[L’absurde] …“C’est ce divorce entre l’esprit qui désire et le monde qui déçoit” (Albert Camus) [1]
1. Introduction: is there a supramolecular chemistry for polyhedrane cages?
A recent review in Angewandte Chemie discusses perfluoropolyhedranes, their appeal, arduous synthesis, specific structural characteristics, unique electron-hosting capacity, limited atom, ion, and molecule hosting capacity, potential uses, and contributions to our understanding of chemistry. It also questions whether, despite the considerable efforts they commanded, they merely epitomize esthetically pleasing but useless molecular objects [2].
One central purpose of that review is to warn Researchers against the erroneous belief that small “cage compounds” [3] such as cubane, adamantane, or dodecahedrane (Figure 1) could easily host atoms, ions, or molecules. Such misconceptions can be induced by the contemplation of developed structural molecular formulas or stick-and-ball representations and by offhand “cage” and “bird cage” (or “box”) language (Figure 2). They can be comforted by the mere existence of a wealth of theoretical studies, even when these lack experimental validation.
The type of molecules we are considering: cubane 1 [4], adamantane 2 [5], dodecahedrane 3 [6, 7], and their perfluorinated analogs 4 [8], 5 [9] and 6 [10].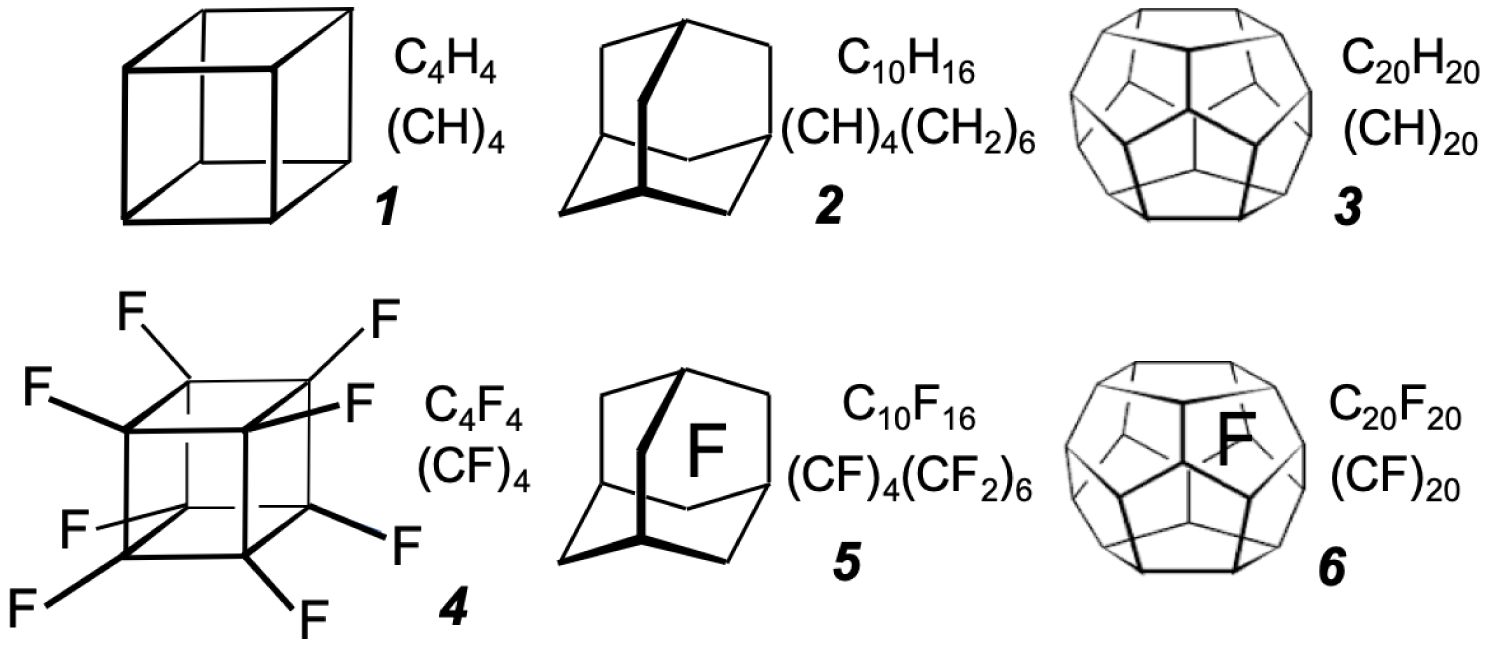
Some representations of (A) adamantane 2, and (B) its perfluorinated analog 5. a from [11, 12]; b from [13]; c compact models drawn on the same scale.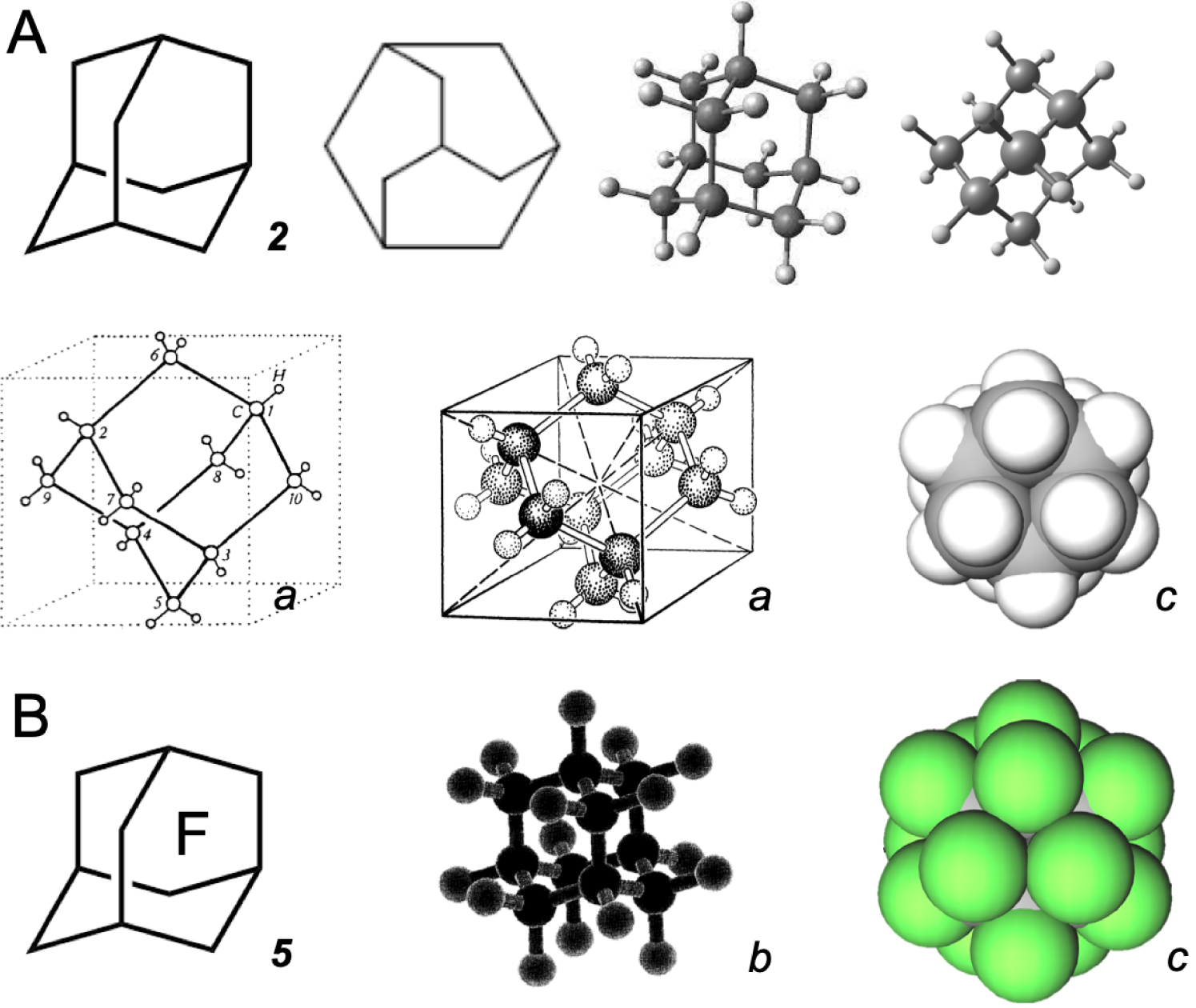
In the following Sections, an overly candid, forthright demonstration of this point by the absurd is proposed, which is merely based on the comparison of van der Waals versus covalent atomic radii (Section 2). This refutation (that would normally be considered too obvious to be published) is justified and spiced up by the critical analysis of both European and United States patents that claim the inclusion of oxygen molecules within voids of the adamantane-type “cage” compound perfluorohexamethylenetetramine 8 (Figure 3) for in vivo O2 delivery (Section 3).
