1. Introduction
The propagation of acoustic waves in liquids generates several physical effects (among particle size reduction, efficient mixing, mass transport, and shock waves) that can affect dispersed particles in very short times. In particular, low-frequency ultrasounds (20–100 kHz) favour the emergence of these physical effects through the unique physical phenomenon called cavitation, which is the birth, growth and implosion of highly energetic microbubbles. Recent studies have shown that ultrasonic irradiation of diverse carbon materials at low frequencies enables the modification of both their texture and surface chemistry. For example, the mechanical damage promoted by sonication on multiwalled carbon nanotubes and carbon black particles was reported [1, 2]. The ultrasonic preparation of carbon nanosheets from flakes of graphite either intercalated or not [3, 4] and from carbon black particles [5] was also mentioned. The ultrasonic preparation of graphene or graphene oxide through exfoliation of graphite under ultrasonic assistance has also been extensively investigated [6, 7, 8]. Recently, Levêque et al. [9] managed to generate nanoparticles of glassy carbon which is one of the hardest carbon materials on Mohs scale under intense low-frequency irradiation. Moreover, a few authors found an impact of ultrasonic waves on adsorption kinetics notably by enhancing the transfer of contaminants toward the activated carbon. For example, Hamdaoui et al. [10] studied the effect of low-frequency ultrasounds (21 kHz) on the adsorption and desorption of p-chlorophenol on a granular activated carbon. They observed that the adsorption capacity and the kinetics were enhanced under ultrasonic irradiation with only tiny modification of the activated carbon particle size distribution. Similarly, the higher desorption rate also observed under ultrasound was explained through the existence of high-speed microjets and high-pressure shock waves produced by acoustic cavitation, subsequently leading to the enhanced cleavage of the intermolecular interactions between the adsorbate and the adsorbent surface.
Although all the above cited studies emphasize the efficiency of ultrasounds to decrease the particle size distributions or to affect the adsorption/desorption mechanisms, no article has been released yet, to the best of our knowledge, on the consequences of using ultrasound on the surface chemistry of these carbon materials. However, it is well known that ultrasounds are responsible for the production of free radicals in solution [11] that may interact with the dispersed solid adsorbent and thus modify its surface chemistry and consequently its adsorption properties.
In aqueous solutions, the homolytic split of H2O molecules during the collapsing phase of the cavitation bubbles leads to the formation of OH° radicals, which are then able to react in the boundary liquid zone surrounding the bubbles [12]. The kinetics of the sonolysis of water saturated with argon, leading to the formation of hydrogen peroxide was found to be faster at 514 kHz than at 20 kHz [13]. Several pure organic solvents or aqueous solutions have also been subjected to ultrasound in a bid to identify species undetectable under silent conditions in order to establish reaction pathways induced by the irradiation. As for example, the sonolysis of a 0.1–3 mol⋅L−1 formic acid solution was found to yield mainly H2 and CO2 in the gas phase as well as trace amounts of oxalic acid and formaldehyde in the liquid phase whatever the ultrasonic frequency [14]. The sonolysis of HCOOH solution at high ultrasonic frequencies (few hundred kHz) also generated additional CO and CH4 in higher amounts than H2 and CO2, acknowledging the intense chemical activity of high-frequency ultrasound compared to the low one [14, 15]. The in situ produced H2 may be used as a chemical means to reduce some oxygenated functional groups usually found at the surface of carbon materials.
Liu et al. reported the oxidation of an activated carbon in sodium hypochlorite solutions of various concentrations under ultrasonic irradiation for 2 h at 40 kHz and the subsequent study of Co(II) removal from aqueous solutions by the prepared oxidized activated carbons [16]. The authors concluded that the ultrasonic assistance of the impregnation of sodium hypochlorite on the carbon materials drastically enhanced the oxidation rate of the carbon leading to a higher adsorption capacity of Co(II) compared with the oxidation by using a single impregnation methodology. We have also previously shown that the oxidation of the surface of a granular activated carbon by hydrogen peroxide was promoted by using a sono-impregnation at 20 kHz [17].
Ultrasound treatment is expected to modify the texture of the activated carbon due to the mechanical effects of the ultrasonic waves, and also the surface chemistry through the reaction with OH° radicals produced by the sonolysis of water or with other types of species originating from other solvent’s degradation.
Supported by the previously published results, we have envisaged that we could either oxidize or reduce the surface of activated carbons by sonication in varying both the nature of the solvent and the ultrasound frequency. In fact, the nature of the solutions of sonication (UHQ water or 35% H2O2 or 98% formic acid) were chosen because we wanted to explore the possible redox reactions of the activated carbon with the chemical species formed by the solvent sonication which are either oxidants (OH° or H2O2 from the sonolysis of water or from the ultrasound irradiation of H2O2 solution) or reductants (H2 and CO from the sono-decomposition of formic acid).
Mason et al. have found that sonolysis of UHQ water produces some amounts of H° and OH° radicals. These radicals recombine themselves yielding H2O2. The reaction of H° with dissolved O2 can also yield H2O2. This produced H2O2 can oxidize the activated carbon surface [12].
H2O2 was chosen as a solvent because of its oxidant power with respect to activated carbon surface. Moreover, Voncina and Majcen-Le-Marechal showed that the sonication of an H2O2 solution emphasizes the production of OH° radicals [18].
Several studies have shown that the sonication in concentrated formic acid yields H2 and CO2 emissions (and also CO at high frequency) from the decomposition of formic acid [19]. We have expected that by using formic acid as a solvent, its decomposition under ultrasound might have hindered the oxidation of activated carbon.
