1. Introduction
Important scientific advances have been devoted to the upgrading of monoterpenes, mainly α- and β-pinene, into high added-value products [1, 2]. In addition to being found in a plethora of essential oils, α- and β-pinene are the main constituents of the crude turpentine oil, a by-product of the Kraft process of paper pulping [3]. Their oxygenated derivatives are versatile bio-based feedstocks used to synthesize flavors, fragrances, agrochemicals and pharmaceuticals [4, 5]. The conversion of these renewable natural olefins to oxidized products has been much explored in the literature [6, 7, 8, 9].
Monoterpene ethers such as the α-terpinyl alkyl derivatives are very attractive products. They have distinctive characteristics depending on the origin of the alcohol: methyl ether has a grapefruit-like aroma and is an ingredient of flavor and cosmetics; ethyl and propyl ethers have a citrus fragrance and grassy flavor, respectively, whilst the butyl ether has a woody scent [10, 11]. Limonene, α- and β-pinene are non-activated olefins, and their conversion to ethers via intermolecular addition reactions of alcohols requires the presence of liquid Brønsted acid catalysts or the use of strongly acidic solids [12].
Etherification reactions are conventionally carried out under homogeneous catalysis conditions using inexpensive Brønsted acids (i.e., H2SO4, HCl). However, these processes lead to problems associated with the neutralization and products separation, the generation of residues and effluents, and the corrosion of reactors [13]. To overcome these drawbacks, much attention has been focused on the development of environmentally friendly solid catalysts [14].
Various approaches taking advantage of heterogeneous catalysis have been proposed, involving highly selective catalysts toward the desired products, which should have a good reusability, low toxicity, simple handling, and easy disposal. Various heterogeneous catalysts have been used for the synthesis of α-terpinyl alkyl ethers, such as zeolites, ions or acid-activated clays, Amberlyst resins, sulfonated silica, micro and mesoporous carbons, poly(vinyl alcohol) containing sulfonic acid groups [15, 16, 17, 18, 19, 20]. Despite the high conversions achieved (ca. 90%), in all these processes, the selectivity of α-terpinyl methyl ether was close to 60%, due to the formation of products resulting from carbon skeletal rearrangement, such as olefins or other ethers [15, 16, 17, 18, 19, 20].
Keggin heteropolyacids (HPAs) are another class of acidic solid catalysts that have been widely used in alkoxylation reactions of monoterpenes [21, 22, 23, 24]. These metal-oxygen clusters have a stronger Brønsted acidity, but due to their high solubility in polar solvents and their low surface area, they have been used as solid supported catalysts [25, 26]. Nonetheless, deactivation problems and leaching, triggered by the polarity of the reaction medium or the water generated by the reactions, may compromise the stability and activity of these catalysts [27, 28].
The use of commercial Lewis acid salt catalysts may be an attractive alternative. Yadav et al. developed a Brønsted acid-free route to obtain ethers from monoterpenes (i.e., α-pinene, β-pinene, limonene), involving a smooth alkoxylation under mild reaction conditions, in the presence of iron(III) chloride, and producing the corresponding ethers with a selectivity higher than ca. 70% [29]. However, the reactions were performed in CH2Cl2, an unfriendly environmentally solvent. Other transition metals have also demonstrated good catalytic activity in the synthesis of terpenic derivatives from the reactions where C–O bonds are formed [30].
Commercially available metal salt catalysts may to be an option to simplify the reactions and reduce the costs of the conversion of monoterpenes to ethers. Tin(II) salts have successfully catalyzed the esterification of biomass derivatives (i.e., glycerol and fatty acids) to chemicals and fuels additives [31, 32]. Tin(II) halides were also active in ketalization reactions of glycerol carried out at room temperature [33, 34]. Although soluble, the SnCl2 catalyst was easily recovered and successfully reused in these reactions.
In this work, several Sn(II) salts were used as acid catalysts to etherify β-pinene with alkyl alcohols. The influence of main reaction parameters was investigated. Among the various Lewis and Brønsted catalysts evaluated, SnBr2 was the most active and selective toward α-terpinyl methyl ether. The efficiency of our method was further established for the synthesis of various α-terpinyl alkyl ethers, obtained in reactions with other alcohols. The activity of SnBr2 catalyst was also assessed in etherification reactions of limonene, camphene and α-pinene with methyl alcohol. The reusability of this catalyst was assessed.
2. Material and methods
2.1. Chemicals
Solvents and reactants used were analytical grade (ca. 99 wt%); methyl, ethyl, propyl, isopropyl and butyl alcohols, were acquired from Sigma-Aldrich. Limonene, camphene, α-and β-pinene (all ca. 99 wt%) were purchased from Sigma-Aldrich. Tin(II) salts (i.e., SnCl2, SnBr2, SnF2, and Sn(OAc)2, 97–99 wt% purity) were bought from Vetec.
2.2. Catalytic runs
All catalytic runs were carried out in a glass reactor (25 mL) fitted with a sampling septum, under magnetic stirring. Typically, the catalyst SnBr2 was dissolved in the alkyl alcohol, and the reactor temperature was adjusted to 323 K. The reaction was started by addition of β-pinene.
To recover and reuse the catalyst, a simple procedure was performed. Ethyl alcohol was removed under vacuum at 323 K leaving behind the solid catalyst and the products. A liquid-liquid extraction allowed the separation of the products. After evaporation of the solvent, the SnBr2 catalyst was collected, dried, weighted and reused.