(A) Some representations of hexamethylenetetramine (CH2)6N4 7. (B) Perfluorohexamethylenetetramine (CF2)6N4 8 and O2. a from [14]; b from [15]; c from CCDC Leibnitz Institute for information infrastructure, entry HXMTAM08; d from US patent 5,243,044; the compact models e are drawn on the same scale.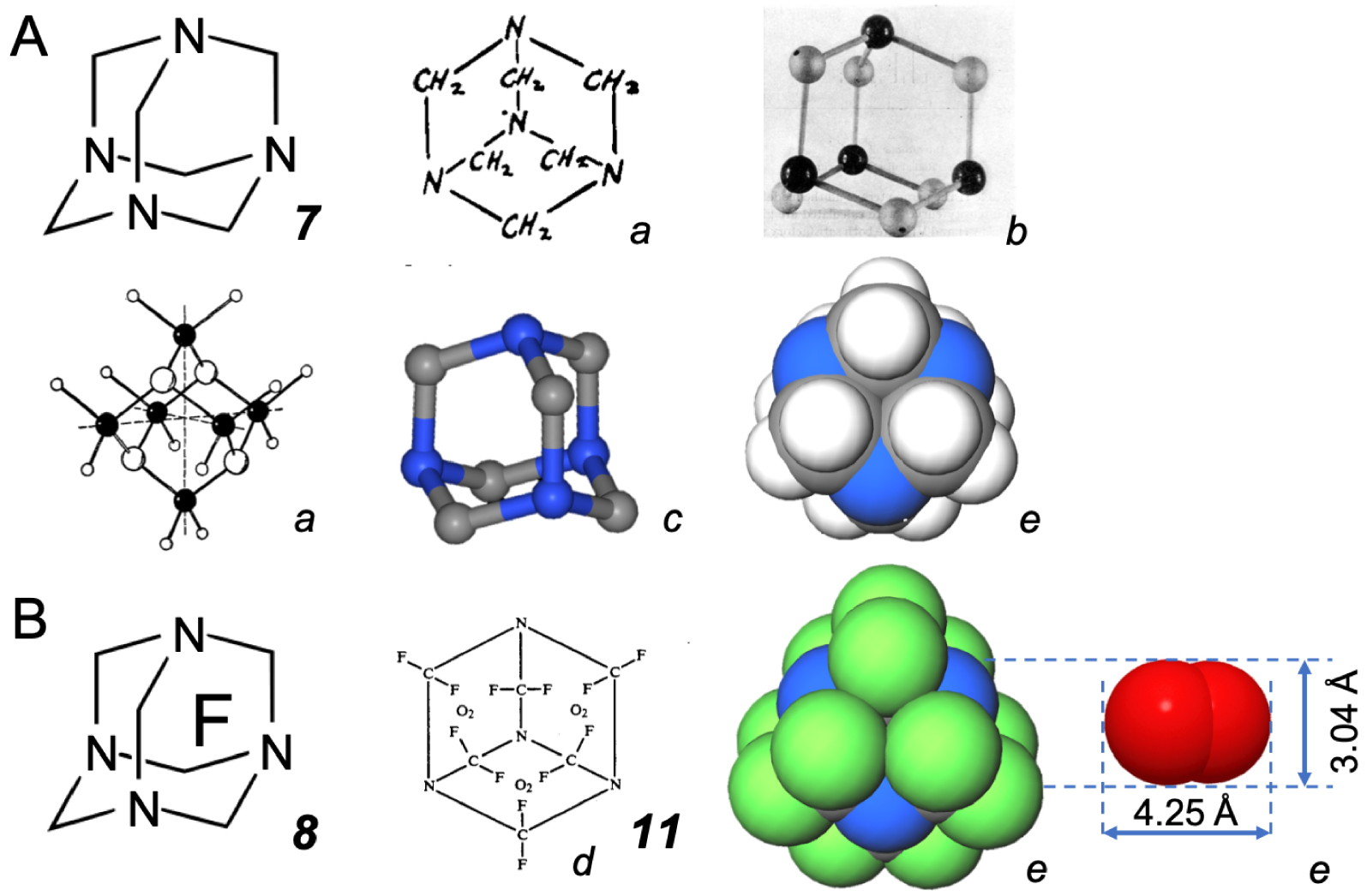
2. The virtues of a demonstration by the absurd
Reasoning by the absurd, the latter meaning stupid, incongruous, ridiculous, irrational, nonsensical, lacking logical sense, utterly opposed to truth and reason, is a type of logical argument, a form of refutation (Reductio ad absurdum) [16] that has been routinely practiced by mathematicians, physicists, philosophers and lay people through millennia. Among them stand again Plato and Euler, who had defined the high symmetry regular (Platonic) polyhedra, but also Archimedes, Aristoteles, and so many others since. The existence of man in an insensitive world is regarded as absurd by existentialist thinkers. The Socratic method, a dialectical method of teaching and conducting political discussion, includes the examination of absurd proposals as a means of fostering critical thinking and exposing logical fallacies. In common speech the term reductio ad absurdum refers to anything pushed to absurd extremes [16]. Confrontation with the absurd provides a very effective didactic tool. Next to nothing impresses a pupil as effectively as a profoundly absurd proposition.
Chemists routinely reason by the absurd when probing their ideas, intuitions, and hypotheses by counting valence electrons, examining molecular orbitals, checking potential interatomic distances (Table 1), considering symmetry rules, etc. They thereby use a “chemical common sense” acquired over the years from learning and rubbing up against the harsh reality taught by the bench. Eliminating wrong possibilities/hypotheses is a spontaneous process for the mature chemist.
Among chemist’s safeguard gears against absurdity: (A) the standard covalent and van der Waals radii of relevant atoms, along with (B) some covalent bond lengths, and (C) distances between non-bound adjacent atoms (all in Å) [17, 18]
| (A) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Atom | C | H | F | O | N | He | Ne | ||
| Covalent radius | a | 0.76 (sp3) | 0.31 | 0.57 | 0.60 (in O2) | 0.71 | 0.28 | 0.58 | |
| Van der Waals radius | b | 1.70 | 1.20 | 1.47 | 1.52 | 1.55 | 1.40 | 1.54 | |
| c | 1.77 | 1.20 | 1.46 | 1.50 | 1.66 | 1.43 | 1.58 | ||
| (B) | (C) | ||||||||
| C–C | 1.54 | C⋯C | 3.40 | ||||||
| C–F | 1.35 | C⋯O | 3.25 | ||||||
| C–N | 1.47 | N⋯O | 3.07 | ||||||
| O=O | 1.21 | C⋯He | 3.10 | ||||||
Such reasoning is seldom published as it is a routine facet of chemists’ trade. A confrontation with the absurd can, however, be useful for “teaching” purposes as, for example, to underline and counter some lingering misperceptions, question some perplexing document, or expose dubious behavior.
For polyhedranes and their perfluorinated analogs, the standard representations (Figure 2) and pervasive (and delusive) “cage” denomination are likely accountable for a sizeable number of theoretical and experimental attempts at introducing atoms, ions, and molecules in the “cage” [2]. Cages, by definition, imply a potential for confinement (or cryptation) and, hence, a possibility for supramolecular host–guest chemistry. Polyhedranes are, however, entirely different from similarly-sized but conformationally flexible cryptand molecules that were precisely designed, from both thermodynamic and kinetic standpoints, for selecting and tightly holding specific ions or molecules [20]1.
A wealth of calculations has probed the viability of endohedral complexes in numerous cage compounds [2]. For example, of H+, H, He, Ne, Li, Li+, Be, Be+, Be2+, Na, Na+, Mg, Mg+ or Mg2+ in C4H4, C8H8, C8H14, C10H16, C12H12 or C16H16 using density functional theory type calculations [21]. Exohedral localization of the aspirant guest was always found preferable to endohedral encapsulation, without exception, and no experimental evidence appears to have been reported yet in support of the formation of any of the above inclusion complexes. Atoms are no hard spheres, but strain energy increases rapidly when atoms or molecules are forced into spaces smaller than their van der Waals diameter. Concerning adamantane 2, the formation of endohedral complexes of small cations, although they were always found less stable than exohedral arrangements, was not excluded, but never demonstrated experimentally [2, 21]. The energy of formation of inclusion complex He@C10H16 (one He atom within a C10H16 frame) was calculated to be 668 kJ⋅mole−1 [21] or 645 kJ⋅mole−1 (and would be accompanied by a substantial C–C bond elongation from 1.54 Å to 1.60 Å) [22]. The inclusion of items as large as an O2 molecule in such small cage compounds has, to my knowledge, never been considered.