The aim of this work was to study the effect of ultrasonic irradiation at two different working frequencies (500 kHz or 20 kHz) in different solvents on the porosity and the surface chemistry of a raw granular activated carbon (referred to as AC). To the best of our knowledge, the influence of the ultrasonic frequency upon the texture and the surface chemistry of a sonicated activated carbon material has never been studied yet in detail. A second aim was to investigate the effect of the AC modifications due to the sonication on the adsorption properties of Ibuprofen (IBP). Indeed, IBP represents one of the most used anti-inflammatory drugs worldwide [20] and it is therefore one of the most commonly detected pharmaceuticals in the environment, with concentrations up to micrograms per litre. The removal of ibuprofen and micropollutants in general, was studied previously using different techniques, advanced oxidation processes (AOPs), such as ultrasonic irradiation [21], ozonation [22], electrochemical degradation, etc., or biological treatments [23], or adsorption on mesoporous silica or activated carbons. The biological treatments are not able to completely degrade the pharmaceuticals present in waste water [21]. An additional tertiary treatment is thus necessary. Because of its wide pore size distributions, specific surface area and hydrophobic character, the Activated Carbon is one of the most efficient adsorbent used in water treatment for the removal of organic micropollutants [24, 25].
The adsorption on activated carbons for removing the remaining micropollutants in waste water presents a satisfying efficiency and does not lead to the formation of possibly toxic derivatives compared to advanced oxidation methods such as ozonation [26]. Thus, the main interest in the adsorption treatment by using activated carbon lies in its non-destructive character to remove the ibuprofen from waste water or from water resource operating for drinking water.
In this article, we have pre-treated activated carbons by ultrasound and their ibuprofen adsorption properties were tentatively correlated to the porous textures, and the surface chemistry.
2. Experimental
2.1. Materials and their sonication treatments
2-[4-(2-methylpropyl) phenyl]propanoic acid, also named ibuprofen (IBP), was purchased from Sigma-Aldrich (>98% purity). This molecule is very slightly soluble in water at neutral or acidic pH (21 mg⋅L−1) [27], but soluble in many organic solvents [28, 29].
A commercial granulated activated carbon was purchased from Sigma Aldrich (ref. 292591, 4-14 mesh). Prior to ultrasound treatment, it was crushed into powder and washed with a 0.1 mol⋅L−1 HCl solution for 24 h to remove the metal salt impurities, then rinsed with distilled water until reaching a constant pH of the filtrate [30] and further dried at 110 °C for 24 h. The dried sample was referred to as AC. Laser granulometry characterization has shown that the AC powder was mainly formed of particles of sizes between 1 μm and 1000 μm (Figure 5).
Experimental conditions of ultrasonic treatments for the different samples
| Solvent (concentration) | Frequency (kHz) | Acoustic power$ (Watt) | mAC∕V solvent (g/mL) | Time (h) | T (°C) | Sample |
|---|---|---|---|---|---|---|
| HCOOH (98%) | 20 | 50 | 2.5/50 | 2 | 33 | AC[98%HCOOH]20 kHz–33 °C |
| HCOOH (98%) | 20 | 50 | 2.5/50 | 2 | 60 | AC[98%HCOOH]20 kHz–60 °C |
| HCOOH (98%) | 500 | 10 | 15/300 | 2 | 27 | AC[98%HCOOH]500 kHz–27 °C |
| H2O2 (35%)* | - | - | 2.5/50 | 5 | 25 | AC[35%H 2 O 2 ] |
| H2O2 (35%) | 20 | 50 | 2.5/50 | 5 | 33 | AC[35%H 2 O 2 ]20 kHz–33 °C |
| H2O2 (35%) | 500 | 10 | 15/300 | 5 | 27 | AC[35%H 2 O 2 ]500 kHz–27 °C |
| UHQ water | 500 | 10 | 15/300 | 5 | 27 | AC[H 2 O ]500 kHz–27 °C |
| UHQ water | 20 | 50 | 2.5/50 | 5 | 33 | AC[H 2 O ]20 kHz–33 °C |
∗Absence of ultrasound treatment and only impregnation, $determined by conventional calorimetric method.
Suspensions of raw granular AC (5 wt.%) were irradiated by ultrasounds at 500 kHz or 20 kHz in different solutions: H2O2 (35 wt.%), Ultra High Quality (UHQ : 18 MΩ.cm) water or concentrated formic acid (HCOOH, 98 wt.%). Treatment times were set to 2 or 5 h at controlled temperatures of 27 °C, 33 °C or 60 °C (Table 1). The sample as referred to AC[35%H2O2] was only prepared by a silent impregnation of the raw AC powder in H2O2 solution to compare with the samples sonicated in H2O2 (Table 1).
For the sonication at 20 kHz, an ultrasonic processor (Sonics and Materials, 500 W Ultrasonic Processor-VC505) of 350 W output with a converter fitted with an ultrasonic titanium probe (19 mm- Sonics and Materials, amplitude set to 43 μm) was used. The tip of the horn was dipped into a cylindrical double-jacketed (to control the temperature) glass cell filled with 50 mL suspensions. For an accurate temperature control, the cooling liquid temperature circulating in the double-jacket, was maintained at 1 °C (or 38 °C), using a cryostat, to adjust the inside reactor temperature to 33 °C (or 60 °C).
The sonication at 500 kHz was performed in a home-made double-jacketed cup-horn reactor [31] consisting of three focused ceramics filled with 300 mL of suspension agitated by a helix stirring rod and maintained at 27 °C by cooling with circulating tap water.
Prior to sonication, the suspensions were deoxygenated with argon gas for 1 h in order to avoid the presence of dissolved carbon dioxide and dioxygen gases. The sonication was performed under argon atmosphere.
After the sonication, the resulting suspensions were filtered off and the recovered carbon powders previously sonicated in formic acid or hydrogen peroxide were rinsed with distilled water and washed for at least one week with boiling distilled water into a Soxhlet extractor equipped with an alumina porous cartridge, until the pH of the water was constant.
2.2. Textural and chemical characterization of the activated carbons
The porosity of the activated carbons was characterized by N2 Adsorption–desorption at 77 K and CO2 adsorption at 273 K using an automatic sorptometer (ASAP 2020, Micromeritics). Prior to measurements, samples have been degassed for 12 h at 523 K under vacuum.