Effect of the Sn(II) catalyst on the conversion and kinetic curves (a) and selectivity (b) of the β-pinene etherification reactions with CH3OH. Reaction conditions: β-pinene (5.0 mmol); CH3OH (14.4 mL; 380.0 mmol); catalyst loading (4 mol %), 323 K.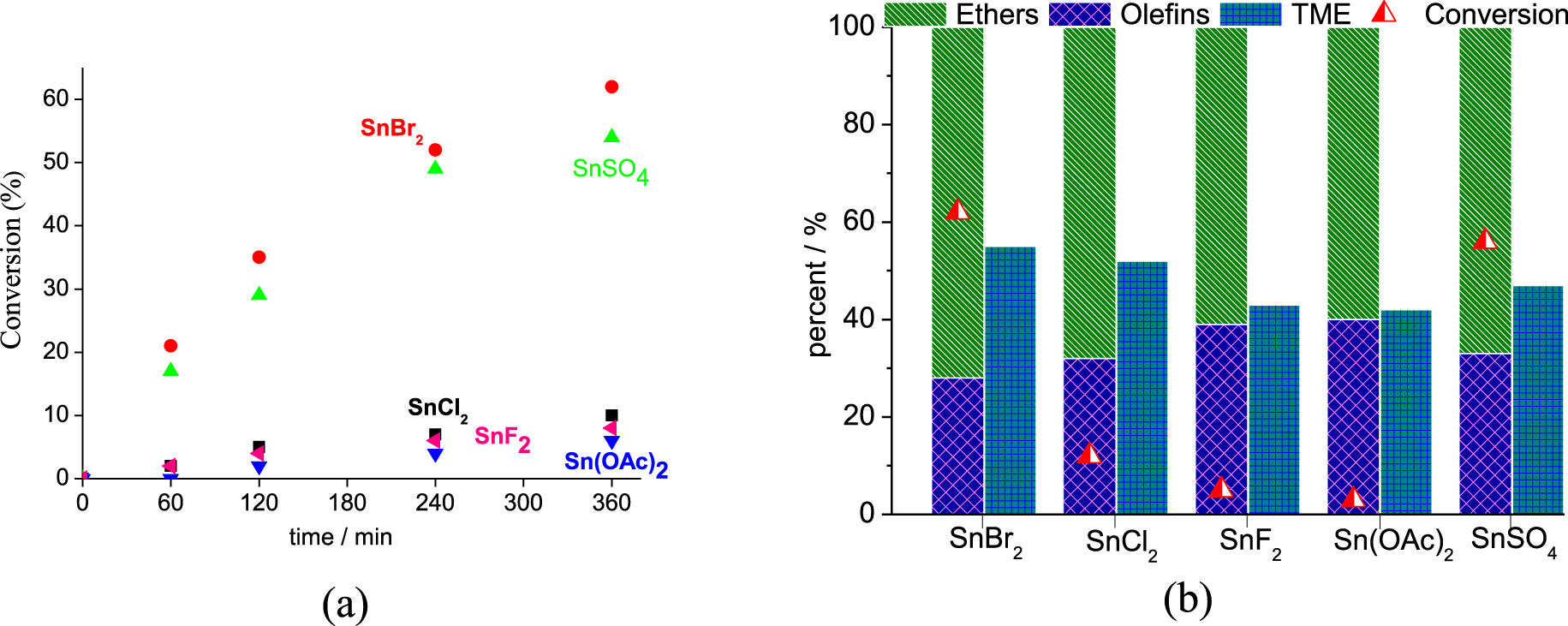
2.3. Method and techniques
The reaction progress was followed by GC analysis (Shimadzu 2010 plus instrument, FID) for 4 h. GC analyses were performed under the following conditions: 80 °C (3 min); rate of temperature increase: 10 °C min−1; final temperature: 260 °C; injector temperature: 250 °C; and detector temperature: 280 °C. Conversion and selectivity were estimated from the corresponding GC peak areas of substrate and products in comparison with the corresponding calibrating curves. Toluene was used as an internal standard. The main reaction products were identified by GC-MS analysis (Shimadzu MS-QP 2010 ultra, mass spectrometer, electronic impact mode at 70 eV, coupled to a Shimadzu 2010 plus, GC).
Measurements of pH, conversion and solubility data of the Sn(II) catalysts on the etherification reactions of β-pinene with CH3OHa
| Entry | Catalyst | Conversion (%) | pH value | Solubility |
|---|---|---|---|---|
| 1 | - | <3 | 7.1 | - |
| 2 | SnBr2 | 62 | 0.5 | partially soluble |
| 3 | SnCl2 | 11 | 1.1 | soluble |
| 4 | SnF2 | 5 | 3.3 | soluble |
| 5 | Sn(OAc)2 | 3 | 5.4 | partially soluble |
| 6 | SnSO4 | 56 | 1.0 | partially soluble |
aReaction conditions: β-pinene (5.0 mmol); Sn(II) salt (4 mol %), CH3OH (10 mL); 4 h; 323 K.
3. Results and discussion
3.1. Effect of the catalyst nature on the reaction of 𝛽-pinene with methyl alcohol
An initial screening of the Sn(II) catalyst was performed in reactions of β-pinene with methyl alcohol at room pressure and 323 K (Figure 1a). It is noteworthy that the reaction conditions were not optimized to provide high conversions. The trend observed was as follow: SnBr2 > SnSO4 > SnCl2 > SnF2 > Sn(OAc)2.
