1. Introduction
Phenylenediamine derivatives 1–3 (Figure 1) are an important class of organic compounds with rich chemical and physical properties [1, 2, 3] and remarkable applications as precursors in color chemistry [4, 5]. Their coordinating ability has been less investigated because phenylenediamines do not form very stable complexes with bivalent metal ions [6]. This poor stability can be explained by the low basicity of the aromatic amines due to the delocalization of the lone pair of the nitrogen atom toward the p orbitals of the aromatic ring, decreasing the electron density on the nitrogen atom available for the bond formation with a metal center. The coordinating ability of aromatic diamines is also conditioned by the position of the nitrogen atoms on the aromatic ring (ortho, meta or para). o-Phenylenediamine ligand 1 coordinates either in a monodentate, chelating bidentate and bridging bidentate fashion in different dinuclear complexes, with possible oxidation of 1 depending on the metal center [7]. Studies of coordination compounds of 2 and 3 in solution and solid state are rare, possibly because these ligands can only coordinate one nitrogen atom to a metal center by means of a weak linkage [7], but also because of oxidation processes forming p-benzoquinonediimine ligands [8]. Falthouse and Hendrickson have reported a series of binuclear Cu(II) complexes in which 2 acts as a bridging bidentate ligand but the instability inherent to the phenylenediamines made it difficult to fully characterize the complexes [9].
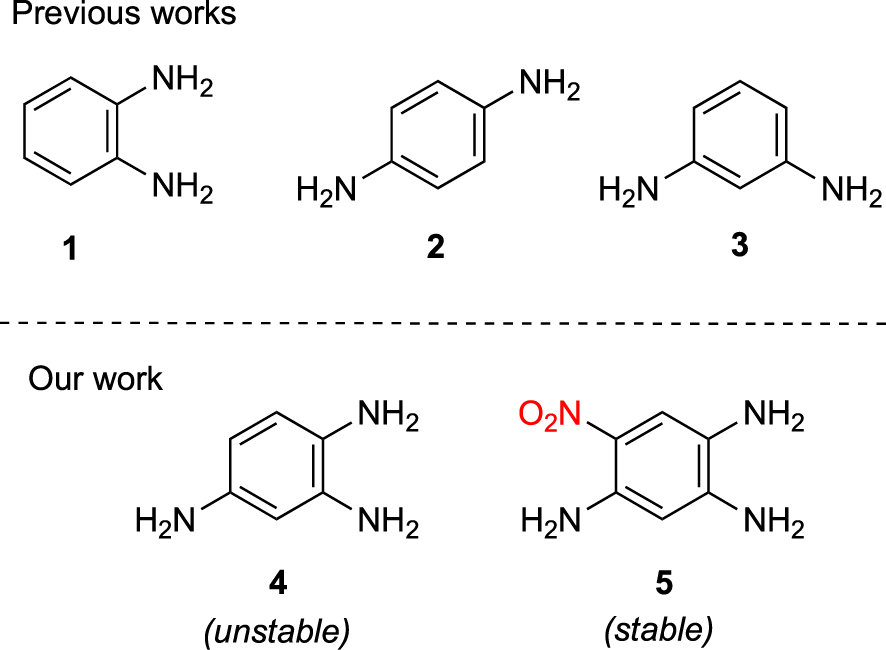
Molecules studies in previous works and our work.
As an extension of these studies, it appeared very attractive to investigate 1,2,4-triaminobenzene 4, which combines the structural elements of 1, 2 and 3, as ligand in coordination chemistry. However, bearing three amino functions, molecule 4 is highly unstable in air and can be easily oxidized, sacrificing its aromatic character in favor of a quinoidal form. To overcome this limitation, we envisaged the use of analogs of type 5 because the incorporation of an electron-withdrawing nitro group should strongly reduce the electron density and consequently prevent air oxidation of the molecule.