The specific surface area was calculated from the N2-isotherms using the Brunauer–Emmett–Teller (BET) equation, assuming the area of the nitrogen molecule to be 0.162 nm2. As negative unrealistic C factors were obtained by applying the BET model in the relative pressure range from 0.05 to 0.3, the BET specific surface areas were preferentially computed in the relative pressure range 0.01–0.05, as for microporous materials [32]. The total pore volume was estimated as the liquid volume of N2 adsorbed at a relative pressure of 0.995. N2 adsorption data at P∕P0 < 0.01 were obtained by using incremental fixed doses of ∼10 cm3⋅g−1 (STP), setting the equilibration interval at 300 seconds. CO2 adsorption data were obtained at P∕P0 ranging from 4 × 10−4 to 3.5 × 10−2, using 45 seconds equilibration interval [33]. Pore size distributions (PSD) of the activated carbon samples were determined by using NLDFT (non local density functional theory) models applied on the adsorption isotherms of N2 at 77 K. In addition, the distribution of pores smaller than 0.7 nm (ultramicropores) was evaluated from CO2 adsorption isotherms at 273 K after degassing at 150 °C (423 K) for 12 h. For that, infinite slit pores model were assumed for CO2 adsorption (pores diameter lower than 1.1 nm), while finite slit pores model was used for N2 adsorption simulations [34].
The pH of each activated carbon (0.5 g) was measured in a distilled water suspension (12.5 mL) after heating at 90 °C and then cooling to room temperature [35].
The pHPZC, i.e. the pH of the solution when the net surface charge equals zero, was determined by the so-called pH drift method [36]. Mixtures of 0.15 g of activated carbons and 50 mL of deoxygenized 0.01 mol⋅L−1 NaCl solutions of initial pH values varying from 2 to 12 were stirred for 48 h under N2 in order to avoid the formation of dissolved CO2. The final pH was measured and plotted against the initial pH. The pHPZC was equal to the value for which pH (final) = pH (initial).
The pKa distribution was determined by titrating a dispersion of each activated carbon (about 100 mg) in 75 mL of 0.01 mol⋅L−1 NaNO3 solution (previously degassed by bubbling N2 gas for 2 h). The pH was adjusted to 3 (by addition of 0.1 mol⋅L−1 HCl) and the titration was conducted under N2 atmosphere, using an automatic titrator (METHROHM, Titrino plus) delivering 0.1 mol⋅L−1 NaOH solution by increment of 0.01 mL. The deconvolution of the curve of the consumed protons versus pH using the SAIEUS code allowed calculating the pKa distribution in the pH range 3 to 11 [37].
The activated carbons were characterized by ATR-FTIR using a Thermo Scientific Nicolet iS10 spectrometer equipped with a germanium crystal. A DTGS KBr detector was used for detection and the incident angle of the beam was 45°. All spectra were collected in the infrared region [600–4000 cm−1] with a spectral resolution of 4 cm−1. 64 scans were accumulated for each analysis.
X-ray photoelectron spectroscopy (XPS) measure- ments were performed using an ESCALAB 250 spectrometer (Thermo Fisher Scientific) at mono- chromatic Al-Kα anode X-ray radiation, on a 150 × 800 μm2 analysis region, under 2 × 10−9 mbar vacuum. The high-resolution scans (0.1 eV) were obtained over the 280.1–299.9 eV (C1s) and 523.1–539.9 eV (O1s) energy ranges with pass energy of 20 eV. After baseline subtraction, the curve fitting was performed assuming a mixed Gaussian–Lorentzian peak shape (the ratio of Gaussian to Lorentzian form was equal to 0.3). The carbon 1s electron binding energy corresponding to graphitic carbon was referenced at 284.6 eV for the calibration [38].
The elemental composition of the different activated carbons was analyzed by the thermo Scientific Flash 2000 CHNS/O Analyzer. In order to analyze oxygen, samples were pyrolyzed at 1000 °C in an oven under helium atmosphere, then measured by gas chromatography.
The particle size distribution (basal size of the raw and modified activated carbons) of the prepared suspensions was directly studied using the Mastersizer 2000 particle size analyzer (Malvern Instruments, range 0.02–2000 μm). The particle size was calculated using the light diffraction theory applied to the spherical shape particles.
2.3. Adsorption experiments
All the IBP solutions were prepared either from UHQ water or in aqueous solutions containing 10 vol. % of methanol (99.9%) in order to increase the IBP solubility.
Adsorption isotherms of IBP on the different activated carbons were performed at pH 3, at 298K according to the experimental procedure previously described [17]. Activated carbons (10 mg) were dispersed in IBP solutions (100 mL) of varying concentrations (5–100 mg⋅L−1) and stirred for 5 days (times to reach equilibrium were determined previously on raw AC [17] and were found less than 1 day) in a thermostatically controlled orbital shaker (New Brunswick Scientific, Innova 40, 250 rpm). After filtration at 0.45 μm on membrane filters (Durapore®-Millipore), the initial and residual IBP concentrations were measured by high performance liquid chromatography (HPLC) using a Waters chromatograph equipped with a high pressure pump (Waters 515), a photodiode array detector (Waters 996) and a Sunfire C18 column (5μm, 4.6 × 250 mm). A methanol/UHQ water solution (80/20, v/v), containing 0.1 vol. % of concentrated phosphoric acid (95 wt.%) in isocratic mode at a flow rate of 1 mL⋅min−1 was used as mobile phase. Detection was operated at 220 nm.
The equilibrium IBP uptake Qads (mg⋅g−1) was calculated from equation:
3. Results and discussion
3.1. Surface chemistry characterization
3.1.1. Results from XPS analysis and elemental analysis
The elemental analysis indicates the carbonaceous nature of the adsorbent. The sonicated activated carbons contain mainly carbon (83 to 89%), oxygen (2.2 to 7.6%), few nitrogen (0.5%) except for AC[98%HCOOH]20 kHz–60 °C, hydrogen (0.4 to 1.8%) and some mineral impurities as the sum of the percentage of these elements is not equal to 100% (Table 2). The high percentage of nitrogen in AC[98%HCOOH]20 kHz–60 °C is attributed to some inhomogeneities in the chemical composition.
