G4212A (Agilent Technologies, Inc., Germany). Chromatographic separation was achieved on a Zorbax Eclipse Plus RRHD-C18 column (50 mm × 2.1 mm, 1.8 μm, Agilent), operated at 30 °C. The mobile phase consisted of a gradient elution of formic acid 0.1% (v/v) (solvent A) and ethanol (solvent B). The gradient program was 0–1 min at 5% of B, 1–7 min from 5% to 42% of B with 3 min of post-time at a flow rate of 0.35 mL/min. The injected volume was 2 μL. The UV detection wavelength was 280 nm for THP and 272 nm for PAL and ROE. Sample preparation was performed by diluting 1 mL of extract obtained in optimizing process in 20 mL of ethanol–water (50:50, v/v). The solution was then vortexed and sonicated for 1 min. The solution obtained (2 mL) was filtered through a syringe 0.22 μm Polyvinylidene Difluoride (PVDF) filter (Millex-GV, Merck Millipore, Germany). Each analysis was performed in triplicate.
2.5 Statistical analysis
All experiments were carried out in triplicate and all the average data are presented in the Tables 2, 3 and 5. Statistical analysis of t-test and analysis of variance (ANOVA) were carried out with a degree of significance of model terms as follows: P < 0.05 significant and P < 0.01 very significant. The verification of the predicted optimized condition of the developed mathematical model was performed using the percentage of relative change (PRC) between experimental and predicted values [21].
Comparison of extractive procedures.
| Extraction method | Conditions | Extraction yields (mean ± RSD, n = 3) | ||||||
| Solvent | Sample (g) | Solvent consumption (mL) | Time (min) | Temperature (°C) | THP | PAL | ROE | |
| UAE | Ethanol 50% | 0.33 | 10 | 10 | 25 | 2.90 ± 0.01 | 0.19 ± 0.01 | 1.12 ± 0.01 |
| MAE | Ethanol 50% | 0.50 | 10 | 15 | 80 | 2.91 ± 0.01 | 0.13 ± 0.02 | 0.79 ± 0.01 |
| Percolation | Dichloromethane | 10.00 | 100 | 240 | 25 | 2.70 ± 0.01 | 0.42 ± 0.07 | 0.74 ± 0.01 |
Predicted and experimental values of the responses at the optimal UAE conditions.
| Compound | Predicted values (%) | Experimental values ± SD (%) | PRCa (%) |
| THP | 2.2363 | 2.2347 ± 0.2444 | 0.06 |
| PAL | 0.1670 | 0.1660 ± 0.0008 | 0.34 |
| ROE | 1.0402 | 1.0437 ± 0.0277 | 0.54 |
a PRC between the experimental values and the predicted values.
2.6 Scanning electron microscopy
To investigate the morphological alterations caused by percolation and ultrasounds on the extracts of S. cambodica tubers, the latter were observed using a scanning electron microscope (SU3500, Hitachi). Samples were mounted on aluminum stubs with colloidal graphite and sputter-coated with gold using a JFC-1200 fine coater (JEOL). The scanning electron microscopy observations were made under high vacuum conditions at an accelerating voltage of 15 kV.
3 Results and discussion
3.1 Single factor experimental design
The effects of different physical parameters on UAE and MAE, including percentage of ethanol, liquid–solid ratio, extraction time, and microwave power on the yields of each alkaloid are shown in Figs. 2 and 3.