Herein, we wish to describe the synthesis and X-ray diffraction analysis of two new complexes (7 and 8), based on the air-stable 1,2,4-triamino-5-nitrobenzene derivative 6. Importantly, the key role of the nitro group on the stability and the reactivity of the ligand is clearly emphasized, illustrating a further aspect to be considered in the coordination chemistry of aromatic amines.
2. Results and discussion
Precursor 6 was first prepared as recently reported in the literature [10]. Its metalation with Ni(acac)2 in toluene at room temperature led to the formation of a beige precipitate (BP) that could be isolated by filtration (Scheme 1). IR analysis of this powder compared to the precursor 6 (Figure 2) revealed the appearance of strong bands around 1380, 1020 and 925 cm−1, which could be attributed to carbon–oxygen and carbon–carbon bonds. In addition, the N–H vibrations above 3250 cm−1 for 6 are shifted for BP whereas the C–H vibrations (2900 cm−1) are not affected. These observations are consistent with the coordination of one or several NH2 group(s) to the nickel center and the presence of acetylacetonate (acac) moieties as ancillary ligand.
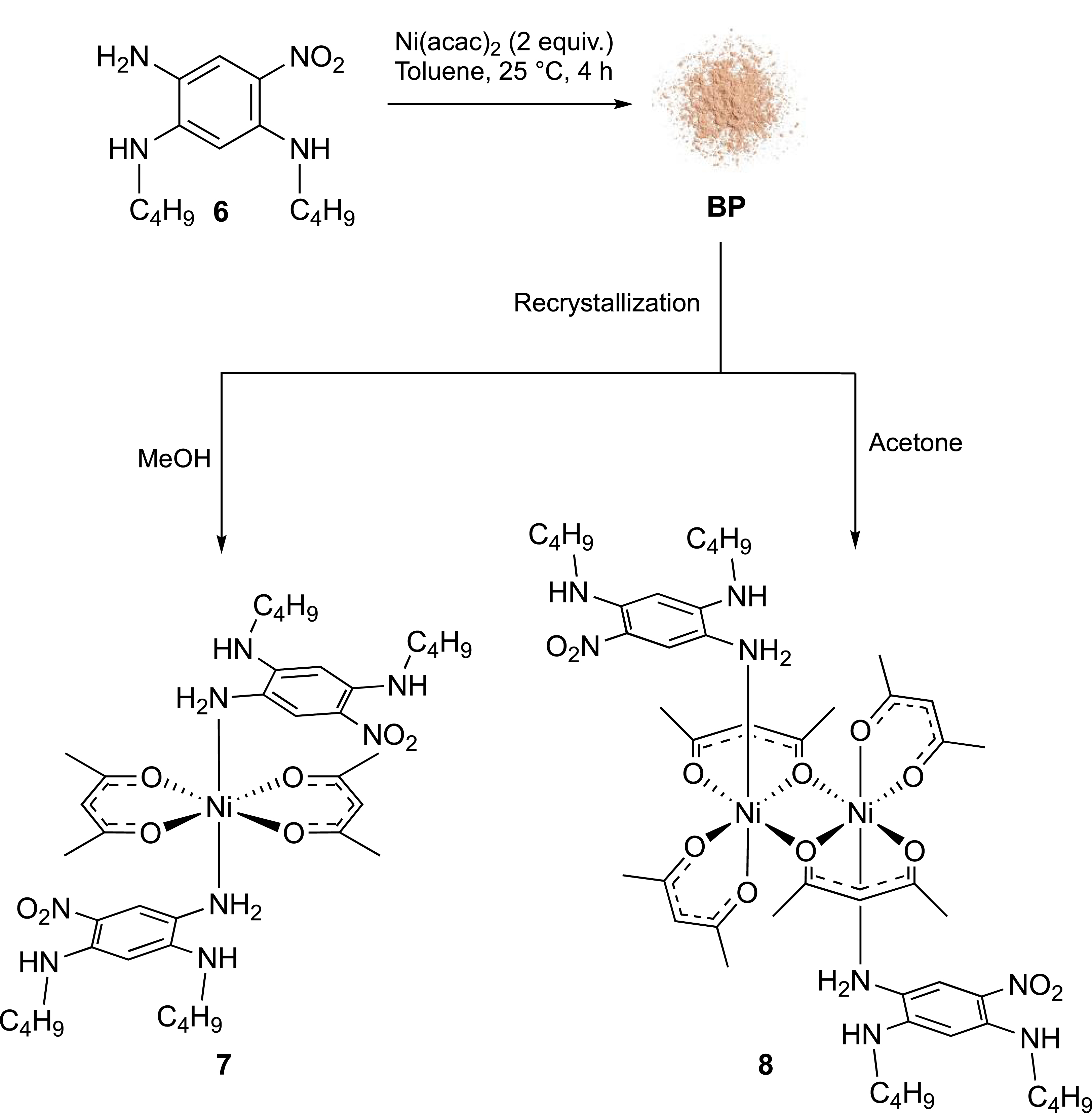
Synthesis of complexes 7 and 8 (acac = acetylacetonate).