On the basis of previous studies, we have verified that the catalytic activity of metal salts under acid-catalyzed reactions (i.e. etherification, esterification) depends on two different aspects; their solubility in the reaction medium, and the decrease in pH value occurring after their addition to the solution [35, 36]. We previously verified that transition metal salt catalysts may react with acetic acid or alcohols, releasing H+ ions in solution, which can itself catalyze reactions such as esterification of glycerol and acetalization of furfural [35, 36]. Herein, to investigate if the same effect was happening, (i.e., the releasing of H+ ions due to reaction of the Sn(II) salts with CH3OH), we carried out measurements of the pH in all the reactions (Table 1).
We would like to highlight that although we are referring to the acidity measurements as “pH value”, it was not strictly correct, because the reactions were performed in alcoholic solutions and not in aqueous solution. Nonetheless, similarly to the other pH values reported herein, the glass electrode is sensitive to H+ ions, regardless of the solvent used. Therefore, a comparison of values measured after addition of the catalyst for each system is still valid.
In the absence of catalyst, regardless of the alcohol excess, no β-pinene conversion was observed (exp. 1, Table 1). Conversely, when a catalyst was present, the most acidic were the most efficient (i.e., SnBr2 and SnSO4, Table 1). We verified that the solutions containing Sn(II) salts with anions derived from strong Brønsted acids (i.e. HBr, HCl, H2SO4), presented the lower pH values. It can be attributed to the reaction of SnX2 salt with CH3OH, which releases HX and consequently reduces the pH value. It is possible that the HX may itself catalyze the etherification reaction. Furthermore, when X = F− or OAc−, the resulting acid (i.e. CH3COOH or HF) is not totally ionizable; consequently, the pH undergoes only a lower decrease. Thus, the Brønsted acid that was generated is a less effective catalyst.
Products obtained in the etherification reactions of β-pinene with CH3OH.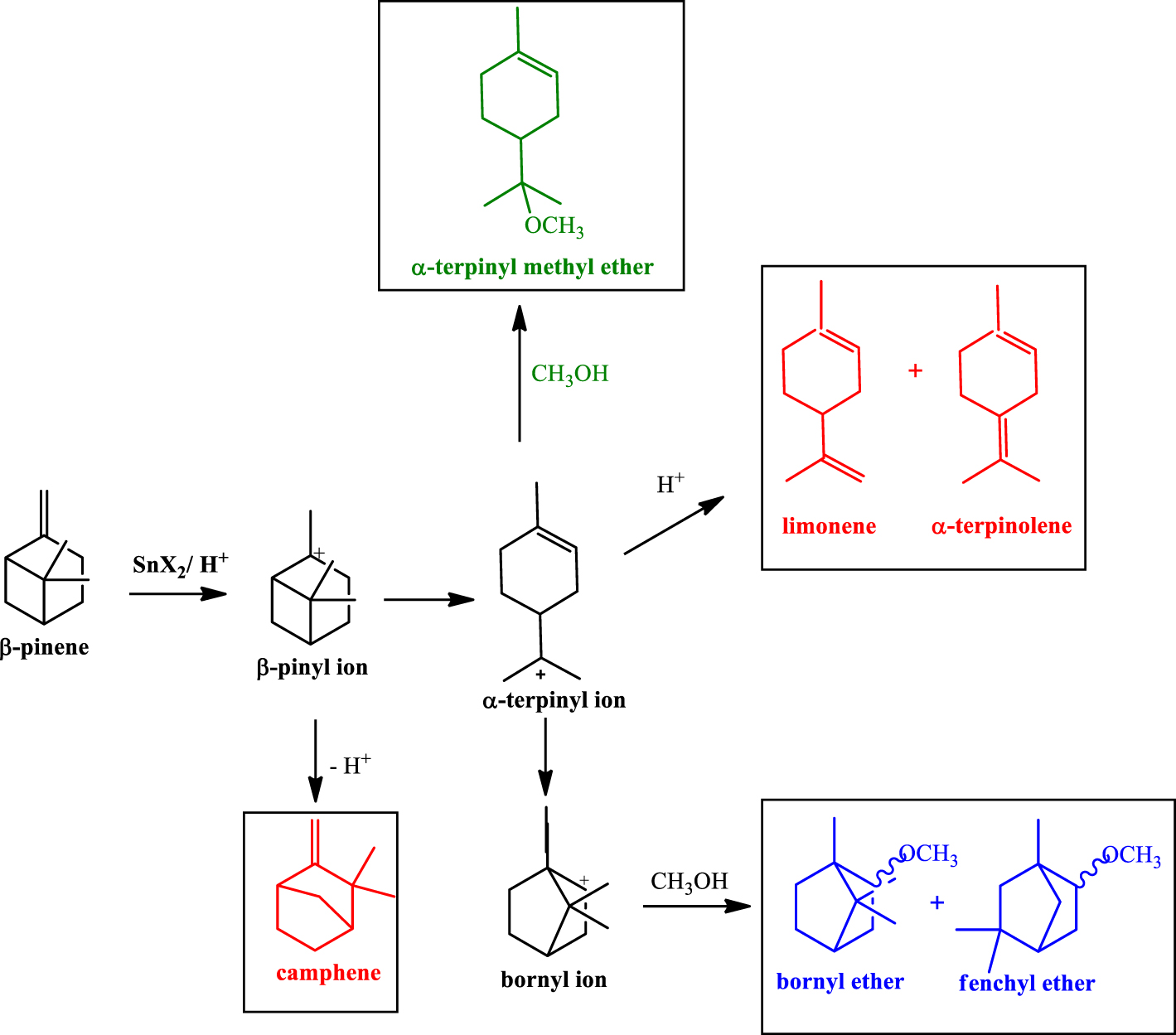
In contrast to other works, we found that the solubility of the catalyst was not an important element [39]. We verified that the partially soluble SnBr2 catalyst was more effective than soluble SnCl2. Although in aqueous solution both HCl and HBr are equally stronger acids, in alcoholic solution HBr was a stronger Brønsted acid than HCl as seen by the pH values, regardless of solubility. This higher Brønsted acidity of HBr is attributed to the lower bond energy of H–Br compared to H–Cl, which is a consequence of the larger radius of the bromine atom.