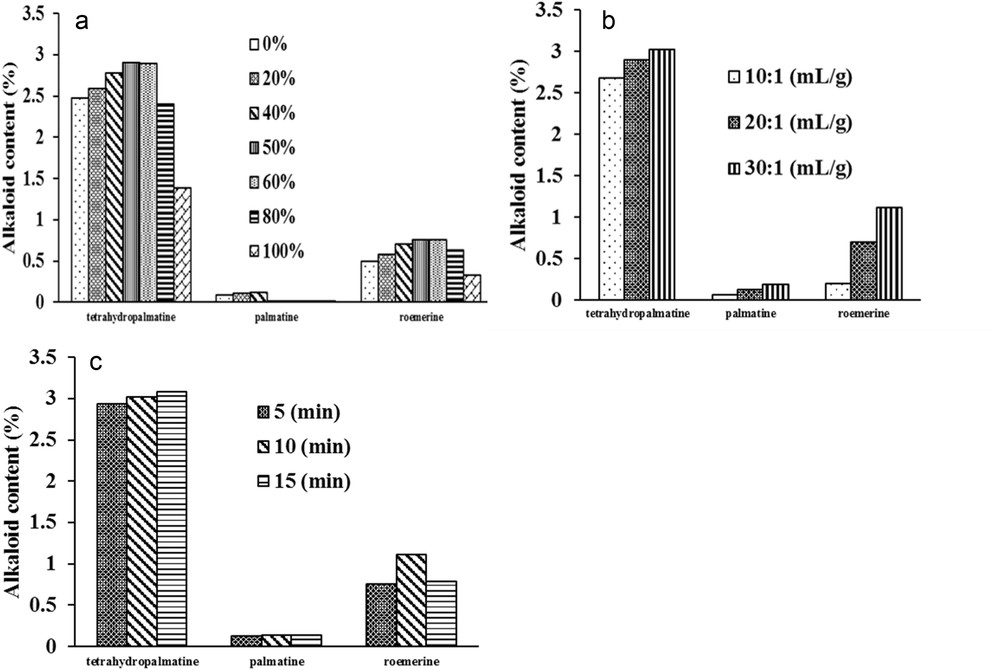
Effect of the (a) ethanol percentage, (b) liquid–solid ratio, (c) extraction time on extraction yields of three alkaloids from Stephania cambodica by UAE.
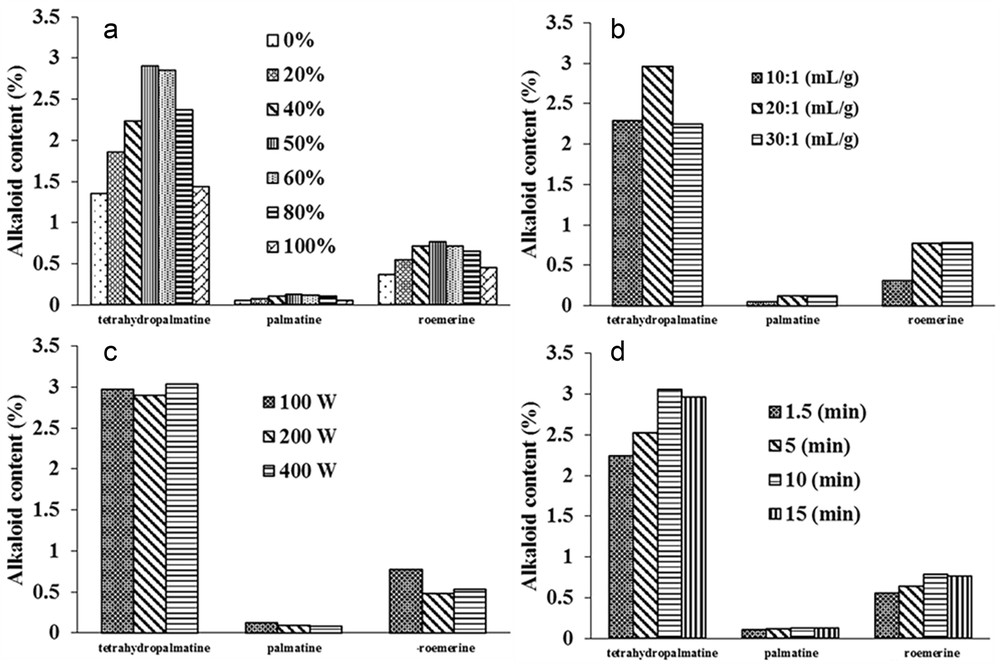
Effect of the (a) ethanol percentage, (b) liquid–solid ratio, (c) power, (d) extraction time on extraction yields of three alkaloids from Stephania cambodica by MAE.
The yields of the alkaloids increase when the percentage of ethanol increased from 20% to 40%, reaching a maximum of between 50% and 60% and then declined as the percentage increased up to 100% (Figs. 2a and 3a). In this solvent system, the adequate proportion of ethanol and water became the determining factor for extraction efficacy. As the percentage of ethanol went up from 20% to 40%, the analyte solubility increased according to the theory of polarity and intermiscibility [22]. However, in UAE, a higher level of ethanol (>80%) could deteriorate the efficiency of cavitation bubble collapse, that is, the main mechanism responsible for accelerating the mass transfer of organic compounds and increasing membrane porosity [23]. In MAE, a smaller quantity of water may result in decreased microwave energy absorption and also in poorer endothermic capacity [24]. These factors led to a lower yield of alkaloids observed in both extractive methods. Through the data obtained, the ethanol–water percentage that provided satisfactory extraction yields for the three analytes was determined to be 50%; it was therefore selected for subsequent experiments. Indeed, the mixture ethanol–water is one of the highly recommended green solvents in chemistry.