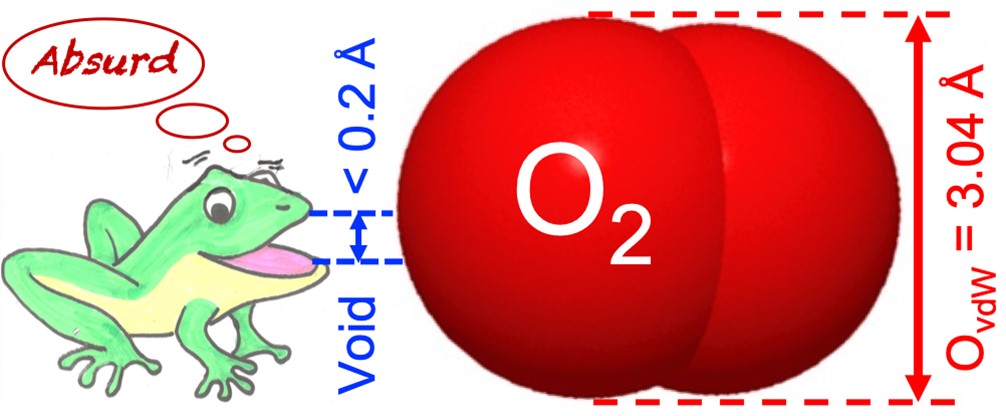
Experimentally, there is no evidence the author knows of that supports the inclusion of anything larger than an electron in adamantane-type “cage” or “bird-cage” structures. For adamantane, the largest intramolecular C–C distance between non-bonded carbons was determined as 3.56 Å (Figure 4A) [11], which, after deduction of the carbons’ van der Waals radii (2 × 1.70 Å), leaves a free central cavity of less than 0.2 Å. As far as I know, the only documented example of insertion in small polyhedranes is that of helium, the smallest possible stable monoatomic molecule (van der Waals diameter 2.80 Å), in dodecahedrane 3 [23]. Although 3 is much larger than adamantane 2 (yet with smaller fenestra) with a distance between opposite carbons of 4.3 Å and a calculated “free-space” central cavity estimated at 1.1 Å [24] or 1.5 Å [7] in diameter, it took bombarding a film of C20H20 with a high energy beam of He+ ions or fast atoms (in He+ form?) to introduce a tiny amount of He, at best one atom of He in 10,000 molecules of dodecahedrane, as determined by mass spectrometry [23]. The calculated interaction energy between He and C20H20 was 138 kJ⋅mole−1, and the pressure inside the dodecahedrane “cage” was evaluated to reach a (highly improbable)2 4 × 1026 atm [25]! The case of larger, apparently loftier structures, including fullerenes, and the recourse to elaborate chemical surgery for the introduction of guests in such frames are discussed in [2]. It is meaningful that the incorporation of O2 in an open-cage C60 fullerene, despite the existence of a comparatively huge, ∼3.7 Å large, inner cavity, required an oxygen pressure of 75 atm for 24 h for a 28% yield prior to HPLC purification [26].
Crystal structures (details) of (A) adamantane 2 and (B) hexamethylenetetramine 7 with explicitly specified interatomic distances, 3.56 Å and 3.28 Å, respectively, between opposite carbon nuclei across the structure, as depicted in [11] (calculated from data of [27] for B). These distances, once the van der Waals radii of the carbon atoms are subtracted, exclude any possibility of insertion of oxygen-size atoms. An O2 molecule (van der Waals radii) is depicted on the same scale for comparison.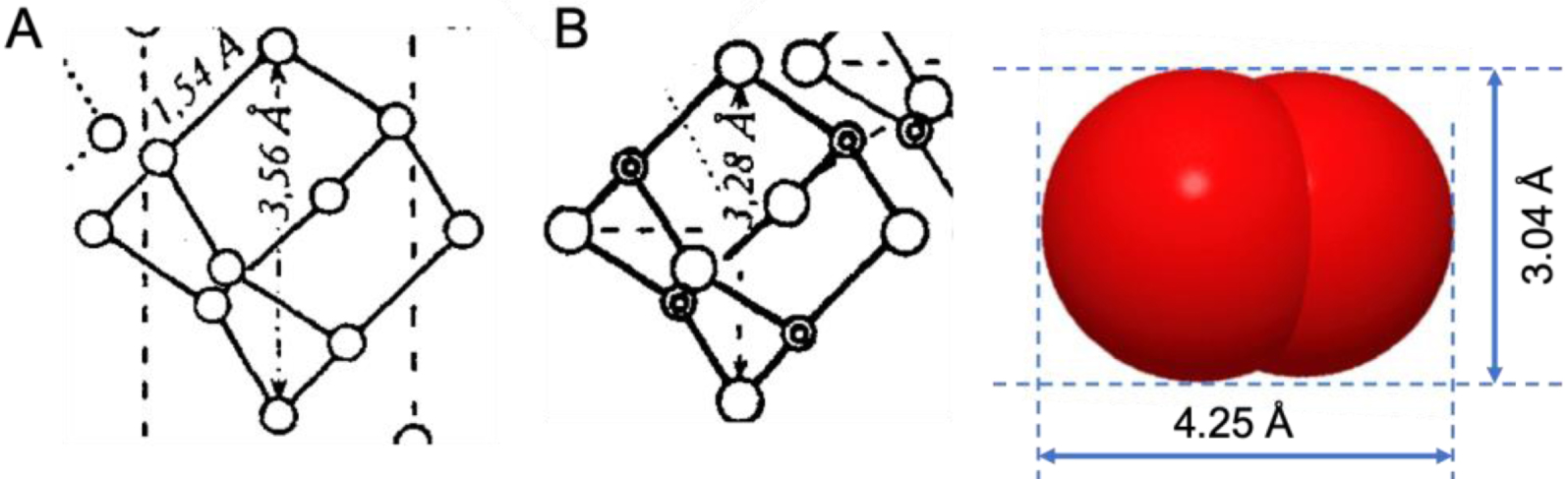
Accomplishing such a feat should be even more challenging, if not hopeless, for perfluorinated cages because of the substantially larger space requirement of fluorine atoms and the dense, poorly polarizable electron sheath that will oppose penetration into (and escape from) the cage through any of their cyclic faces. Inspection of interatomic distances within perfluoropolyhedranes, or glancing at compact molecular models, should again suffice to forewarn the most naïve against considering any easy, stable, and reversible host–guest assemblies. Molecular electrostatic potential surfaces calculated for perfluorocyclohexane 9, representing a face of (and potential entry port into, and exit port from) perfluoroadamantane 5 (Figure 5), confirm that passage through the cycle is essentially barred by the negative electrostatic potential shell formed by the bulky fluorine atoms [28]. To the best of my knowledge, there is no reported example of a host/guest complex of perfluoropolyhedranes.