As reported in the literature, the formation of α-terpinyl methyl ether (i.e. TME, Figure 1b) governed the selectivity of the β-pinene etherification; TME was the major product, which was obtained from the nucleophilic attack of CH3OH on the α-terpinyl carbocation. This carbocation also generated isomerization products (i.e., limonene and α-terpinolene) (Scheme 1).
The carbon skeletal rearrangements undergone by β-pinene followed by methyl alcohol attack gave other ethers (i.e., bornyl and fenchyl ethers). Camphene was an isomer also detected in these reactions. Figure 1b presents in detail the selectivity for α-terpinyl methyl ether, all the ethers, and the olefins. The SnBr2 catalyst was the most selective toward TME (ca. 61%) and all the ethers (ca. 76%) (Figure 1b).
3.2. Effects of the concentration of SnBr2 on the 𝛽-pinene etherification with methyl alcohol
Although catalyst concentration does not affect the conversion of the reactions that reach an equilibrium, it is important to know the lowest concentration which gives the highest conversion in the shortest reaction time. The catalyst loading varied over a range from 2.0 to 10 mol% (Figure 3).
In the range of concentrations studied, an increase in catalyst loading enhanced the initial rate of reactions as well as the final conversion. An almost complete conversion of β-pinene was achieved after 6 h of reaction with 10 mol% of SnBr2.
Effects of catalyst loading on the kinetic curves and selectivity of the SnBr2-catalyzed β-pinene etherification with CH3OH. Reaction conditions: β-pinene (5.0 mmol); reaction volume (10 mL); temperature (323 K).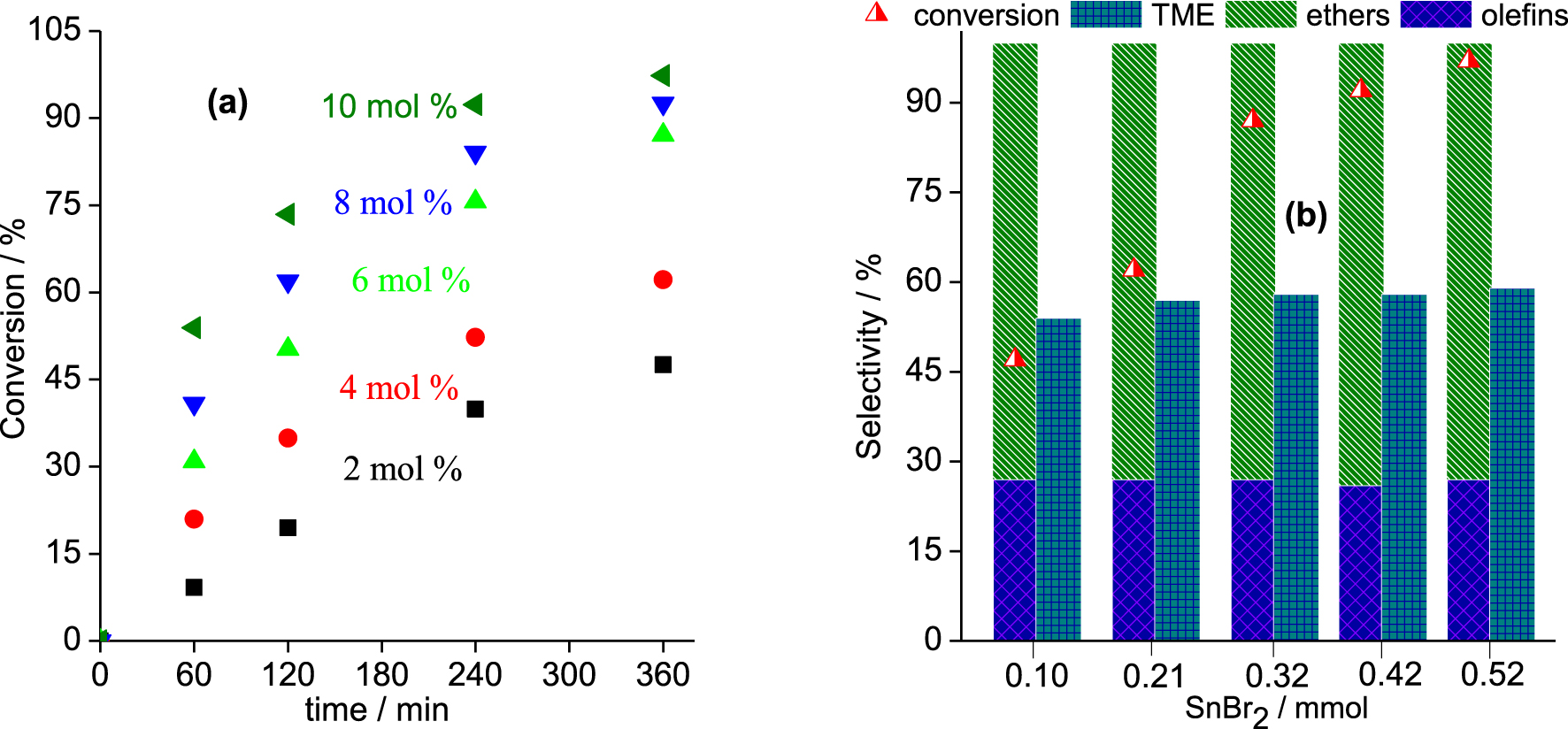
However, as demonstrated in Figure 2, regardless of the catalyst concentration, the reaction selectivity remained practically unaltered. In all the runs, the α-terpinyl methyl ether was always the major product, with a selectivity similar to those reported in the literature for Brønsted acid-catalyzed β- or α-pinene etherification reactions [21, 22, 23, 24].