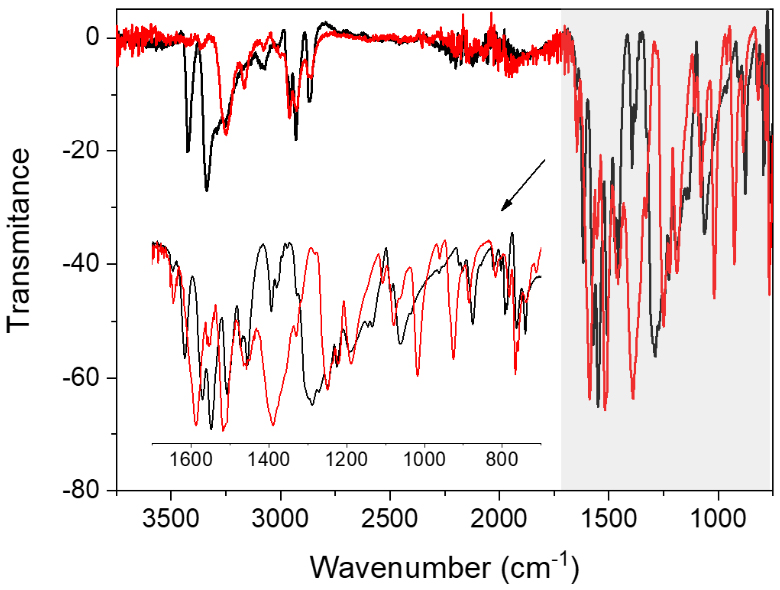
IR spectra of precursor 6 (black) and BP (red).
Next, we characterized BP in solution. Its 1H NMR spectra in acetone-d6 and methanol-d4 are reported in Figure 3, showing signals of aliphatic chains of ligand 6 and peaks of the acac moiety in the 1–5 ppm region, together with the presence of square planar low-spin complexes (i.e., diamagnetic character). Unfortunately, further investigations by mass spectrometry could not afford usable data due to the presence of numerous peaks in both methanol and acetone solutions.