Molecular electrostatic potential surface for perfluorocyclohexane, C6F12, 9 in chair (D3d) conformation, along with ball and stick, and compact models, adapted from [28].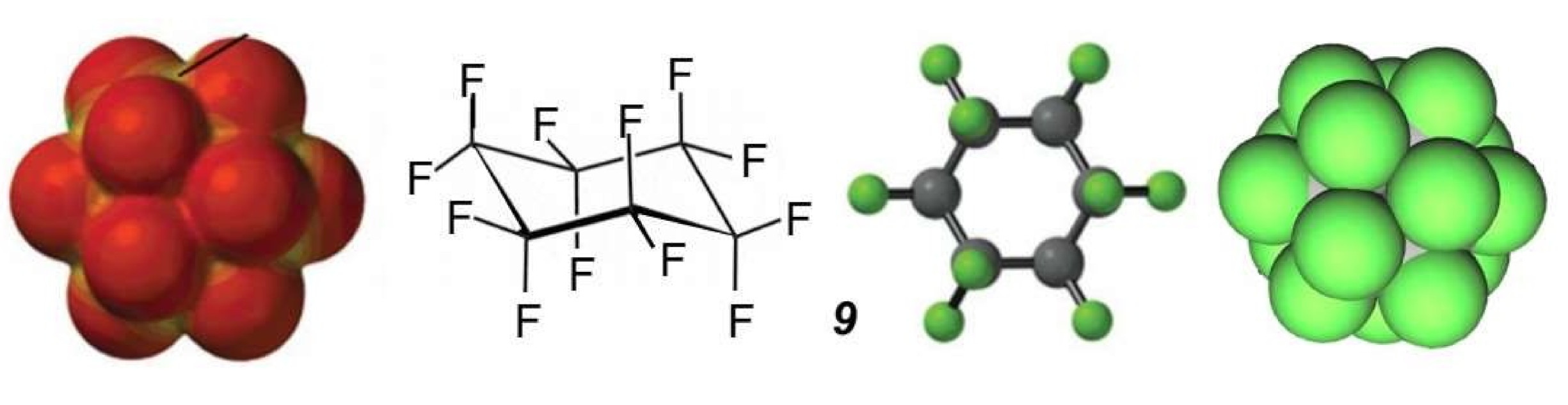
One can safely conclude that compounds such as 1 to 6 can spontaneously “encage” nothing except, in the notable case of perfluorinated polyhedranes, electrons [29], as recently demonstrated with perfluorocubane [8]3. This conclusion can be further validated by a simple demonstration by the absurd for those who would still be tempted to see here an easy entry to supramolecular cryptation of atom-size objects in small polyhedrane “bird-cages”. Let’s probe the idea of introducing, let’s say life-saving oxygen O2 in say perfluorohexamethylenetetramine 8 (Figure 3). By 1963, no less than ten X-ray, electron, and neutron diffraction studies of the structure of hexamethylenetetramine 7 in the solid and vapor states were already available [14, 31]. The structures of adamantane 2 [5, 11, 32] and perfluoroadamantane 5 [13] have also been established. The interatomic distances between opposite carbon nuclei across the structure, 3.56 Å for 2 and 3.28 Å for 7, had even been explicitly specified [11, 27] (Figure 4). Once the van der Waals radii of the carbon atoms are subtracted, these distances leave essentially no empty room inside the “cages”.
Perfluorination led, in the case of adamantane, to a slight 0.02 Å lengthening of the C–C bonds that was assigned to the electron-withdrawing capacity of the fluorine atoms [13]. Substitution of the four vertex carbons by nitrogen atoms when switching from (CF2)6(CF)4 5 (Figure 1) to (CF2)6N4 8 (Figure 3) is expected to shrink the molecular frame slightly due to nitrogen’s smaller covalent radii. In hexamethylenetetramine 7, the C–N bond was found to be shorter by about 0.03 Å in the crystal than in the gas [15, 33]. In any case, the variations in molecule size observed in adamantane-type frames due to substitution of C by N or of F by H, or changes in physical state or anisotropic effects, are on the order of a few hundredth of Å only, far from the about three Å required for O2 inclusion. Inspection of all the (abundant) published structural data concludes that there is no space available (and no access) for O2 to such “cages”4. Studies of hexamethylenetetramine co-crystals have since generated further structural information, including about the limited interatomic bond length variations in various environments [34].
The idea of introducing O2 in such structures is thus absurd simply because the smallest dimension for non-binding contact of O2, that is, the van der Waals diameter of an oxygen atom is 3.04 Å (Figure 4), while the free central cavity in 8, if any, is known to be at best a few hundredth of Å across. Absurdity derives from a confrontation [1], here of the size of the guest atom with the size of the empty hole that is expected to host it. The conclusion is clear-cut: there is no room inside hexamethylenetetramine to lodge an O2 molecule in conditions currently accessible to the average chemist. The use of 8 for enhanced oxygen transport for therapeutic purposes [35] is therefore also illusive.
Note that van der Waals space can actually be “squeezed out” from molecular crystals if extreme pressure is applied, as through shock-wave technologies [36]. But we are then talking of pressures of 105 to 106 atm, when oxygen turns into a metal. Such pressures are without common measure with the 2–3 atm. typically applied for hyperbaric oxygen therapy. Considering such an amount of squeezing to introduce O2 in 8 would be absurd in every way, including, of course, medical practicability, not to speak of patient persistence.
Interestingly also in this context is that the inverted adamantane isomer 10 (Figure 6), in which one of the H atoms is covalently bound (not hosted) inside the “cage”, was computed to endure a substantial 440 kJ⋅mole−1 strain (yet was predicted to have a half-life of 30 ms at 298 K and of 2 days at dry-ice temperature, assuming that it could be obtained and isolated) [37]. The author of the calculation suggests experimental verification (or refutation) of 10 as a novel synthetic challenge, thus illustrating again the privilege of theoreticians, who are seldom asked to substantiate their prognostications with experimental evidence5. It took a much larger, 22-atom [36]adamanzane frame, flexible enough to allow internalization of the nitrogens’ lone pairs, to produce an H+-containing adamanzane cation, including sodide 11 (an “inverted” sodium hydride) at low temperatures [38].
In-adamantane 10 and its computed structure [37], and a 22-atom-large adamanzane frame that hosts a proton in sodide 11 [38].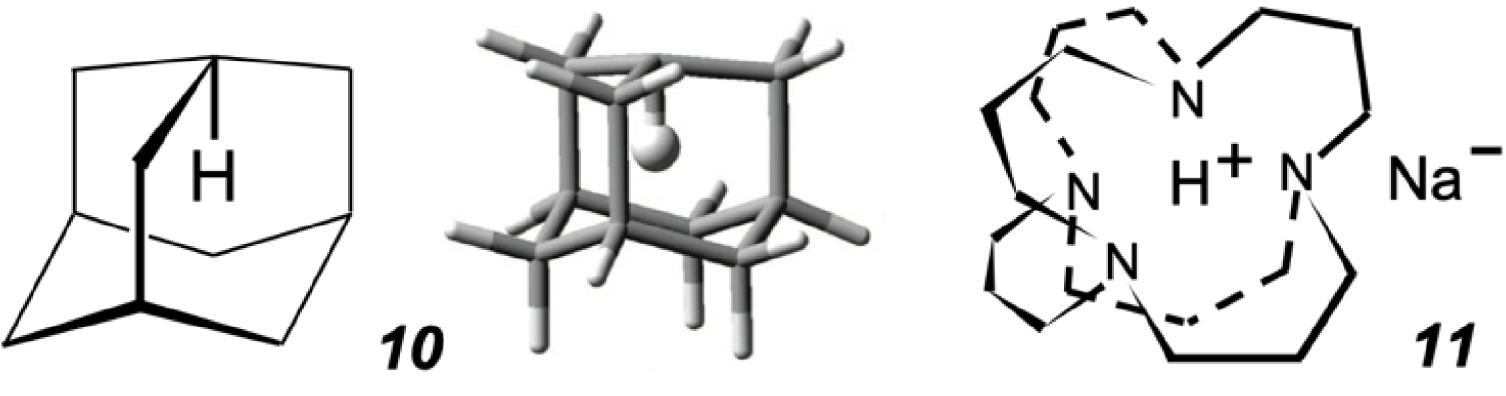
3. Perplexing patents analyzed
Yet, unlikely as it may sound, there exists a European patent (EP 0 261 802 B1, filed 25/8/1987, granted 8/1/1997), a legal document, that alleges “enhanced O2 carrying capacity” by perfluorohexamethylenetetramine 8. This patent claims that 8 (which has never been mentioned anywhere else) can transport O2 molecules “in two ways” (Figure 7): the usual, long-known one in which O2 is located between the perfluorinated molecules, and a hitherto unheard-of way in which O2 is trapped in “voids or pockets” within the molecular structure of “individual molecules” as shown in 12 (and referred to as the “first way of transport”) (Figure 7A)6. Since the well-understood inter molecular O2 transport way by fluorocarbons is described in the Angew. Chem. paper [39] cited by the Inventors, the inventive step of the patent can only be the O2 transport mode within molecules of 8. Fluorocarbons have played a foremost role in the quest for “blood substitutes” and other oxygen delivery-based therapeutics [35, 40], prompting the production of numerous patents of unequal merits.