The effect of liquid–solid ratio on the extraction yields of alkaloids is demonstrated in Figs. 2b and 3b. In MAE, the extraction yields of THP increased significantly from 10:1 to 20:1 (mL/g) (p < 0.01) and decreased when the ratio was up to 30:1 whereas there was no significant change in the other two alkaloids from 20:1 (mL/g). However, in UAE there was a significant increase in the quantity of the three alkaloids ranging from 10:1 to 30:1 (mL/g) (P < 0.05). Consequently, the optimized liquid–solid ratio was 30:1 and 20:1 (mg/L) in UAE and MAE, respectively.
As demonstrated in Fig. 3c, in MAE the extraction yields were not significantly different when using power more than 100 W (p > 0.05). As a result, 100 W microwave power was selected for the ulterior tests.
The effects of time in UAE are shown in Fig. 2c. The extraction yields of THP and ROE were enhanced from 5 to 10 min. There was no significant difference between THP and PAL extracted in 10 and 15 min (p > 0.05). However, the extraction efficiency for ROE declined markedly when the time was up to 15 min (p < 0.05). Fig. 3d displays a gradual increase in the alkaloids within the time range of 1.5–10 min in MAE. The difference was highly significant for the yields of THP and ROE extracted from 1.5 to 10 min (p < 0.01). No significant enhancement in extraction yields of the three alkaloids was observed at 15 min (p > 0.05). In this study, we could not substantiate the optimal time of 1.5 min for the extraction of THP and PAL by MAE, as reported by Xie et al.[16]. The result suggested that for the time of 10 min, extraction yields for most analytes in UAE and MAE are sufficiently great.
3.2 Selection of UAE method for optimization by RSM
On the basis of the single experiment results, the satisfactory extraction could be obtained from the following conditions: liquid–solid ratio 30:1 mL/g, ethanol 50%, sonication time 10 min in UAE and liquid–solid ratio 20:1 mL/g, ethanol 50%, time 15 min, power 100 W in MAE. To select an appropriate extraction to be investigated by RSM, we compared the yields of the alkaloids extracted in optimal conditions of UAE and MAE with that of the conventional extractive procedure (Table 3). In addition to using green solvent systems, short working time, and small sample quantities, UAE and MAE are as efficient as percolation (p > 0.05). Moreover, UAE and MAE are more ecofriendly and chemicals used are less toxic.
MAE was as efficient as UAE for the extraction of THP; however, UAE significantly enhanced extraction yields of PAL and ROE compared with MAE. Furthermore, UAE was realized in a shorter extraction time at a lower temperature. That is, UAE prevents a degradation of thermally labile compounds and possible coextraction of unwanted constituents in the tuber, such as polysaccharides [25], which is a significant advantage for subsequent analytical methods used in the quality control of S. cambodica such as UHPLC [5]. More importantly, operating conditions with UAE are easy to implement as adopted in a number of pharmacopoeias. Although it was reported that degradation of secondary metabolites in UAE occurs at a frequency of more than 20 kHz [26], this is relatively mitigated by the equipped cooling system within short extraction time. UAE was hence selected in the optimization process by Box–Behnken design.
3.3 Optimization of UAE conditions by Box–Behnken design
Box–Behnken design of three factors and three levels was used in this study to investigate the combined effects of the process variables on the response variables in UAE. The factor levels were determined based on the aforementioned results of single factor experiments. The design matrix of 17 runs and the responses are shown in Table 2.