Elemental analysis of raw AC and the sonicated samples (weight %, Error range: + ∕−0.1)
| Sample | N | C | H | O |
|---|---|---|---|---|
| AC | 0.5 | 88.1 | 0.5 | 2.6 |
| AC[98%HCOOH]20 kHz–33 °C | 0.5 | 86.2 | 0.5 | 3.5 |
| AC[98%HCOOH]500 kHz–27 °C | 0.5 | 88.9 | 0.4 | 2.2 |
| AC[98%HCOOH]20 kHz–60 °C | 2.3 | 85.0 | 1.8 | 3.7 |
| AC[35%H 2 O 2 ]20 kHz–33 °C | 0.5 | 79.8 | 0.6 | 7.6 |
| AC[35%H 2 O 2 ]500 kHz–27 °C | 0.5 | 82.9 | 0.5 | 4.4 |
| AC[H 2 O ]20 kHz–33 °C | 0.5 | 83.5 | 1.0 | 3.5 |
| AC[H 2 O ]500 kHz–27 °C | 0.5 | 86.3 | 0.7 | 3.4 |
The carbon content (Table 2) decreases after the treatments at 20 kHz due to an increasing amount of oxygen owing to the oxidation of the carbon surface. The oxygen content increase is higher for the samples sonicated in hydrogen peroxide (twice or three times the oxygen content of raw AC) because of the strong oxidizing power of this reactant (Figure in “supplementary material”). For all the carbons sonicated at 20 kHz, either in formic acid or in water, only a little increase in oxygen content was observed (increase from 2.6% to about 3.6%). This little increase might be due to the breaking of C−C bonds because of cavitation at the surface of particles and to the mechanical shocks between the particles. This breaking might be followed by a reaction of the instable formed dangling bonds with OH° radicals leading to the formation of C−OH groups (of phenol groups for example) at the activated carbon surface.
The activated carbon treated in concentrated formic acid at high frequency (500 kHz) and at temperature close to 30 °C presents a low oxygen content, similar to the one of raw activated carbon, which means that it has not been oxidized during the sonication treatment. By contrast, the activated carbons treated in concentrated formic acid at 20 kHz have been slightly oxidized. For example, AC[98%HCOOH]20 kHz–60 °C has a relatively high oxygen and hydrogen content (3.7% and 1.8%, respectively), which could mean that this sample is rich in phenol groups.
Surface chemical groups were also characterized by XPS. The deconvolutions of C1s signals have given peaks assigned to C−C (284.6 eV), C−O (285.3–285.91 eV), C=O (286.25–286.91 eV), O−C=O (287.25–287.76 eV), carbonate (288.58–291.54 eV) bonds (the peak assigned to carbonate was also attributed to π-π* transitions). The deconvolutions of O1s signatures have given peaks attributed to O=C (531.36–532.65 eV), O−C (532.63–533.49 eV), chemisorbed O probably due to the presence of water (at 533.43–534.09 eV), metal oxides from impurities (530.67–531 eV) and occluded (entrapped) CO or CO2 gas (534.46–537.02 eV).
Figure 1 displays for each sample, the total weight percentage of the carbon linked to oxygen atoms in C−O, C=O and O−C=O (from carboxylic and lactone) bonds, and the total weight percentage of oxygen linked to carbon atoms either in O−C or in O=C bonds, retrieved from C1s and O1s XPS analyses. The sonication at 20 kHz or 500 kHz in various environments (H2O, H2O2, HCOOH) leads either to an oxidation of the activated carbon due for example to the oxidizing power of H2O2 or to an absence of modification of the surface chemistry for both AC[98%HCOOH]500 kHz–27 °C and AC[H2O]500 kHz–27 °C samples (Figure 1). The weight percentages of carbon bonded to oxygen (C−O, C=O and O−C=O signals) have increased for all samples except for these two latter samples in which the content in oxygen bonded to carbon atoms was found very slightly modified compared to pristine AC. As expected, the weight percentage of carbon (C−C) (Figure 2) measured for these two samples is close to the one obtained for raw sample AC. The ultrasound irradiation at 20 kHz has led to a strong oxidation in the presence of hydrogen peroxide and a medium oxidation in the presence of formic acid (Figure 1). Indeed, the mass percentage of carbon from XPS is greatly reduced after treatment by H2O2 and HCOOH at 20 kHz (Figure 2).
Mass percentages of carbon bonded to oxygen atoms (C1s), and oxygen bonded to carbon atoms (O1s) obtained by XPS analysis of raw and modified activated carbon.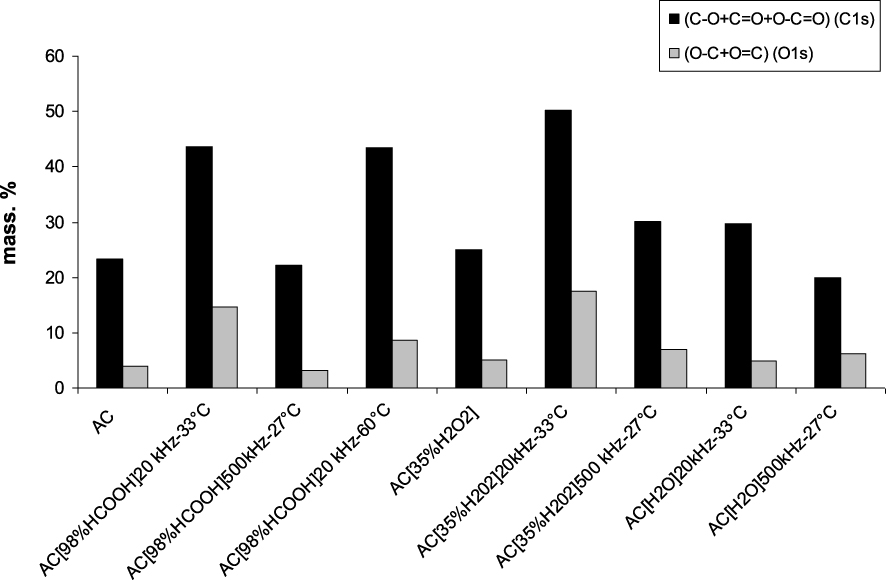
Weight percentages of carbon (C−C), (C−O) and (C=O) (C1s) obtained by XPS analysis of raw and modified activated carbons.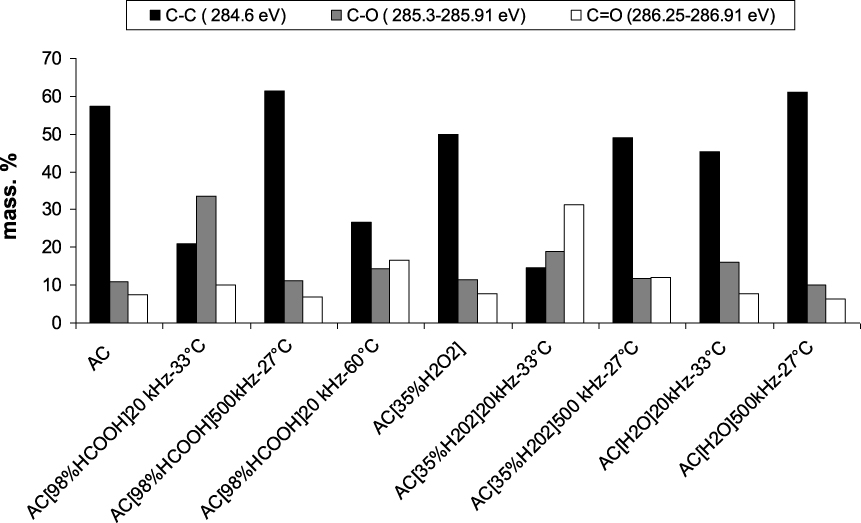