Drawings found in patent EP 0 261 802 B1: (A) Skeleton formula 12 of perfluorohexamethylenetetramine 8 transporting O2. (B) Scheme wherein the perfluoro molecules are denoted by large circles, moving in a capillary, and the oxygen molecules are denoted by dots located both in the interstices between the large perfluoromolecules and also within the circles (and referred to in the patent as being the “first-way of O2 transport”).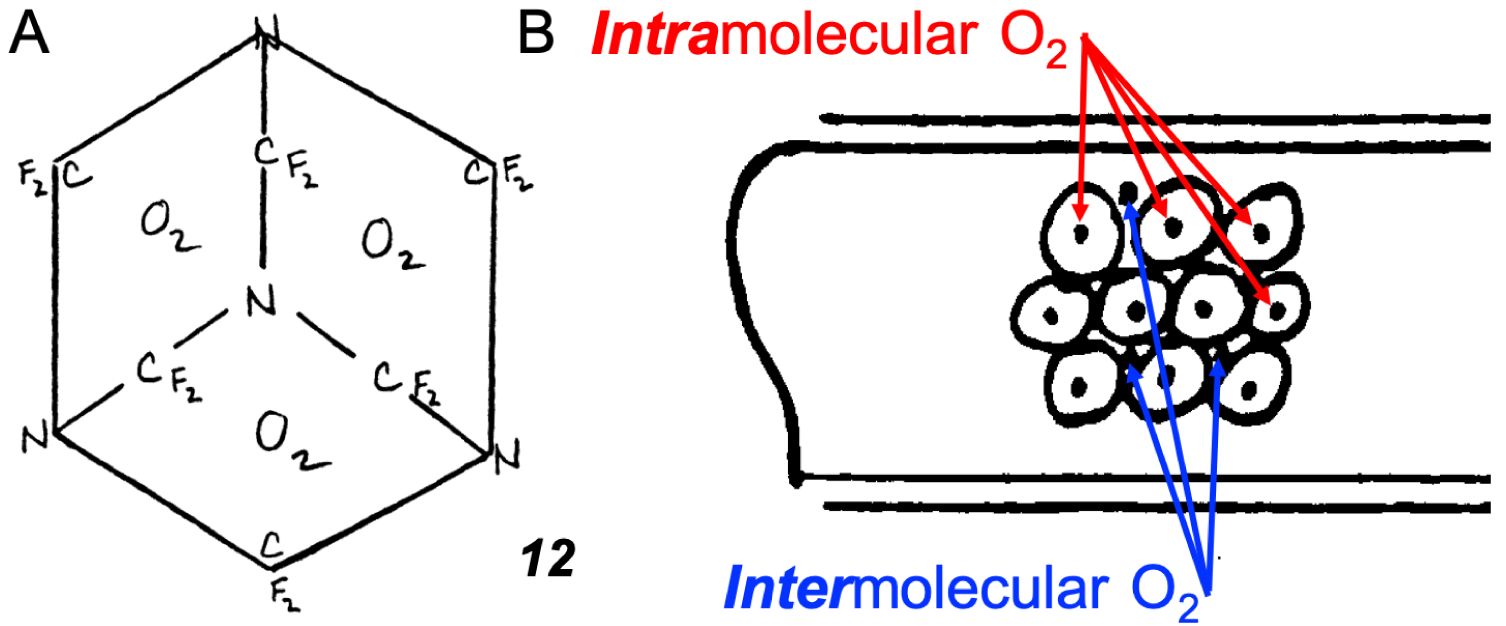
No experimental support was provided for this “finding”, no characterization or any information about compound 8, entity 12, or their emulsions, no O2 dissolving capacity measurement, and no emulsion stability or droplet size and size distribution data that are so critical for parenteral preparations, not to speak of toxicity data7.
The Inventors paid little attention to the available dimensional characteristics of guest “cages” 2, 5, or 7 that are closely related to 8, as exposed in Section 2 8, which predict that hosting O2 in perfluorohexamethylenetetramine is, for all practical purposes, impossible.
The patent provided no data supporting the announced improved O2 transport capacity. Actually, the available experimental and semi-empirical data demonstrate that the O2-dissolving capacity of cyclic and polycyclic fluorocarbons is significantly lower than for linear ones of similar molecular weight [40, 41, 42]. In particular, no enhanced O2 dissolving capacity was measured for perfluorodimethyladamantane (actually a mixture that included open-cage bicyclic fluorinated compounds) [41]. Using a semi-empirical group additivity method [43] available to the Inventors, the O2 solubility of 8 can be estimated to an expectedly low value of about 42 vol% (assuming that 8 is in liquid form). Generally speaking, all the O2 solubility values measured so far for perfluorocarbons, including polycyclic ones, are in line with Hildebrand’s regular solution theory [43, 44, 45].
Yet, in spite of the unfeasibility of claimed contraption 12, far from satisfactory descriptive section, absence of any experimental characterization of the products, and lack of demonstration of utility and of industrial feasibility, the patent was granted.
The same holds for a similar United States patent (US patent 5,243,044, filed 17/2/1989, granted 7/9/1993) by the same Inventors that again describes the same “two-way” O2 transport mode, the first way being again the physically impossible transport inside the “molecular basket”, for the same and a panoply of other polycyclic perfluorocompounds, some of which defy chemists’ common sense. Again, none of the claimed host compounds or O2-loaded contraptions of type 12 were characterized, proved to carry any extra O2, and to be useful and industrially feasible. But this patent (and a few similar ones) was also granted ….
The fact that such patents could be filed—and granted—further demonstrates (by the absurd) that our warning against an over-optimistic valuation of the hosting capacity of “cage” compounds is far from superfluous.
We briefly discussed patent EP 0 261 802 B1 in the manuscript of our review on perfluoropolyhedranes [2] for its obvious didactic value. However, an anonymous Contradictor—a Referee, who did not signal any error, lacuna, inaccuracy, or other scientific issue, observed that the patent “seemed to be a granted patent” (and hence, irrefutable?), and resented our mention of a Google search. In defense of our paragraph, we brought the following scientific, didactic, ethical, and social awareness-based arguments to the fore:
- A key didactic point of our review is to warn people against the erroneous idea that “cage” compounds can easily “encage” atoms, molecules, or ions. The impossible supramolecular compound claimed in the patent is a case example, a proof by the absurd, that very effectively supports our point. We think that the extreme absurdity of this example should impress the Reader and prompt caution.
- Patents are legal documents. It is likely that the “inventors” are no chemists, but that Patent Office Examiners, who are believed to be expert chemists, can let pass such unsubstantiated nonsensical claims is unexpected and perplexing. In our opinion, the possible non-infallibility of Patent Offices merits to be signaled.
- Such patents are not innocent. Based on problematic claims, some ghost companies deceive naïve investors in their disclosures. We believe that such fabrications should not be covered or ignored but exposed, particularly when chemical science is misused to such ends.
- The Referee did not criticize our writing because what we wrote was wrong, i.e., that the claimed insertion compound cannot exist, but because it was “badly written” and mentioned a Google search.
- It happens that one of us, early in his career, co-authored a crystal structure study of an adamantane-like inorganic “cage” compound [46](see also [47]). The measured interatomic distances immunized him against any temptation of exploring insertion chemistry for such compounds, but he since witnessed multiple attempts, experimental and theoretical, to do so.
- More generally, we believe that it is our responsibility as scientists to warn our colleagues against certain persistent misconceptions, criticize irrational claims, and expose unethical behavior for better science and social awareness.
Eventually, the following expunged paragraph, which retained our proof by the absurd as illustrated by the patent was proposed:
“A 1997 patent (EP 0 261 802 B1) claims “perfluoro hexamethylenetetramine” ((CF2)6N4) “wherein O2 is trapped in the molecular structure”, for which inclusion structure X (12 in Figure 7) was asserted. No preparation procedure or characterization was provided, not to speak of O2 solubility measurements”.
4. When absurd meets absurd
However, the above-cited Contradictor again disputed our amended paragraph, but this time he purported that “the patent is entirely reasonable in its description and does not claim O2 in the middle of the molecule”. Apparently blind to the schemes provided by the Inventors (Figure 7) and to the patent description (and deaf to our arguments), he proposed the arrangement depicted in Figure 8 as likely representing the Inventors’ intent.