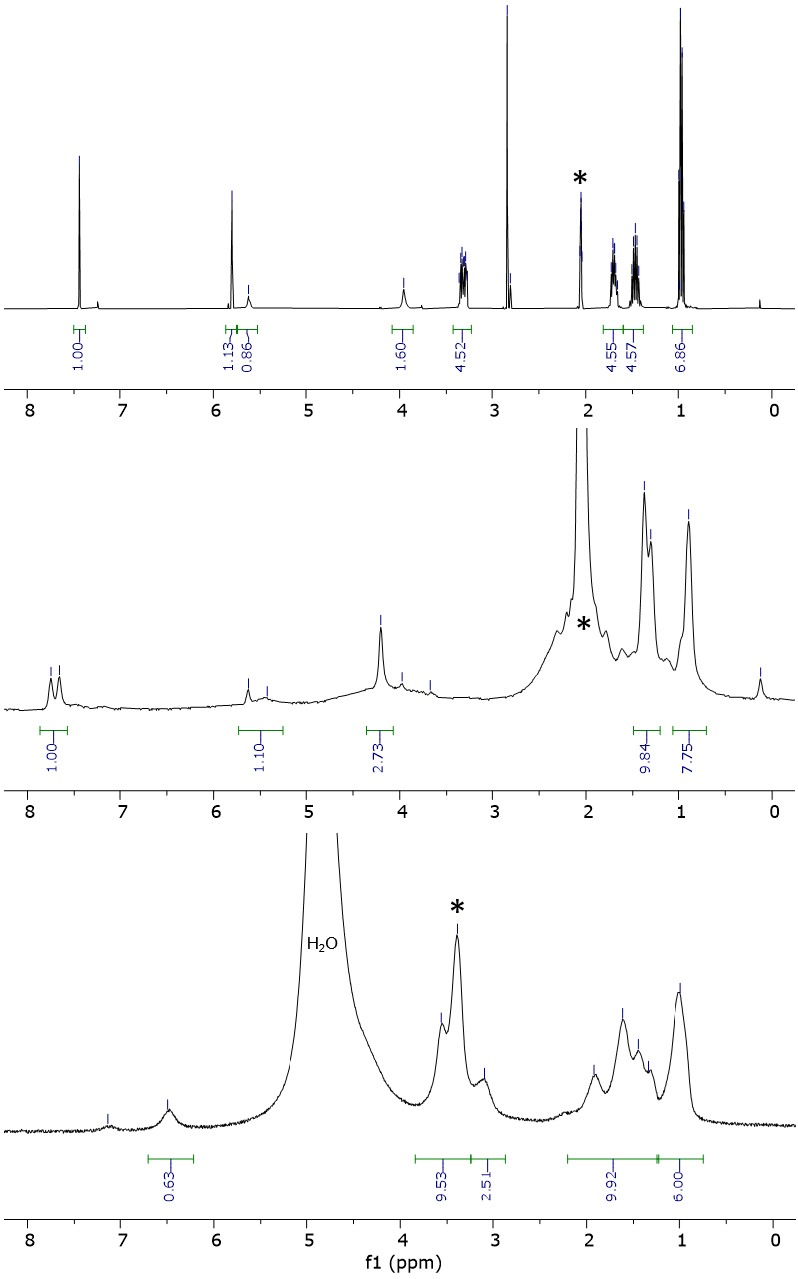
1H NMR (400 MHz) spectrum of 6 in acetone-d6 (top) and BP in acetone-d6 (middle) or methanol-d4 (bottom). The solvent’s residual peak is indicated with an asterisk.
Interestingly, the UV–Vis spectroscopic data recorded in methanol and acetone revealed the same absorption profile for BP and 6 (Figure 4). This observation proved the instability of the complex BP in solution by means of a weak linkage, suggesting that 6 probably coordinates only one nitrogen atom to a metal center (unlike 1) [7]. Crystallization attempts of BP in different solvents were then performed to gain insights into the nature of the coordination mode of 6.
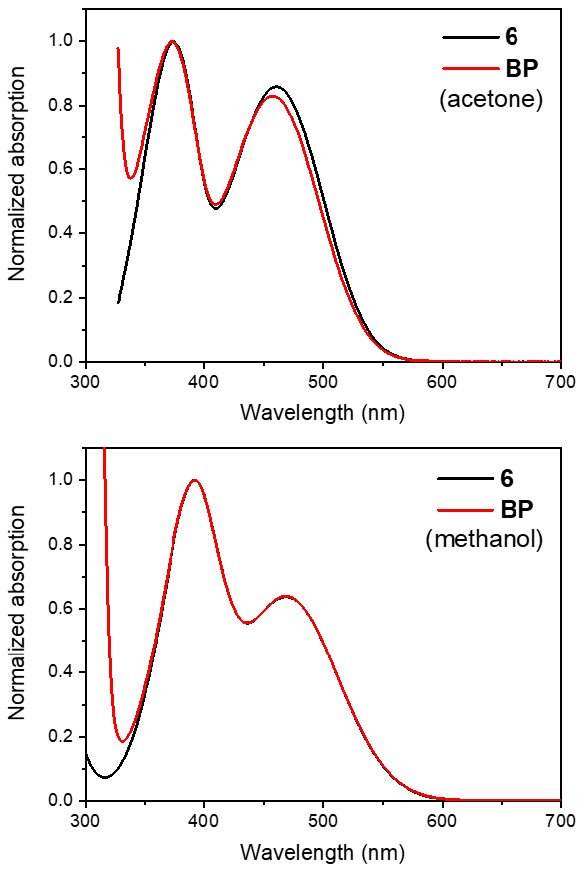
UV–visible absorption spectra of 6 and BP dissolved in acetone (top) or methanol (bottom).