3.3. A comparison of the catalytic activity of Lewis and Brønsted acids in etherification reactions of 𝛽-pinene with methyl alcohol
As demonstrated in the previous section, Lewis acid metal salts generate H+ ions, which may help to catalyze the etherification reaction of the β-pinene. Therefore, it appears relevant to know how the reaction will proceed if true Brønsted acids are present at the beginning of the reaction. Thus, we carried out the etherification reactions in the presence of typical Brønsted acids (Figure 3). Traditional Lewis acids were also evaluated. The acidity of reaction medium was also measured.
Lewis and Brønsted acid-catalyzed etherification reactions of β-pinene with methyl alcohol. Reaction conditions: β-pinene (5.0 mmol); catalyst (10 mol%); CH3OH (10 mL); 6 h; 323 K.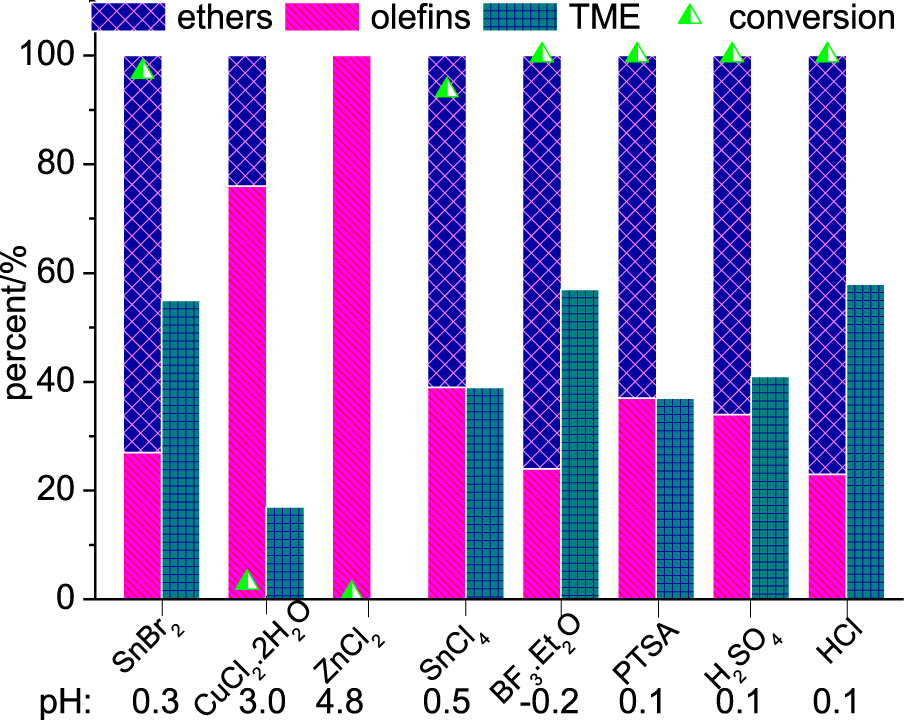
Typical Lewis acids such as BF3⋅Et2O and SnCl4 were also evaluated. Although high conversion was achieved, SnCl4 was less selective toward TME. The main drawback of these catalysts is their low water tolerance and their difficult handling. Although Cu2+ and Zn2+ cations have a high Lewis acidity, their chloride salts were practically inactive. The pH measurements corroborated this result.
Conversely, the reactions performed in the presence of common Brønsted acids (i.e., HCl, H2SO4, PTSA) reached high conversions and HCl was the most selective toward TME. Nevertheless, they are all corrosive liquid acids and require neutralization steps that generate large amounts of residues. Therefore, among the different acids assessed, SnBr2 deserves special attention because it is solid when pure, has low or no corrosivity and may be potentially recoverable and reusable.
3.4. Impact of temperature on the SnBr2-catalyzed 𝛽-pinene etherification with methyl alcohol
We assessed the effect of temperature avoiding to exceed the boiling point of methyl alcohol. Even when the reactions were carried out under reflux, no additional pressurization was performed on the glass reactor.