The XPS analyses have also shown that an oxidation by H2O2 is more effective if it is coupled to an ultrasonic treatment than if H2O2 is used alone. Indeed, the weight percentage of oxygen is higher for sample AC[35%H2O2]20 kHz–33 °C (14.37%) than for sample AC[35%H2O2] (11.97%). Voncina and Majcen-Le-Marechal [18] showed that the ultrasonic irradiation at 20 kHz in the presence of H2O2 increases the production of the OH° radicals formed by the homolytical split of H2O2, which explains the strong oxidation of the activated carbon in the presence of H2O2 coupled with an ultrasonic treatment. The oxidation in H2O2 medium is mainly attributed to the oxidizing ability of this chemical agent.
Amounts of surface groups by the distribution of pKa method, pH and pHPZC of raw and sonicated activated carbons
| Sample | pKa distribution (mmol⋅g−1) | pHPZC | pH | ||
|---|---|---|---|---|---|
| 3 < pKa < 7 | 7 < pKa < 11 | Total | |||
| AC | 0.03 | 0.20 | 0.23 | 7.80 | 9.05 |
| AC[98%HCOOH]20 kHz–33 °C | 0.01 | 0.15 | 0.16 | 7.20 | 6.70 |
| AC[98%HCOOH]500 kHz–27 °C | 0.10 | 0.15 | 0.25 | 7.35 | 7.50 |
| AC[98%HCOOH]20 kHz–60 °C | 0.05 | 0.46 | 0.51 | 7.45 | 7.90 |
| AC[35%H 2 O 2 ]20 kHz–33 °C | 0.36 | 0.54 | 0.90 | 8.00 | 8.00 |
| AC[35%H 2 O 2 ]500 kHz–27 °C | 0.17 | 1.09 | 1.26 | 7.80 | 7.70 |
| AC[H 2 O ]20 kHz–33 °C | 0.05 | 0.20 | 0.25 | 7.30 | 7.40 |
| AC[H 2 O ]500 kHz–27 °C | 0.03 | 0.17 | 0.20 | 7.30 | 7.20 |
For samples sonicated in water, the formation of some radicals could be partly responsible for the oxidation of the activated carbon surface. It is well known that the sonolysis of degassed water creates H° and OH° radicals which recombine either inside the cavitation bubbles to form hydrogen or at the bubble–liquid interface to form hydrogen peroxide [12]. The sonication at 20 kHz might lead to the C−C bond breaking of the aromatic planes through the mechanical effects of ultrasounds and cavitation, and further oxidation at the edge of the layers by reaction with water or OH° radicals or gas emitted during the solvent or solute decomposition (for example, formic acid). The sonication in concentrated formic acid might hinder this oxidation due to the H2 emission [14] from the decomposition of the formic acid which could act as reducing agents. The sonolysis of water at high frequency is known to produce large amounts of H2 (from H° radicals) and H2O2 (from OH° radicals) compared to the sonolysis of formic acid (also giving CO2 and CO emission) [14].
The absence of modification of the surface chemistry of the activated carbon after sonication at high frequency (500 kHz) in concentrated formic acid or in water (Figure 1 and Figure 2) could be related to the absence of mechanical effect of the high-frequency ultrasonic waves compared to 20 kHz sonication [10]. Furthermore, the increase in the frequency is supposed to induce a better efficiency in the sonolysis of water producing radicals in the cavitation bubbles. As the absence of modification of the surface chemistry is observed either in 98% HCOOH or in water solvent, the radicals formed through the water sonolysis might be inefficient for the oxidation of the activated carbon whether the C−C bonds were broken or not. Moreover, recent studies [14, 15, 19] have shown that under high-frequency ultrasounds the sonolysis of formic acid produces H2 and CO2 in the gas phase. The formation of CO was also highlighted. The reducing gases such as CO and H2 could thus inhibit the oxidation of the material which would contribute to the absence of oxidation of the surface of the activated carbon.
3.1.2. pHPZC and pKa distribution
The pH values are found to be almost neutral (values around 7) for many of the sonicated samples (Table 3) compared to pristine (pH = 9), but also slightly basic for the samples sonicated in formic acid at 500 kHz–27 °C or 20 kHz–60 °C (values between 7.5 and 8), and in hydrogen peroxide at 500 kHz or 20 kHz (values about 7.7–8). As a conclusion, the activated carbons have become more neutral after their sonication in formic acid or in water except for the two samples prepared by sonication in H2O2. The oxidation in H2O2 under sonication at 20 kHz has led to the formation of a basic surface (pHPZC = 8) possibly because of the formation of phenol and carbonyl groups (also evidenced by the infrared characterization), in agreement with the Boehm titrations from our previous study [17].