Single crystals suitable for structural determination could be obtained by slow evaporation of a solution of BP in methanol. The X-ray diffraction analysis revealed the formation of a 1:2 (metal (Ni)/ligand (6)) complex 7 in which the nickel ion is found in an octahedral geometry and surrounded by two acac moieties coordinated in a planar fashion with respect to the metal center (Figure 5).
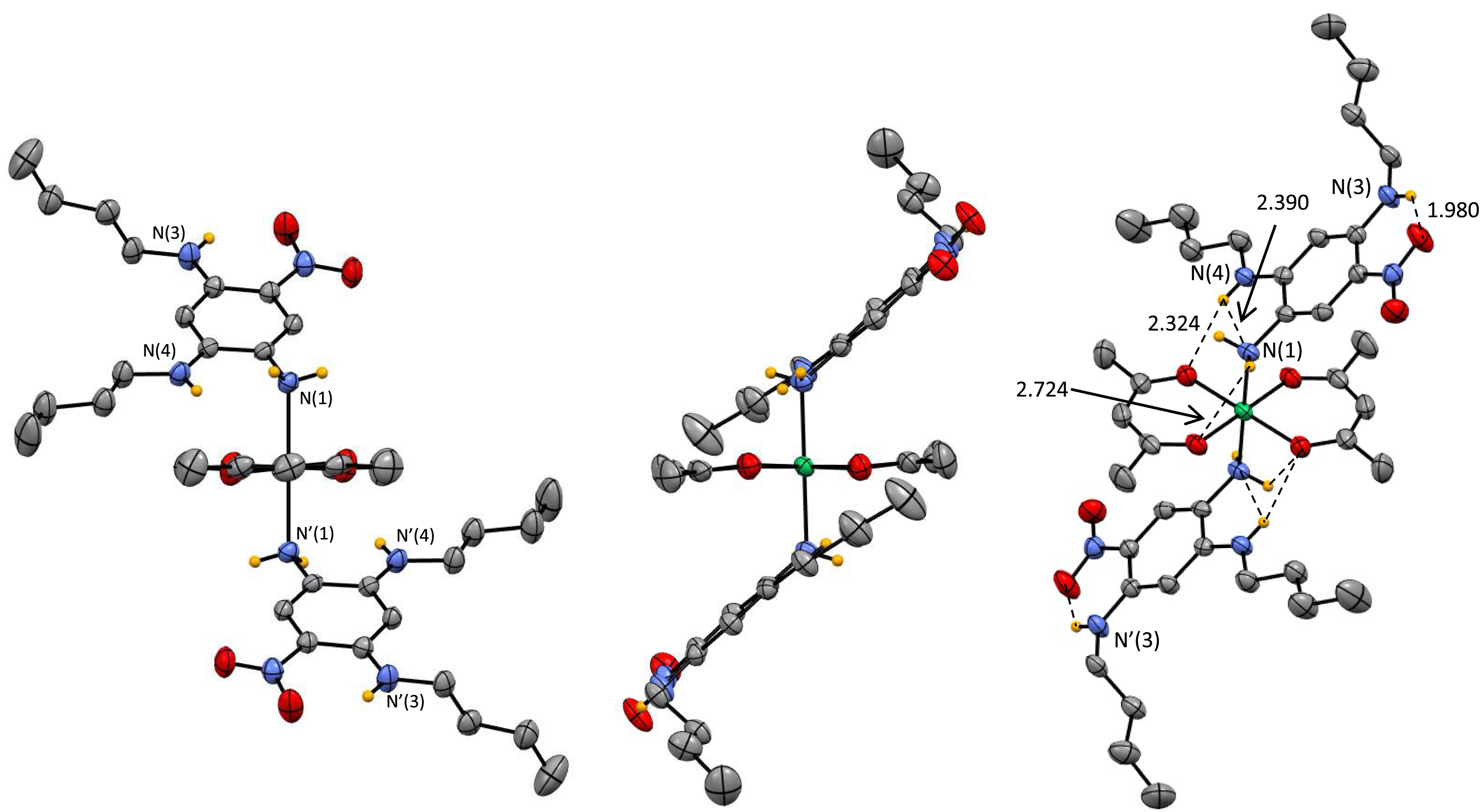
Single-crystal X-ray structure of 7 (recrystallization of BP in methanol). Selected bond lengths are indicated in Å.