Effect of temperature on the SnBr2-catalyzed β-pinene etherification with CH3OH. Reaction conditions: β-pinene (5.0 mmol); reaction volume (10 mL); SnBr2 (10.0 mol%).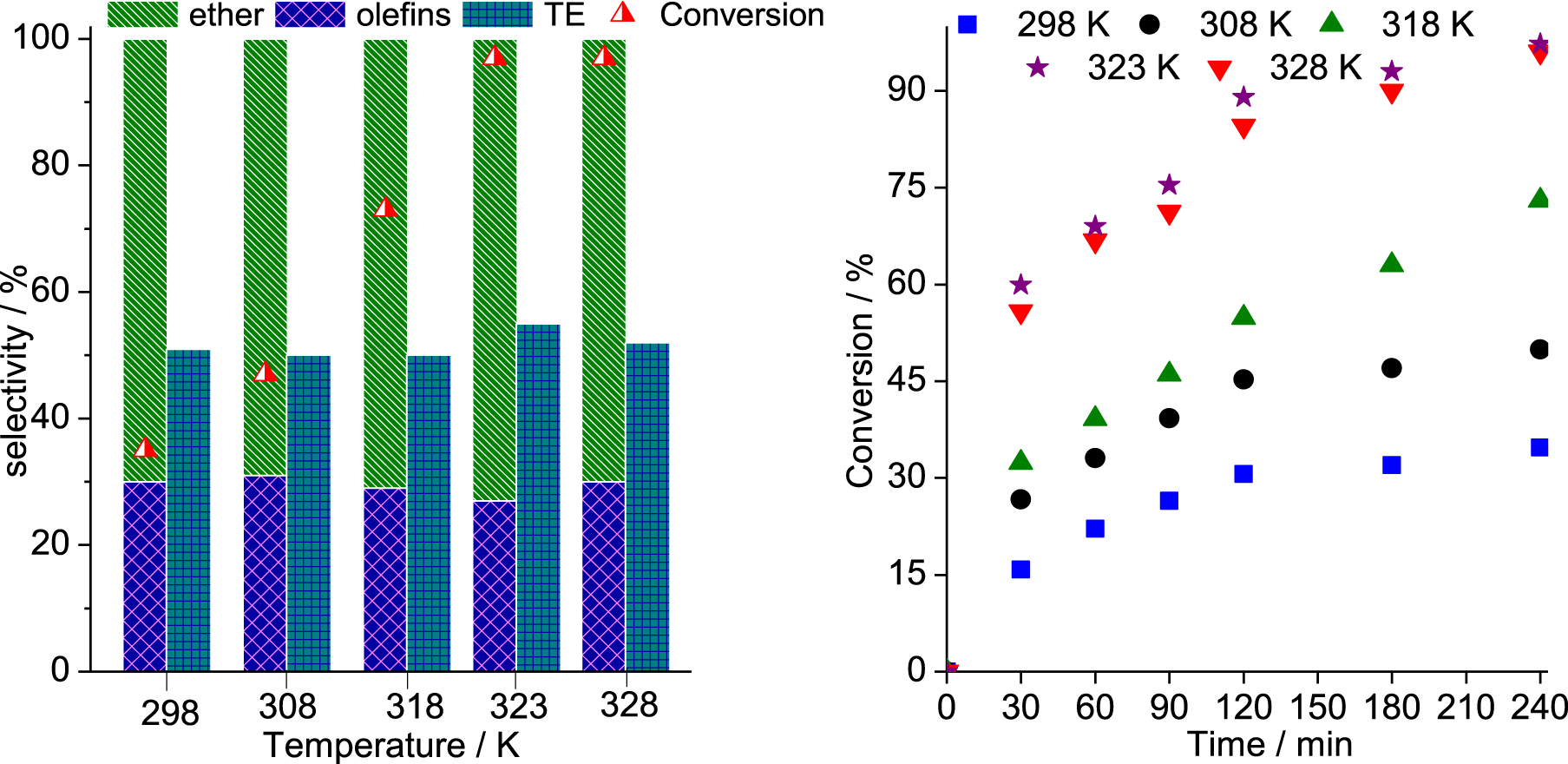
Reactivity of different alcohols in the SnBr2-catalyzed β-pinene etherification reactions. Reaction conditions: β-pinene (5.0 mmol); SnBr2 (0.52 mmol); alcohol (10 mL); T (323 K).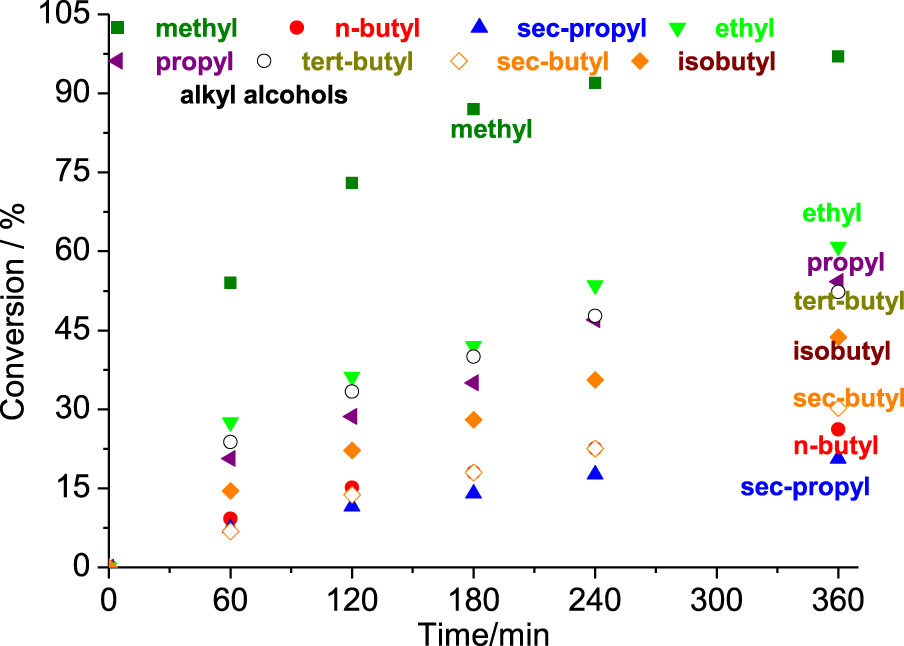
The temperature impacted differently the selectivity and the conversion of the reactions. While the selectivity was only slightly affected, TME being always the main product, the conversions were progressively improved when the reactions were carried at higher temperature (Figure 4). The initial rate of reactions was enhanced by increasing the temperature from 298 to 323 K; however, at 328 K, no significant gain was noticed.