The pKa distributions of the sonicated carbons were quite similar to the raw AC one except for the carbons which were sonicated in H2O2 (at 20 kHz and 500 kHz) and in formic acid at 20 kHz–60 °C. The pKa distribution of these latter samples reported in Figure 3 in comparison with the raw AC has brought out the presence of basic groups of pKa corresponding to the phenol one (pKa close to 11). This is consistent with the elemental analysis of these activated carbons (Table 2) indicating a high oxygen content.
Distribution of acidity constants.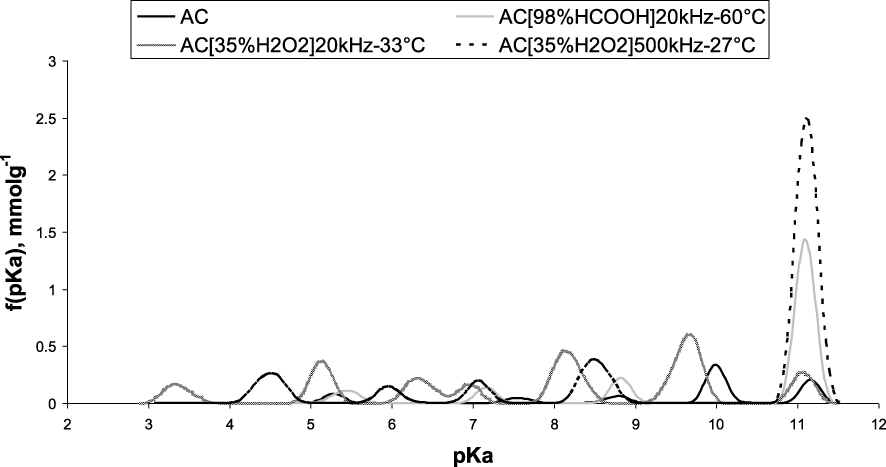
The amounts of surface groups (in mmol⋅g−1) and their pKa distributions (Table 3) indicate that the activated carbon treated in H2O2 at 20 kHz is the richest in carboxylic groups (0.36 mmol⋅g−1) as it contains the largest number of surface groups of pKa < 7. The activated carbon treated with H2O2 at 500 kHz is the richest in phenolic groups (1.09 mmol⋅g−1) and in oxygenated groups (1.26 mmol⋅g−1). A previous study [17] reported an increase in the carbonyl group amount after the oxidation through H2O2 impregnation coupled or not with ultrasonic irradiation. The presence of phenolic groups created through the ultrasonic treatment (0.27 meq⋅g−1 from reference [17]) could be attributed to the reaction between the carbon atoms and the OH° free radicals mainly generated by the H2O2 degradation by the ultrasonic irradiation [12]. Activated carbons sonicated in HCOOH or in water (at 20 kHz–33 °C and at 500 kHz–27 °C) showed the lowest amount of surface groups. This confirms XPS or elemental analysis results for the sonication at 500 kHz. However, the very slight oxidation observed by XPS after sonication in water or HCOOH at 20 kHz has not been detected by the pKa distribution analysis.
3.1.3. Infrared spectroscopy
All the infrared spectra were very similar to the pristine except the one of AC treated in H2O2 and sonicated in formic acid at 500 kHz (Figure 4). Whatever the sample, the infrared spectra showed the presence of a large band between 1000 and 1250 cm−1 attributed to the C−O stretching in acids, alcohols, phenols, ether and esters. The band at 1600 cm−1 is assigned to C=C stretching modes of aromatic rings or to C=O groups stretching vibrations conjugated with aromatic rings [39].
The infrared signal of the sample sonicated in hydrogen peroxide at 20 kHz (Figure 4) exhibits a band at 1720 cm−1 due to the C=O stretching vibration (of ketone, ester and/or aromatic carboxylic groups), attributed to the H2O2 oxidizing effect [17].
3.2. Porosity and texture characterization
3.2.1. Porosity
The nitrogen adsorption–desorption isotherms of the raw and modified carbons (shown in supplementary material) are typical of microporous–mesoporous adsorbents (type IV in the IUPAC classification). The most characteristic feature of a type IV isotherm is the hysteresis loop (H4 type) in the desorption branch at relative pressures above 0.5. The adsorption–desorption hysteresis on ACs isotherms showed clearly that liquid nitrogen was condensed in slit-shaped mesopores.
ATR spectra of raw AC and some modified AC derivatives.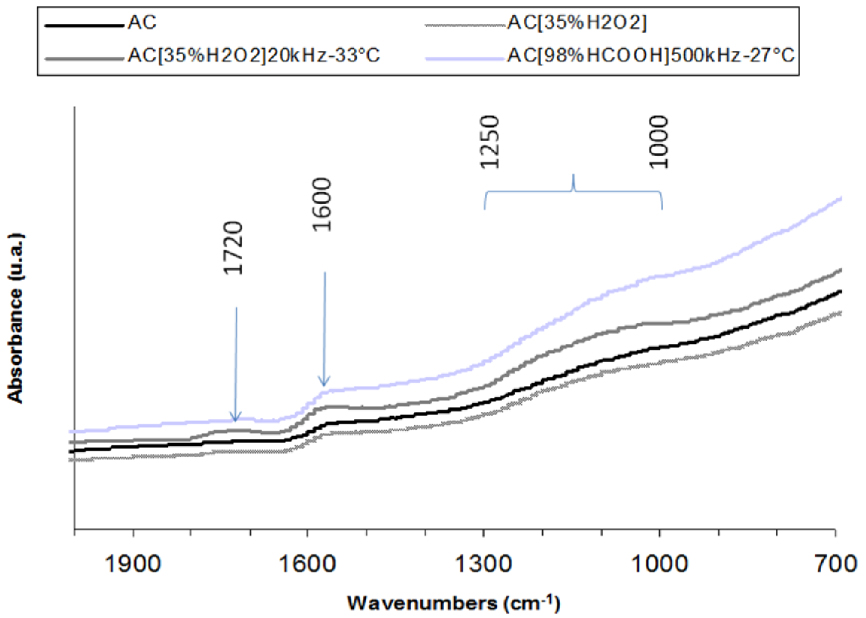
The BET-specific surface areas (Table 4) have slightly increased after sonication compared to pristine AC (increase of 0.6–4%) except for AC sonicated in water at 20 kHz. The N2 adsorption-desorption isotherms of AC and its sonicated derivatives (Figures a, b and c in supplementary material) possess quite similar profiles but they are very slightly shifted to higher adsorption volume after sonication. No significant differences have been observed because the adsorption of N2 at 77 K depends mainly on the adsorption in micropores (pores of diameter lower than 2 nm) which represents the internal surface of the activated carbons. These micropores which are located inside the particles are not affected by the sonication. In fact, the mechanical effect of ultrasounds has involved a break of the particles which has led to a slight increase in the external surface and thus concerns the mesoporosity (pores of diameter higher than 2 nm and lower than 50 nm).