The Ni coordination sphere is completed by two additional ligands 6 in which a single amino group N(1) (and N′(1)) is coordinated. The monodentate coordination mode of 6 can be explained by a closer look at its molecular structure. Since only the amine function N(1) in meta position is not conjugated with the nitro group, it coordinates specifically with a metal center due to the availability of its lone pair, in contrast to the two other amine functions in para and ortho positions, which are highly conjugated with the nitro group. This hypothesis is confirmed experimentally by examination of the bond lengths between the nitrogen atoms of the amines and their corresponding aromatic carbons. The distance N(1)⋯C(Ar) of 1.437 Å clearly indicates a lack of conjugation and availability of the lone pair whereas the distances N(4)⋯C(Ar) and N(3)⋯C(Ar) are much shorter due to the conjugation with NO2 (1.355 and 1.350 Å, respectively). A close examination of the hydrogen-bonding interactions in 7 revealed H-bonds involving N(1), N(2) and N(3) (as H-donor or H-acceptor) that clearly stabilize the molecular architecture in the solid state (Figure 5).
Remarkably, we observed that recrystallisation of BP in acetone instead of methanol led to the formation of complex 8 having the same octahedral geometry but a different stoichiometry (2:2 versus 1:2) (Figure 6). By analogy with 7, ligand 6 is monodentate since only the amine function N(1), in meta position with respect to the nitro group, is coordinated to the Ni center owing to the availability of its lone pair. Numerous N–H⋯O bonding interactions involving N(1), N(2) and N(3), ranging from 1.976 to 2.251 Å, contribute to the stabilization of the complex.
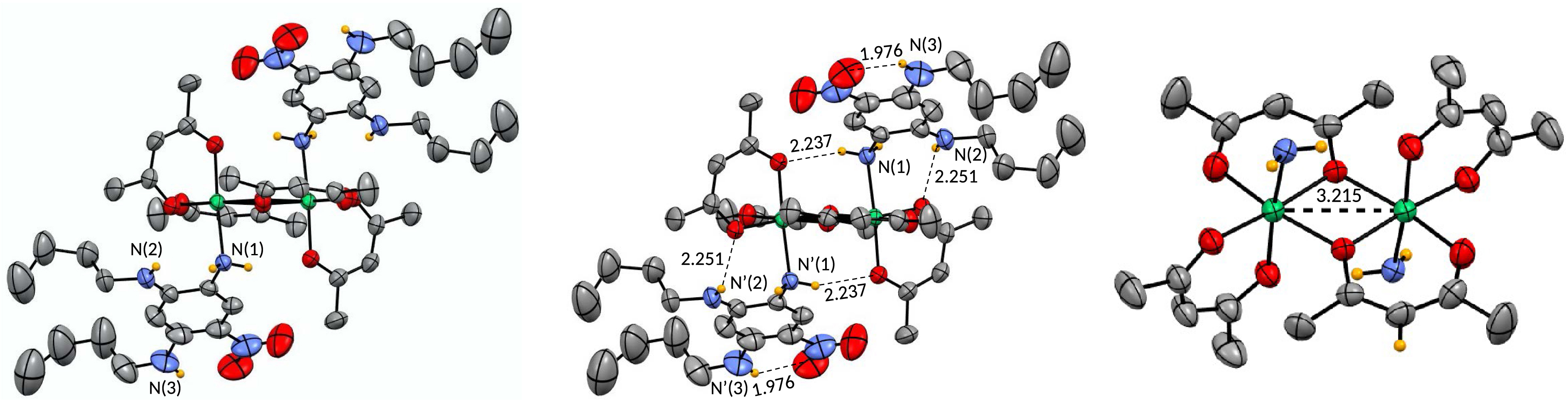
Single-crystal X-ray structure of 8 (recrystallization of BP in acetone). For the ortep view on the right, the aromatic amino ligand 6 is omitted for clarity except for the amino function coordinated to the Ni center. Selected bond lengths are indicated in Å.