Conversion and selectivity for α-terpinyl alkyl ether in SnBr2-catalyzed etherification reactions. Reaction conditions: β-pinene (5.0 mmol); alcohol (10 mL); time (6 h); temperature (323 K).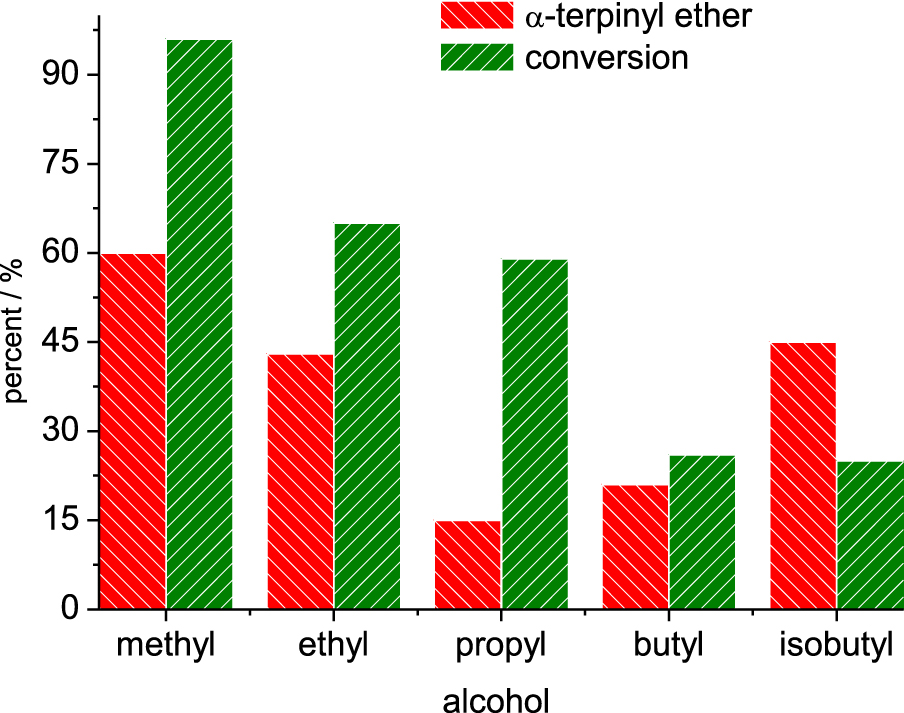
Conversion and selectivity in SnBr2-catalyzed etherification reactions of β-pinene with alkyl alcohols. Reaction conditions: β-pinene (5.0 mmol); alcohol (10 mL); SnBr2 (10 mol%); time (6 h); temperature (323 K).
3.5. Effect of the alcohol on the SnBr2-catalyzed 𝛽-pinene etherification
The performance of the SnBr2 catalyst in β-pinene etherification reactions with different alcohols was evaluated at 353 K (Figure 5).
The length of the alcohol carbon chain and the steric hindrance on the hydroxyl group affected the reactivity. For the linear primary alcohols, the following trend was observed: methyl > ethyl > propyl > isobutyl > n-butyl. However, the secondary alcohols reacted as follows; sec-butyl > sec-propyl.
When alcohols with longer carbon chains were used as substrates, the reactivity toward the formation of α-terpinyl alkyl ether was gradually decreased (Figure 6). It was mainly observed in reactions with primary alcohols. Moreover, no formation of α-terpinyl alkyl ether was observed in reactions with secondary alcohols.
Despite the low conversion of the reactions with longer-chain alcohols, new products of nucleophilic addition were formed, which resulted from addition of the bromide ion present in the SnBr2 catalyst to the bornyl and terpinyl carbocations. Bornyl bromide and α-terpinyl bromide were identified by GC-MS analysis.
While the selectivity to α-terpinyl ether gradually decreased due to the lower alcohol reactivity, the isomerization of β-pinene to isomers such as α-pinene, α-terpinolene, camphene, and limonene, was being concomitantly improved (Figure 7).
Conversion and selectivity of major product obtained in SnBr2-catalyzed etherification reactions of monoterpenes with methyl alcohol. Reaction conditions: monoterpene (5.0 mmol); methyl alcohol (10 mL); time (6 h); temperature (353 K); sealed tube. The selectivity is given for the major product.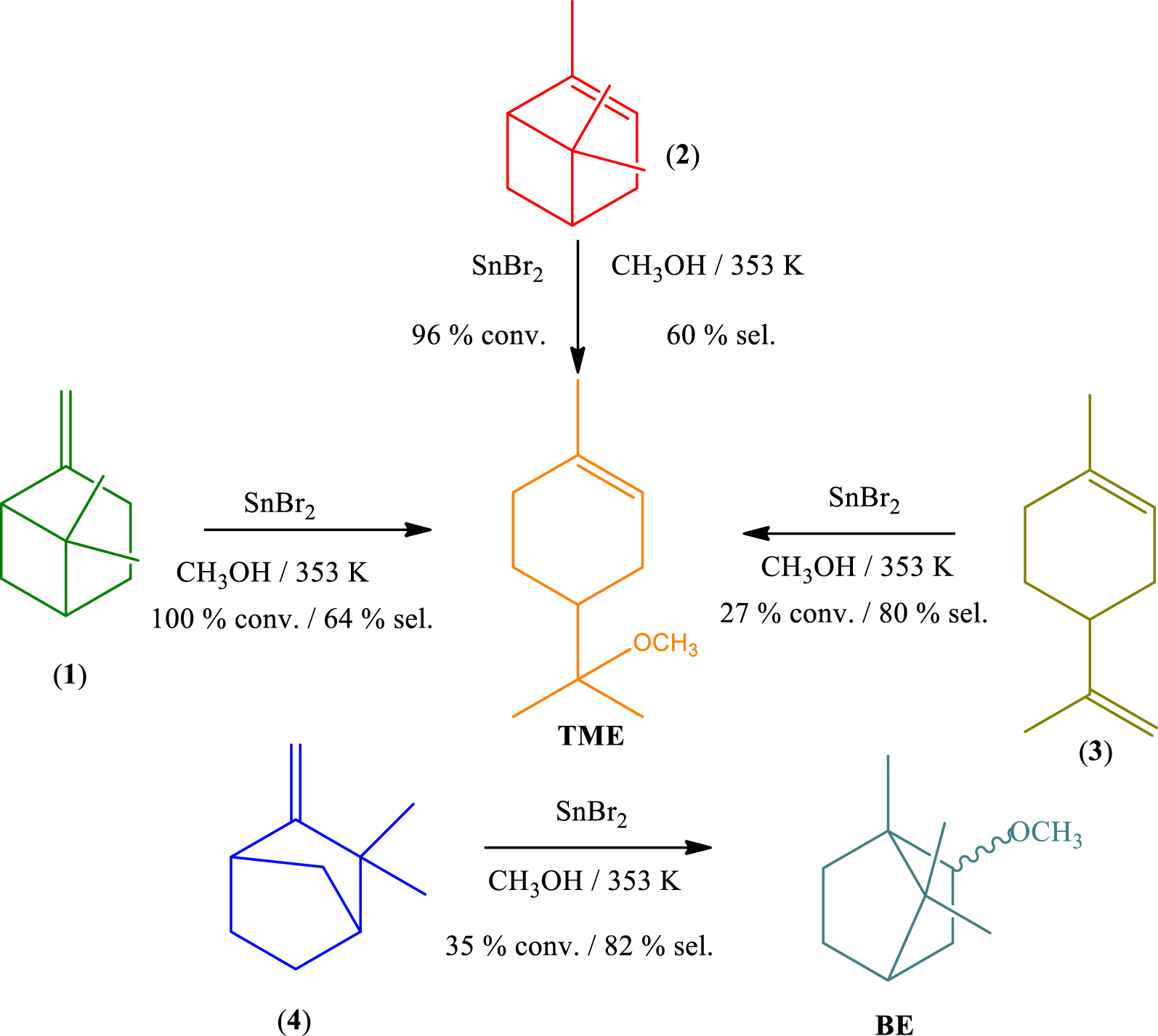
3.6. Effect of the monoterpene on the SnBr2-catalyzed etherification reactions with methyl alcohol
The catalytic activity of SnBr2 was also assessed in etherification reactions between CH3OH and monoterpenes with different carbon skeletons and double bonds. The main results of conversion and selectivity are displayed in Scheme 2. Unlike other catalytic tests that were carried out at temperatures lower than the boiling point of methyl alcohol, these reactions were performed at 353 K, in a sealed glass tube reactor.