Textural properties obtained from N2 adsorption/desorption at 77 K and CO2 adsorption at 273 K
| Sample | SBET (m2⋅g−1) | Total pore volume (cm3⋅g−1) | Micropore volume (cm3⋅g−1)a | Ultramicropore volume (cm3⋅g−1)b |
|---|---|---|---|---|
| AC | 745 | 0.47 | 0.29 | 0.18 |
| AC[H 2 O ]20 kHz–33 °C | 737 | 0.56 | 0.29 | 0.14 |
| AC[H 2 O ]500 kHz–27 °C | 771 | 0.49 | 0.30 | 0.15 |
| AC[35%H2O2] | 781 | 0.56 | 0.26 | 0.14 |
| AC[35%H 2 O 2 ]20 kHz–33 °C | 762 | 0.55 | 0.30 | 0.14 |
| AC[35%H 2 O 2 ]500 kHz–27 °C | 750 | 0.48 | 0.29 | 0.15 |
| AC[98%HCOOH]20 kHz–33 °C | 763 | 0.53 | 0.30 | 0.14 |
| AC[98%HCOOH]20 kHz–60 °C | 764 | 0.49 | 0.30 | 0.15 |
| AC[98%HCOOH]500 kHz–27 °C | 778 | 0.54 | 0.31 | 0.15 |
aDubinin Radushkevich, bfrom CO2 adsorption at 273 K (DFT model).
Indeed, the total porous volumes (Table 4) have also slightly increased in the range 2–20% after the sonications although the micropore volumes were almost unchanged. Thus, the porous volume increase is explained by a slight increase in the mesoporous volumes. This also means that the slight increase in the BET surface area related to the sonication treatment is attributed to an increase in the external surface owing to the reduction of the particle size.
The ultramicropore volume estimated from CO2 adsorption isotherms of the sonicated samples (Table 4) shows a slight decrease (0.03–0.04 cm3∕g) which appears to be not significant. However, in case of oxidized samples (treated with H2O2) this might be explained by a blockage of the small micropore volume (i.e. the ultramicropores) due to the formation of oxygenated surface functional groups.
3.2.2. Laser granulometry
The ultrasonic treatment typically induces a significant reduction of the particle size that is due to acoustic cavitation [40, 41]. The granulometric distribution plot (Figure 5) shows that after manual crushing the raw AC has included particle sizes in the range 1 μm to 1000 μm.
The samples sonicated at 500 kHz or impregnated in H2O2 (without ultrasound) have a rather similar size distribution (d50 ∼ 500 μm). Surprisingly, their size distribution displays larger particle size proportion than the pristine one (mode at ∼400 μm), attributed to an aggregation phenomenon occurring either by oxidation (in H2O2) and/or by 500 kHz sonication. High-frequency ultrasound has a non-significant mechanical effect on the particle size reduction whatever the solution, which is consistent.
By contrast, the ultrasonic treatment at 20 kHz has induced a particle size reduction due to the cavitation phenomenon. Indeed, the cavitation bubbles obtained at 20 kHz are known to implode on the solid particle surface forming high speed liquid jets that affect the AC surface [42]. These intense shocks induce a mechanical crushing. Thus, the samples sonicated at 20 kHz in H2O and in formic acid (modes at 3 μm and 8 μm, respectively) have submicrometric particles and show the smallest particle sizes. A bimodal particle size distribution (modes at 16 μm and 160 μm) is found while sonicating at 60 °C in formic acid (sample AC[98%HCOOH]20 kHz–60 °C). A trimodal distribution (d50∼5 μm) is observed for AC sonicated at 20 kHz and 33 °C (in H2O2) exhibiting modes at 250 μm, 16 μm and 3 μm.
Particle size distributions (percentage of particle volume as a function of particle size) for the different activated carbons.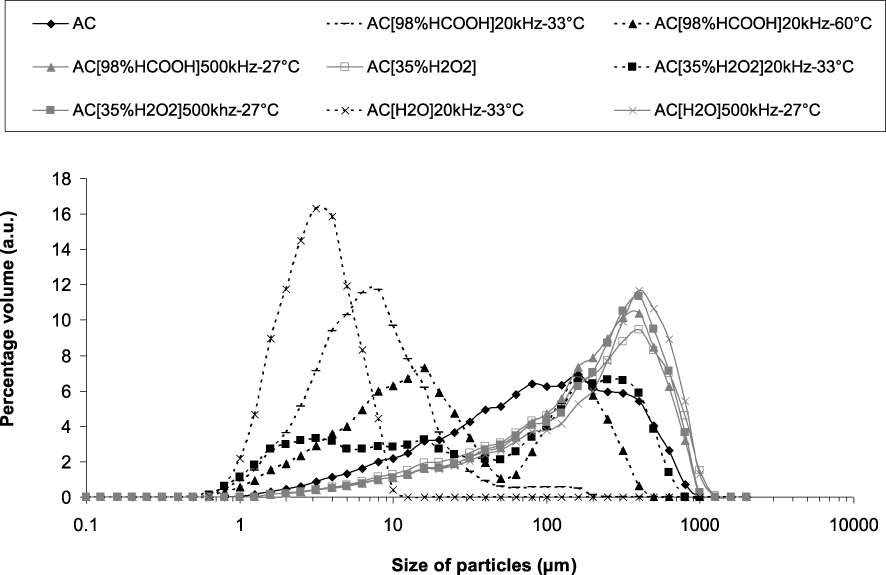
The greater particle size obtained by the 20 kHz sonication in formic acid at 60 °C compared to the one at 33 °C can be related to the more efficient acoustic cavitation in low vapour pressure solvents. Indeed, the temperature increase makes the solvent become volatile (vapour pressure raise) and reduces the energy of implosion of the cavitation bubbles.