A debatable proposition for O2 insertion in perfluorohexamethylenetetramine emulsion droplets intended to justify patent EP 0 261 802 B1 claims (van der Waals radii of F in blue, N in mauve and O in red added).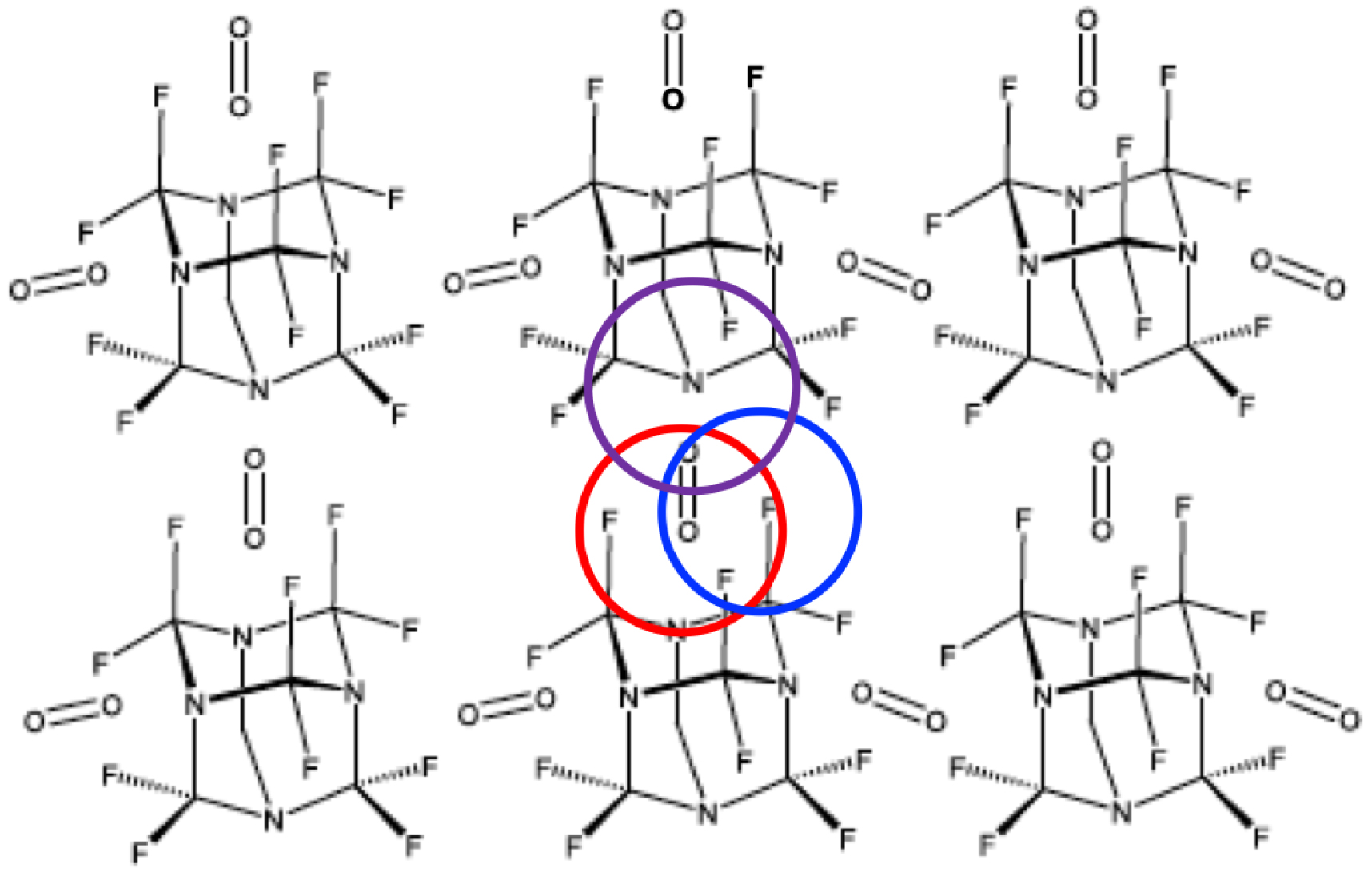
In my opinion, the proposition drawn in Figure 8 is unrealistic, and in effect absurd, on both scientific and legal grounds:
From a scientific viewpoint, one may first notice that the regularly ordered arrangement shown in Figure 8 evokes a solid state crystal structure rather than the liquid state inherent to emulsion droplets described by the Inventors. Also problematic is that the non-covalently-bonded interatomic distances (e.g., O–F, O–N) appear (in the absence of 3-dimensional views) rather similar to the intramolecular ones (C–F, C–N or O=O), while van der Waals radii require significantly different distances (Table 1, Figure 8). Most surprisingly, the arrangement of Figure 8 totally ignores the Inventors’ description of a two-way O2 transport mode, including the novel one depicted in Figure 7, in which O2 is accommodated within individual molecules and described as the first way in which 8 was supposed to transport O2.
From a patentability standpoint, the situation is also quite confounding. The principal criteria for patentability in Europe and the United States are similar. They include:
- Novelty: The invention must be new, different from what is already known, and should not have been disclosed prior to the patent application.
- Non-obviousness/Inventive step: It must not be obvious to someone skilled in the art.
- Utility: It must have a practical purpose.
- Industrial applicability: It must be able to have industrial application.
- Adequate disclosure: It must be clearly described in the patent application to allow reproduction by someone skilled in the art.
Additionally, anything that is contrary to natural laws (e.g., perpetual motion) is deemed non-patentable.
Two locations could a priori be envisaged for O2 molecules relative to compound 8 in a Socratic probing discussion: inside the “cage” and outside the “cage” (Figure 7), knowing that both locations could be occupied simultaneously. As shown above, the first option is readily discarded on scientific grounds: O2 is too large to fit into 8, as demonstrated in Section 2.
The second O2 localization option is in-between the molecules of 8. This O2 entrapment option can be discarded straightaway from a patentability standpoint given the existence of abundant prior art concerning the transport of O2 by intermolecular O2 solubilization in perfluorocarbons, including in perfluoroadamantane derivatives and perfluorocyclic amines [41, 43, 45, 35, 40, 42]. By citing a 1978 review in Angew. Chem. on fluorocarbon-based blood substitutes [39], the Inventors of EP 0 261 802 B1 indicate that they do not claim already publicized intermolecular O2 solubilization as their invention. By proposing this O2-solubilization option exclusively, our Contradictor demonstrates that the patent is invalid since his interpretation embodies plentiful prior art.
Our observation “that the patent was granted is perplexing” is thus legitimate in all cases (Figure 9): either the Inventors mean (without proving it) that O2 is carried, at least in part, within the interstices of (uncharacterized) compound 8, and the patent is problematic, or the Contradictor is correct (the Inventors mean intermolecular O2) and there should be no patent, due to profuse prior art9.
Contraption 12 is, in principle, not patentable.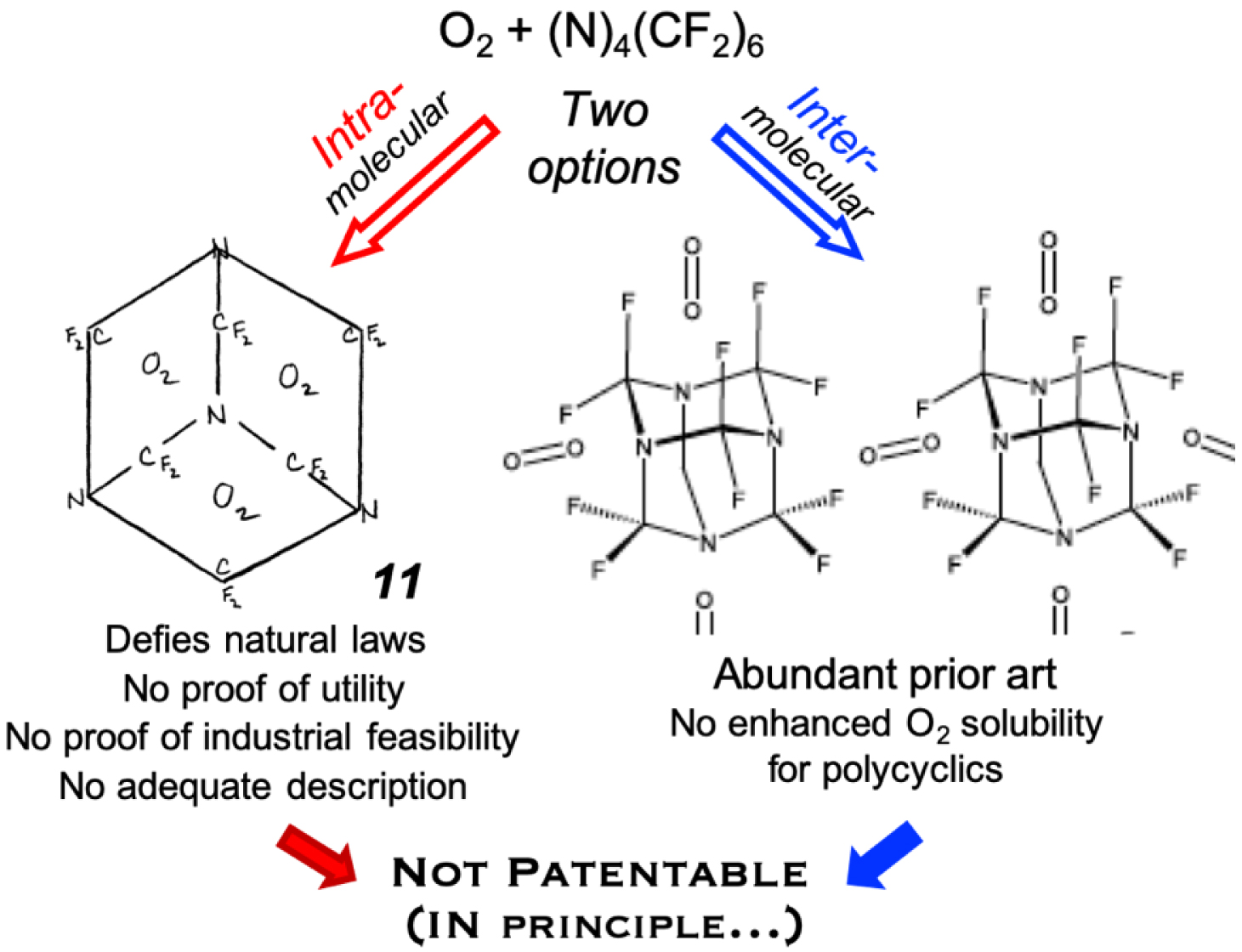
The patents cited are also un-reasonable on other grounds. In particular, the foreseeable [43] vapor pressure of 8, around 360 mm Hg at 25 °C, is way above the ca. 20 mm Hg limit above which fatal pulmonary gas embolism is known to occur for effective doses of fluorocarbons [48, 49]. The useful range of molecular weights of fluorocarbons for effective dose intravascular use as a blood substitute had thus been established to a narrow 460–520 (barely more than the molecular weight of one CF2) [49], as duly noted by the Inventors themselves in US patent 4,900,824 (filed Feb. 17, 1989, date of patent Feb. 13, 1990); yet the molecular weight of 8 is 356. So much again for the claimed utility of the invention as a blood substitute component. One should note that the above information was widely available at the time when the patents were filed and examined. Also, oxygen therapeutics means not only O2 transport but O2 delivery as well, and hence, a low enough activation barrier for release of the O2 load. What about the availability of an O2 molecule that is supposedly trapped within the rigid molecular “cage” structure of 8? And what about the shape of the O2 content versus O2 partial pressure curve, linear as usual for perfluorocarbons? Or somewhat s-shaped, reflecting saturation of the Inventors’ “first way” of O2 transport?