Under these reaction conditions, β-pinene (1), α-pinene (2), and limonene (3) yielded α-terpinyl methyl ether (TME) as main product. While (1) and (2) presented a very similar reactivity, limonene was less reactive, suggesting that the carbon skeletal rearrangement undergone by (1) and (2) (see Scheme 1), which resulted in the terpinyl carbocation, was more favorable than the formation of this carbocation from limonene (3). Nonetheless, contrary to (1) and (2), which may generate products derivatives from bornyl and fenchyl carbocations, limonene can give only terpinyl derivatives. Consequently, the selectivity of TME was higher in reactions starting from limonene (3) than α- or β-pinene (1 and 2).
The SnBr2-catalyzed etherification of camphene (4) provided selectively the bornyl ether (BE, ca. 82%), with fenchyl ether being the secondary product. Although this reaction has been very selective, more so than described elsewhere in the literature, the conversion was lower; for instance, when catalyzed by silica supported heteropolyacids, an almost complete conversion of camphene was achieved at the same temperature [37].
4. Conclusion
Several tin(II) salts were assessed for the β-pinene etherification with methyl alcohol, with SnBr2 being the most active catalyst toward the α-terpinyl methyl ether (TME), the main product. The activity of SnBr2 was compared to that of other Lewis (i.e., SnCl4 and BF3⋅OEt) and Brønsted (HCl, H2SO4 and PTSA) acid catalysts. Although some of them have been equally active or selective, they presented serious drawbacks such as high corrosiveness, low water tolerance and the necessity of neutralization steps after use, problems that are absent when SnBr2 is the catalyst. We demonstrated that some tin(II) salts (i.e., SnBr2 and SnSO4) have the ability to generate H+ ions from reactions with methyl alcohol, which was a pivotal aspect for the performance of catalyst in this reaction. The reactivity of other alkyl alcohols was also studied, and we verified that it depends on the steric hindrance and size of the carbon chain. TME was obtained as main product only when linear primary alcohols were used. Brominated products (α-terpinyl and fenchyl) were detected with alcohols of lower reactivity toward ethers. Other monoterpenes were also esterified in SnBr2-catalyzed reactions. Camphene was mainly converted to bornyl methyl ether. The selectivity of monoterpenes toward TME followed the following trend: limonene > β-pinene α-pinene; however, limonene provided the lowest conversion. The reaction selectivity was impacted by the carbon skeletal rearrangement and isomerization. This proposed method is based on the inexpensive, noncorrosive, and commercially available SnBr2 catalyst, which was efficient to convert monoterpenes and alcohols to alkyl ethers.
Acknowledgements
The authors are grateful for the financial support from CNPq and FAPEMIG (Brazil).
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.




 CC-BY 4.0
CC-BY 4.0