3.3.1 Fitting the model
Second-order polynomial models for the three alkaloids were developed by multiple regression analysis. The significance and good fit of model were assessed accordingly using ANOVA and the results are shown in Table 4. All models are highly significant (p ≤ 0.01) and the lack of fit statistics is insignificant (p > 0.05), which suggest the three models are adequate and reliable. The R-squared values of the models are all more than 97%, indicating that the predicted values correlate well with the experimental data. The values of the adjusted R-squared are also very high to advocate for a high significance of the model. According to our statistical analysis, each alkaloid content follows the reduced second-order polynomial equations:
| (2) |
| (3) |
| (4) |
ANOVA of the quadratic polynomial models and regression coefficients for the extraction yields of the three alkaloids.
| Coefficients | THP | PAL | ROE |
| βo (intercept) | 2.1 | 0.15 | 1.01 |
| Linear | |||
| β1 (liquid–solid ratio) | 0.072∗∗ | 0.014∗∗ | 0.063∗∗ |
| β2 (percentage ethanol) | −0.34∗∗ | −0.018∗∗ | −0.11∗∗ |
| β3 (extraction time) | 0.1∗∗ | 0.0057∗ | 0.068∗∗ |
| Quadratic | |||
| β11 | 0.0006025ns | 0.008003∗∗ | −0.047∗∗ |
| β22 | −0.33∗∗ | −0.033∗∗ | −0.23∗∗ |
| β33 | −0.077∗ | 0.005373ns | −0.048∗∗ |
| Cross product | |||
| β12 | −0.002675ns | 0.003125ns | 0.007825ns |
| β13 | −0.017ns | −0.0016ns | −0.043∗∗ |
| β23 | 0.027ns | 0.00475ns | 0.012ns |
| p value of model | <0.0001∗∗ | 0.0001∗∗ | <0.0001∗∗ |
| R2 | 0.9873 | 0.9716 | 0.9924 |
| Lack of fit | 0.4559 | 0.9351 | 0.7057 |
| Adj-R2 | 0.9709 | 0.0796 | 0.9827 |
3.3.2 Response surface analysis
The two-dimensional contour and surface plots were constructed based on the fitted models. Figs. 4–6 present the plots with one variable kept at medium level and the other two with their experimental range. The significance of each coefficient for the models is shown in Table 4.
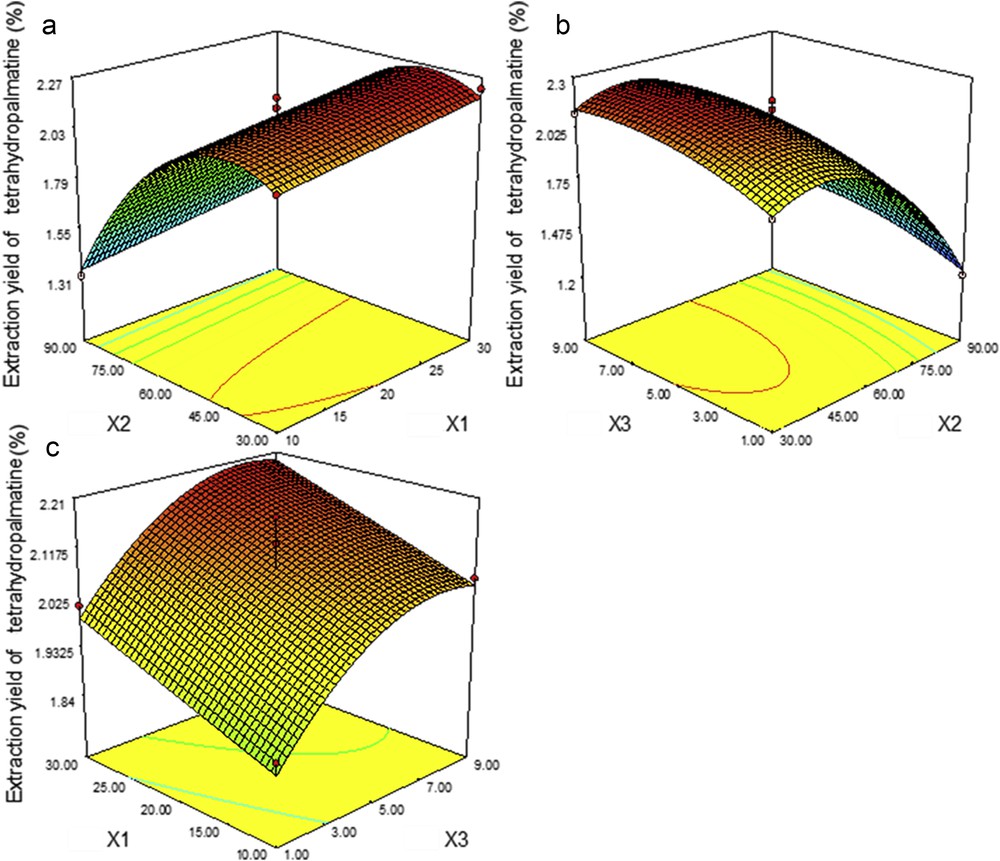
Response surface plots representing the effects of (a) X2 and X1, (b) X3 and X2, and (c) X1 and X3 on the extraction yields of THP. X1, liquid–solid ratio; X2, ethanol percentage; X3, extraction time.