Altogether, the above-discussed patents are nonsensical and host/guest complex 12 defies chemical wisdom and published knowledge The Inventors could not and did not observe what they claimed to have discovered. They did not establish their “findings”’ utility and industrial feasibility, and the descriptions provided do certainly not allow a person skilled in the art to reproduce the claimed O2 complex 12. The claims are not practical, functional, and applicable (appliqué in French; angewandte in German). No wonder indeed if Google, 25 years later, still found no smidgeon of perfluorohexamethylenetetramine’s miraculously enhanced O2-engulfing capacity besides in these patents10.
Yet, the patent, despite being contrary to natural laws and current knowledge and not meeting most patentability criteria, was granted. And the Editors followed the anonymous Contradictor’s advice11.
5. Should the Reader not be allowed to judge by himself? And some further current issues relating to scientific editing and publishing
Whether the granting of the patent was justified is a question that our Editors felt they could not answer. They did not consider the Journal to be a suitable platform for discussing such a conflicting scenario and decided that the editorial team did not have the capacity to further moderate an exchange between the Referee and us. We were coerced, politely but definitely, to remove any reference to the patent from our manuscript, thus depriving the Reader of a critical discussion of highly questionable but legal documents.
And yes, a scientific paper should be opinionated, critical, and thought-provoking (as often encouraged by Journals), and it should identify data gaps, unsolved problems and absurd claims, especially when it comes to warning the Reader against certain tenacious misconceptions (Figure 10). Should Authors not be allowed to freely express their doubts12 and opinions about improbable claims, even when (or especially when) the document is unsatisfactory? Should the Reader not be considered a learned, mature individual capable of judging on his own, especially when the matter is debatable?
(A) No material bird (even monoatomic) can be lodged decently in “bird-cage” compound perfluorohexamethylenetetramine 8. (B) Amusingly, it is with a barbed wire cage by Chema Madoz in 2003 that Le Monde Diplomatique in its Mai 2023 issue (p. 3), that is when our review in Angewandte Chemie was first published, illustrates an article on la liberté d’expression.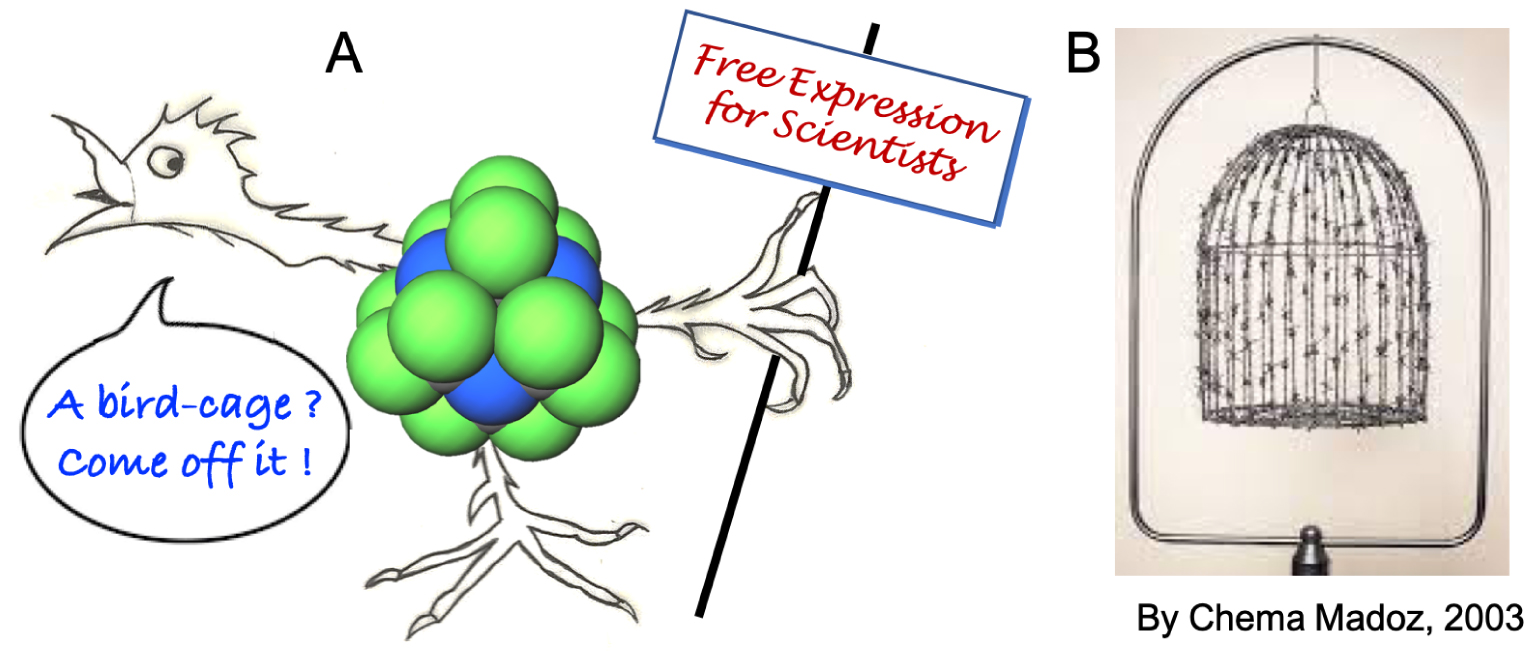
Whether or not the paragraph about perplexing patent EP 0 261 802 B1 was comprised in our review about perfluoropolyhedranes is of little consequence. The case against the easy occupation of such “cage” compounds is strong enough without. But the reasons why a great decent chemistry journal should impose removal of—in effect censure—an Author’s didactic approach and reasoned (cartesian) skepticism on the basis of a single referee’s untenable position can legitimately be asked. It can certainly not be for lack of understanding of basic chemistry; or of common sense; or of prevention against thought-provoking, out-of-the-box (or cage) considerations; or fear of discussing controversial issues; or restriction about letting reveal the non-infallibility of patented nonsense or possible inattention during a patent examination process; or chickening out from exposure of unethical behavior, with potential effects on naïve investors’ savings; or disrespect for Authors’ opinions; or disregard for the Reader’s right to be informed, exposed to controversial issues, and aptitude to conclude independently. So what?