The most striking feature of 8 is the coordination mode adopted by two of the six acac ligands, one of whose oxygen atoms coordinates to two metal centers, acting as a bridging ligand (Figure 6). As a result, the two Ni centers are in proximity (d(Ni⋯Ni) = 3.215 Å). To the best of our knowledge, this arrangement was only observed for modified acac in which electron-donating substituents in the 3-position of the pentane-2,4-dionato group were added [11].
3. Experimental section
3.1. General
Solvents (HPLC grade) and reagents were purchased from Sigma Aldrich and were used as received. Compound 6 was prepared according to published procedures [10]. NMR spectra were recorded on a JEOL ECS400 NMR spectrometer at room temperature. IR spectra were recorded on an Agilent Cary 630 FTIR equipped with an attenuated total reflectance (ATR) sampling. Suitable crystals were mounted on a Rigaku Oxford Diffraction SuperNova diffractometer and measured at 293 K at the Cu radiation (𝜆 = 1.54184 Å). Data collection, reduction and multiscan ABSPACK correction were performed with CrysAlisPro (Rigaku Oxford Diffraction). Using Olex2, the structures were solved with the ShelXT structure solution program using Intrinsic Phasing and refined with ShelXL using least-square minimization.
3.2. Synthesis of BP
Ni(acac)2 (m = 204 g, 0.792 mmol, 2 equiv) was added to an orange–red solution of 6 (100 mg, 0.396 mmol, 1 equiv) in 20 ml of toluene under vigorous stirring. The reaction mixture was stirred at room temperature for 4 h. The precipitate obtained was then isolated by filtration on a sintered glass funnel and dried in vacuo to afford BP as a beige solid (m = 184 mg).
4. Conclusion
We have prepared and crystallized novel nickel complexes 7 and 8 incorporating a new aromatic triamino ligand 6. This ligand behaves in a monodentate fashion in coordination chemistry because of the presence of the electron-withdrawing group NO2 that “deactivates” the two amine functions in ortho and para position. Although scarce in the literature due to a weak linkage inherent to the monodentate aromatic amines, complexes 7 and 8 could be fully characterized by X-ray diffraction, probably because of the presence of numerous intramolecular H-bonding interactions. The structure determination clearly established two different metal (Ni)/ligand (6) stoichiometry depending on the solvent used for crystallization, and the formation of a 1:2 complex (7) or a 2:2 adduct (8) could be observed in methanol and acetone, respectively. This work might open new perspectives in coordination chemistry of aromatic polyamines by exploiting the NH2/NO2 balance to tune the reactivity of the ligand and/or the stability of the metal complexes.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
This work was supported by the CNRS and the French “Ministère de la Recherche et de l’Enseignement Supérieur.”
Acknowledgements
The authors thank M. Giorgi (Spectropole, Marseille) for the X-ray diffraction studies.





 CC-BY 4.0
CC-BY 4.0