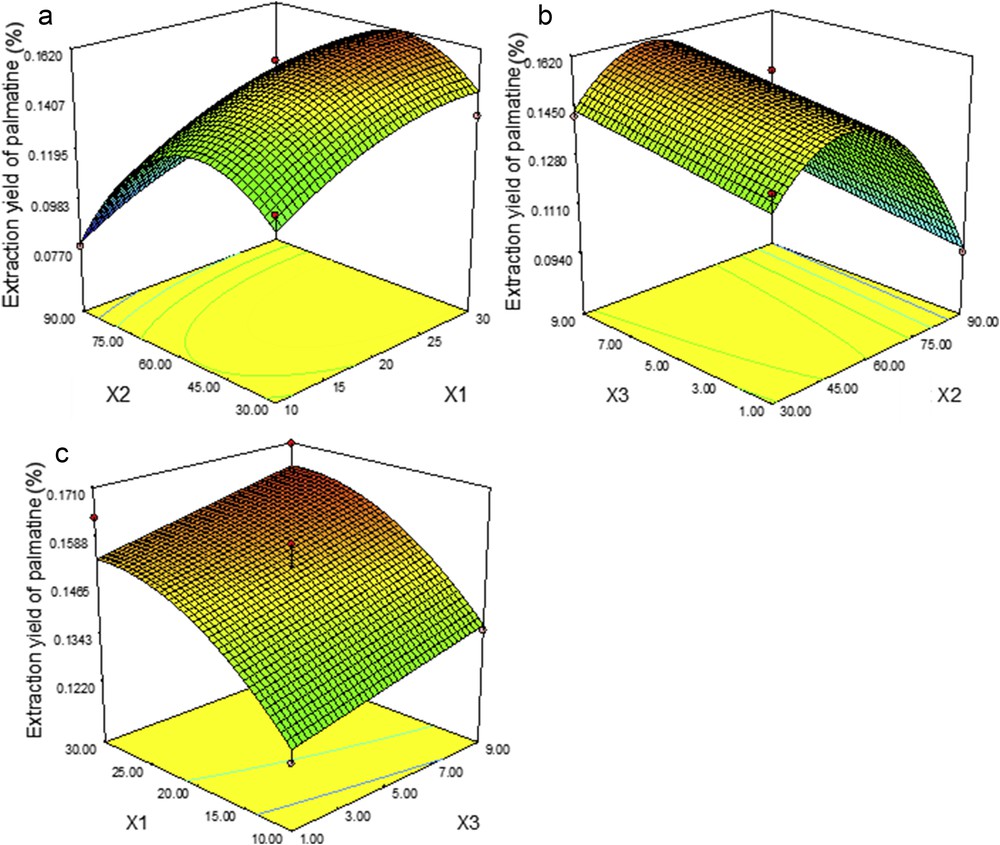
Response surface plots representing the effects of (a) X2 and X1, (b) X3 and X2, and (c) X1 and X3 on the extraction yields of PAL. X1, liquid–solid ratio; X2, ethanol percentage; X3, extraction time.