The author willingly recognizes that Editors are faced with increasingly trying challenges. The number of papers (and aggressive competing journals, including predatory ones) has exploded; the papers, systems and matters submitted are increasingly complex; the wealth of information found on the net is not always critically assessed and understood by authors, while a fair amount of prior work is inexcusably ignored; some authors may not find time for both writing and reading; the volume of often unduly lengthy, sometimes undigested or irelevant, repeatedly duplicated considerations and copy-pastes that encumber some articles tends to thrive; the space devoted in papers to their “marketing” (and over-marketing) tends to escalate, while experimental parts and hard data are too often relegated to Supplementary Information; a substantial gap, sometimes, between the proclaimed findings and the actual results delivered; an appetite has developed for scoopy announcements and arty cartoons at the expense of scientific rigor; and conversely, a lack of inclination for correcting bad science and battling enduring misconceptions (or “unicorns”) [51, 52]; the recruitment of Referees that are both competent, available and open-minded, and willing to support a publishing system in which they may be losing faith, is increasingly difficult; the dealing with inapt or failing Referees can be frustrating13; the commercial objectives of many scientific journals seem to compete increasingly with scientific considerations; there is a rising resentment among authors who feel to be exploited by greedy publishers and perceive journals as lucrative and unfair cash-machines that participate to the merchandizing of knowledge; such resentment is fueled, in part, by the excessive impact of unaudited bibliometrics on young scientists’ recruitment, tenure, promotion, and funding; the heavily discriminating open versus paid access to publishing and to information encouraged by publishers is definitely unfair; the increasing interdisciplinarity of research projects can leave knowledge vacancies and a dilution of responsibilities; referees are often recruited in the same community (e.g. medicine or biology) as the authors, while some concepts, techniques and instrumentation they use should be reviewed by specialists of the latter (e.g. physicists, analysts), failing which, artefacts and the meaninglessness of some data escapes the reviewing process; the formation of coteries that hamper access to a domain of new-comers with non-conform ideas or criticizing the coterie’s papers, thus perpetuating inbreed thinking, is not infrequent; the “proliferation of bullshit” [53] appears irrepressible14; certain forms of social networking manipulate the peer-review system and citation records; an increasing number of fraud factories and paper mills that churn out fake data and papers to order, are downright deception-oriented; the advent of “intelligent” and “creative” writing and drawing software (that sometimes compete with the bench) can introduce further hurdles; AI-generated data, images and text become increasingly difficult to detect15; although AI software in development may also help identify fake papers and paper mills; etc. [51, 52, 54, 55, 56, 57, 58]; but relying uncritically on AI rather than on human judgment for editorial tasks would be dangerous and was called a “trivial nonsense” if only because we do not know how the algorithms are made [59].
Altogether, we are witnessing a rapidly changing state of affairs for which we are finally all responsible: scientists, editors, publishers, research institutions, and funding agencies all together. And should work all together at remediating. This certainly requires increasing attention, a commitment to robust science, respectful open exchanges, improved transparency, refined discernment, and enhanced accountability. The development of open-minded human relations between authors, editors, and reviewers should certainly be fostered. Editors have also, in this author’s opinion, in addition to publishing scientifically sound, high-quality research, a societal role to play by exposing and combating misconduct, fraud, and other unethical behavior, thereby also contributing to the preservation of public trust in science and scientists in times of increasing conspiratorial anti-science activity [60]. Some do.
The author hopes that the cautionary tales, analyses, and opinions expressed here may prove useful.
Declaration of interests
The author do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Acknowledgements
Many thanks to the Comptes Rendus de l’Académie des Sciences (Chimie) and their Editor, Dr. Pierre Braunstein, for hosting these opinions freely and offering full open access to them for free.
Note added in proof
The referee’s reports on this paper were of interest due to the broad diversity of opinions expressed and to the many facets of the manuscript that were overlooked or chosen to be discounted. None of the referees found any interest in didactics, preventing misconceptions, correcting wrong science, discussing related societal consequences, or pondering issues related to scientific publishing in the current fast- changing world, while their field, chemistry, is most decried and that momentous mutations are looming. In line with the author’s plea for greater transparency in the evaluation process of scientific papers and increased constructive exchanges between editors, referees, and authors we proposed with the editor to disclose these reports on an experimental basis as Supplementary Information. After being asked, the referees kindly agreed to the experimentation (one of them reluctantly) and under the condition of anonymity, although they did not know in advance (nor did the author) that their reports (and his answers) would eventually be published.
The author deems that systematic transfer to each referee of the reports of all referees, along with the author’s and editor’s reactions, or better, making these exchanges available to the readers, for example as Supplementary Information or in the Journal’s website, is appealing for several good reasons:
- for much-wanted enhanced transparency of the editorial paper assessment process;
- to allow exposure of divergent visions and arguments;
- to provide the referees with a sense of the amazing variability of opinion, input, and attitude that can happen among them;
- to offer them a means of self-assessing their performance, attentiveness, contribution to improving the paper they reviewed, or penchant for censorship;
- to help them appreciate how an author may perceive, consider, and use their advice, suggestions, judgments, and (anonymous) ukases;
- to thereby incite them to (next time) read their report over aloud, as recommended to referee’s by PNAS before firing;
- to gratify the Reader with additional information, opinions, controversial visions, and matters to consider.
Such openness could also incite more young scientists to participate in the peer-reviewing process. The author is grateful to Professor Etienne Ghys (Académie des Sciences) for drawing his attention to a recent study showing indeed that publishing peer-review reports increased the willingness of younger scientists and non-academic scholars to accept to review, and yielded more positive and objective recommendations, and more constructive reports [61]. Obviously a win-win situation for all.
1 Dodecahedrane has the same number of atoms (but many more vertexes) as Lehn and Sauvage’s [2.1.1]-cryptand that was designed to selectively engulf the Li+ ion.
2 If confirmed, this would be many orders of magnitude larger than the pressure at the center of the sun, 2.7 × 107 GPa (2.7 × 1012 atm); molecular frames are hardly expected to withstand such pressures.
3 But even electrons are sensitive to roominess [30].
4 Were it possible to produce them and inject them parenterally, contraptions 12, given the gigantic amount of energy that would be packed in them, would qualify as picometric intravascular bomblets.
5 It is biting that computer chemists ingenuously bring forward, among the motives for their studies, the potential use of strained high energy density molecules as advanced explosives, and then suggest that experimentalists go and see.
6 Repeated in German in EP 0 261 802 B1 “Die Verbindung nach Anspruch 2, worin Sauerstoff in der Molekul struktur eingeschlossen ist”, and in French “Composé selon la revendication 2, dans laquelle de l’oxygène est piégé dans la structure moléculaire”, follows the structure reproduced in Figure 7A. And US Patent 4.900.824 says: “The oxygen molecules pack into voids or cavities” ….” in two ways, first, by selecting structures which because of their molecular shape pack poorly together and leave voids in the liquid, and second, by building voids or pockets into the molecular structure itself so as to accommodate an oxygen molecule into the interstices of individual fluorochemical molecules.” (italics added).
7 Perfluoro[3.3.3]propellane, another undocumented (and challenging) cage compound, can also be present in the composition of the patented emulsions.
8 All the structural data presented in Section 2 were available when the patent was filed and examined.
9 The Editors of [2] gracefully satisfied the author’s request to forward these observations to Contradictor, but the latter has not yet manifested himself.
10 That such patents were granted—and by both EU and US offices—is disconcerting, and inevitably questions the patent offices’ attentiveness. Even the titles of the patents contain errors: hexamethyltetramine for hexamethylenetetramine in the EU patent; and propellene for propellane in the US patent.
11 The notion of handling a controversy with an anonymous, blind, and deaf personage could also feed an interesting debate. An increasing number of journals ask the Referees if they agree to communicate their names once the paper is issued. This is a win-win situation in which the authors are happy to advertise positive opinions and the referees see their expertise and efforts acknowledged; moreover, it introduces some much-needed transparency in the refereeing process.
12 “Of all [science’s] many values, the greatest must be the freedom to doubt” [50]. This may even count for granted patent claims.
13 The Referees lottery for paper [2] gratified us with three dissimilar but representative breeds of reports: (over)laudatory, futile, and absurd. And, unfortunately, chemist Referees seldom care about didactics, censorship, correcting misconceptions, editorial challenges, publication ethics, human exchanges, or societal issues related to science.
14 “Bullshit is unavoidable whenever circumstances require someone to talk without knowing what he is talking about” [53].
15 Some readily available sites identify plagiarized text and concurrently offer services to rephrase that text.




 CC-BY 4.0
CC-BY 4.0