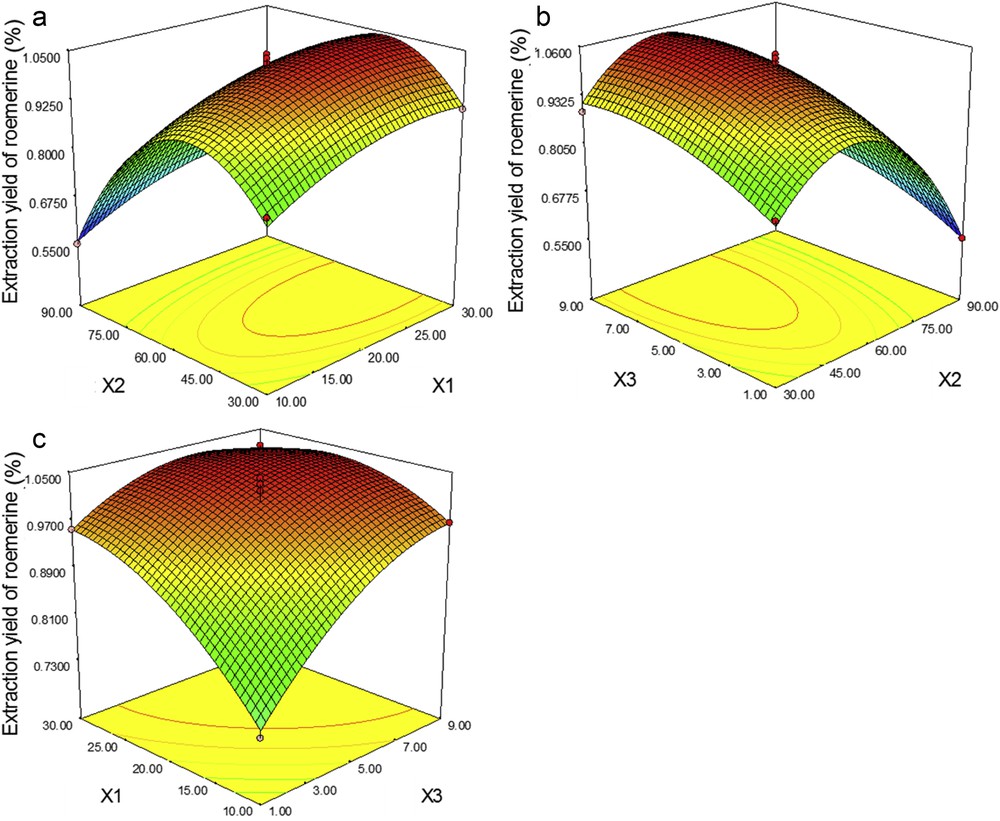
Response surface plots representing the effects of (a) X2 and X1, (b) X3 and X2, and (c) X1 and X3 on the extraction yields of ROE. X1, liquid–solid ratio; X2, ethanol percentage; X3, extraction time.
Fig. 4 illustrates the response surface and contour plots depicting the effects of liquid–solid ratio (X1), ethanol percentage (X2), and extraction time (X3) on THP. X2 has the most important effect (p ≤ 0.01), followed by X3 (p ≤ 0.01) and X1 (p ≤ 0.01), respectively. X2 and X3 also exert significant effects (p ≤ 0.01) at the quadratic level. When X2 increases, the yields of THP initially increase, reaching a maximum at ethanol 45%–55%, and then decline. When X2 and X1 are kept at low and medium levels, the extraction yield is enhanced markedly by prolonging X3 from 1 to 5 min and attains the peak from 7 min. We observed that at high X2 the yields vary slightly with increases in X3 and X1. The maximal yield is reached at a liquid–solid ratio of 29:1 mL/g, ethanol 49%, and an extraction time of 8.38 min.
Fig. 5 shows the response surface and contour plots for PAL. The compound yields are significantly affected by all the three factors at a linear level and by X1 and X2 at a quadratic level. The yields of PAL increase following the increase of X2, but begin to decrease at about ethanol 60%. The maximal yields can be obtained at ethanol 51%, a liquid–solid ratio about 29:1 mL/g, and an extraction time of 9 min.
Fig. 6 displays the response surface and contour plots for ROE. All the three factors exhibit significant effects (p ≤ 0.01) on extraction and also have highly significant quadratic effects (p ≤ 0.01). Among the interactions of factors, only the cross product X1X3 (p ≤ 0.01) is significant, which means that the yields increase gradually together with increments of X1 and X3. Alongside with an increase in X2, the extraction yield increases, reaching the peak value at about ethanol 50%. The ROE yields were finally optimized at a liquid–solid ratio of over 22:1 mL/g, ethanol 50%, and an extraction time longer than 7 min.
From these results, the percentage of ethanol is the most influential factor on the extraction of the three alkaloids. The remarkable enhancement of the extraction yields was observed between low and medium levels of ethanol percentage. At a higher level, the efficacy of UAE becomes less important, presumably owing to the increasing vapor pressure of the solvent, which perturbs the cavitation process [23].
3.4 Verification of the model-predicted optimal condition
Derringer's desired function methodology was used to predict optimum extraction conditions for the three alkaloids simultaneously. The predicted optimal conditions were as follows: liquid–solid ratio 26.6:1 mL/g, ethanol 52%, and ultrasonication time 9 min. The validation of the model was checked in triplicate confirmatory experiments carried out under adjusted optimum conditions: liquid–solid ratio 25:1 mL/g, ethanol 50%, and time 9 min. The results expressed by PRC in Table 5 show that the predicted values are in close agreement with experimental values, proving that prediction by the established model is reliable.
3.5 Structural changes after extraction
The various extraction methods produced different physical changes in S. cambodica tubers. Fig. 7 displays the micrographs of samples from S. cambodica tubers before and after the different extraction methods. An undamaged structure could be observed in the untreated material (Fig. 7a). Breaks, wrinkles, and fragmentation of cell walls were observed in the samples treated by percolation (Fig. 7b) and ultrasounds (Fig. 7c), with bigger damages observed in the samples having followed the second treatment. In other words, the rupture of cell walls and pits were more intense in samples treated by UAE than in those treated by percolation. This structural change leads to higher extraction yields in UAE than in percolation by the enhancement of diffusion and washing out (rinsing) the cell contents [23].
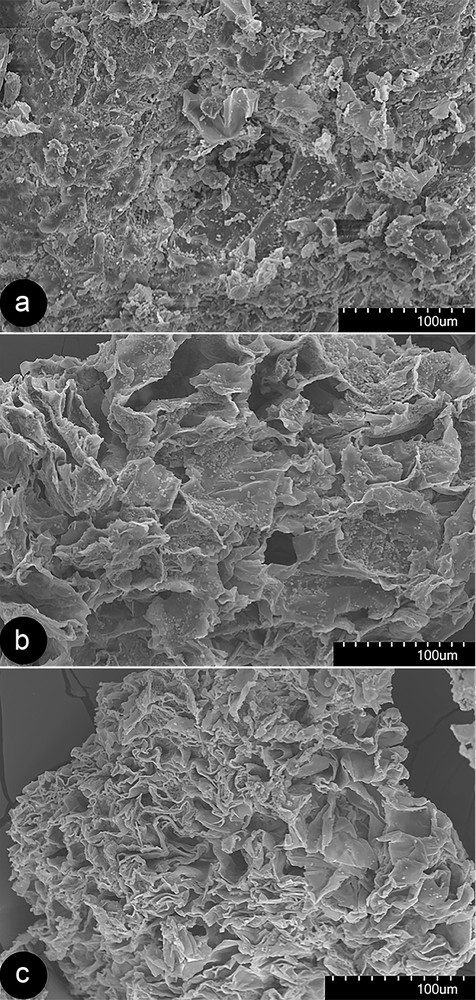
Scanning electron microscopy images of (a) untreated Stephania cambodica tuber extract, (b) sample treated by percolation, and (c) by ultrasounds. Accelerating voltage (kV), magnification, and working distance (mm) are (a) 15, 320, 23.4; (b) 15, 320, 23.7; (c) 15, 350, 15.6.
4 Conclusions
An ultrasonically assisted extraction of three bioactive alkaloids viz. THP, PAL, and ROE from S. cambodica tuber was developed and optimized using a combination of single factor experiments and Box–Behnken design. The proposed optimal conditions to obtain maximum yields of the three alkaloids were determined to be as follows: liquid–solid ratio 26.6:1 mL/g, ethanol 52%, and ultrasonication time 9 min. In comparison with MAE and conventional extraction, the proposed UAE presents significant advantages in terms of green solvent, small amount of a plant material, short processing time, and low temperature. These findings suggest that UAE is an ecofriendly technique suitable for preparation of extracts rich in the three bioactive alkaloids, their subsequent analytical control, and pharmacological and clinical investigation.
Acknowledgments
The study was supported by French government scholarships (bourse du gouvernement français). The authors acknowledge the assistance of Pr. Cheng Sun Kaing, Pr. Tep Rainsy, Dr. Bun Hot, and Pr. Tea Sok Eng. Special thanks are addressed to Pr. Iliadis Athanassios for his useful advice on the design.