Furthermore, the increase in particle size is related to the surface oxidation which promotes agglomeration. The surface functional groups may form bonds together (hydrogen bonding and van der Waals bonds) and subsequently the activated carbon particles may agglomerate (Figure 5). Among the samples sonicated at 20 kHz, the most oxidized one (AC[35%H2O2]20 kHz–33 °C) possesses the largest particle size.
Moreover, the 20 kHz–33 °C ultrasonic treatment of AC either in water or in formic acid was the most efficient for the size reduction because the carbon surface was very poorly oxidized through sonication in these non-oxidizing solvents allowing a good dispersion of the small milled particles. The average particle size obtained after the ultrasonic treatment in formic acid is higher than in H2O. This can also be related to the higher viscosity of concentrated formic acid compared to water which could reduce the implosion energy of the bubbles upon cavitation [43].
3.3. Adsorption isotherms of ibuprofen
The ibuprofen adsorption isotherms (Figure 6) were studied at pH 3 either in UHQ water or in a water/methanol mixture (90/10 vol.). Many studies have concerned the adsorption mechanism of ibuprofen on activated carbons [17, 44, 45, 46, 47]. The main contribution to adsorption is due to hydrophobic repulsions from the solvent which decreases as pH increases [45]. But our previous studies [17, 44, 46] have shown that the adsorption is also governed by dispersive interactions and the contribution of hydrogen bonding owing to the presence of the carboxylic function in ibuprofen. Thus, it can explain that the oxidation of the surface yielding carboxylic groups and carbonyl groups was found to increase the adsorption uptake and decrease the enthalpy of adsorption through the hydrogen bonding interaction. The previous studies of the sites of adsorption of ibuprofen in activated carbons have demonstrated that the adsorption occurs mainly in micropores (supermicropores and larger ultramicropores) [17, 44, 45]. The models established by statistical physics have allowed to simulate the adsorption isotherms of ibuprofen on activated carbons using a monolayer model [46], or a double layer model [48] in agreement with the accommodation of the adsorbate in the micropores.
Ibuprofen adsorption isotherms at pH 3 and at 298 K (experimental points and Langmuir–Freundlich fits) in a water/methanol (90/10 v/v) mixture (a), and in UHQ water (b), for raw AC and for AC derivatives sonicated in formic acid, in water and H2O2.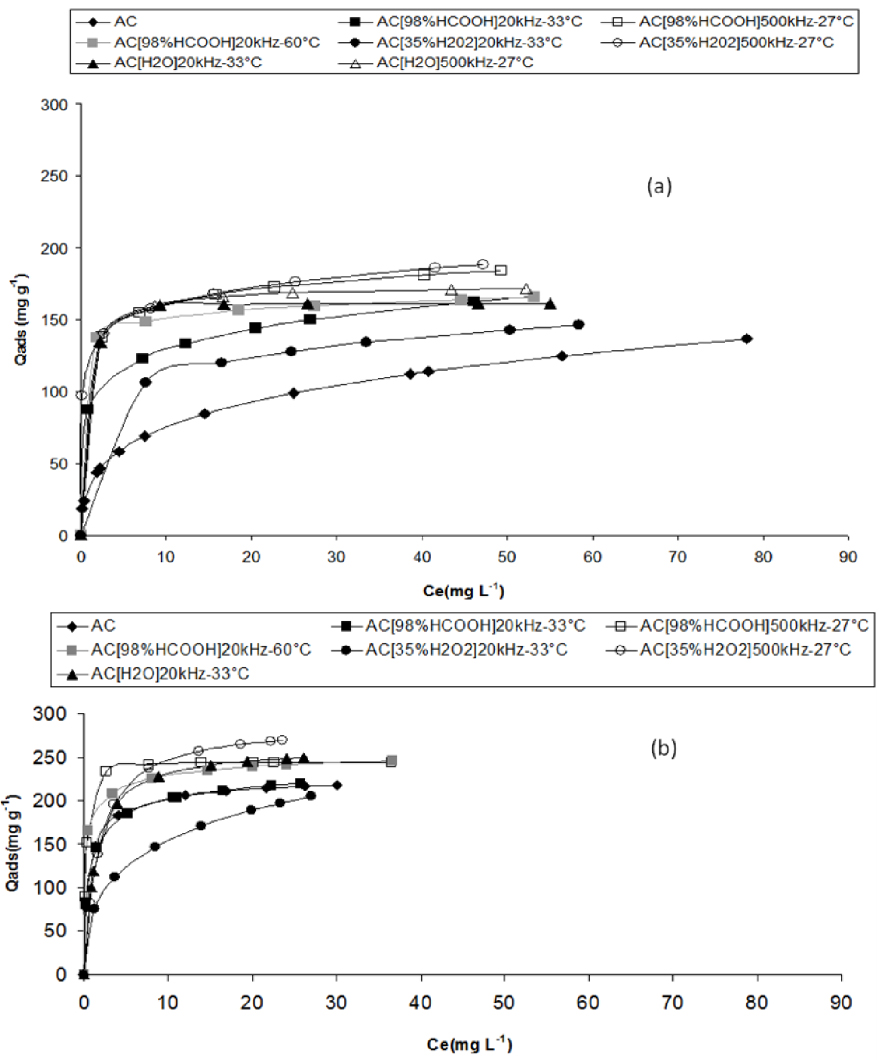
The evolution with temperature of the absorption equilibrium uptake of the ibuprofen adsorption was investigated in our previous publications on activated carbon cloth [44, 45] and particularly on the AC sample [17]. The increase of the uptake together with temperature characteristic of an “apparent” endothermic phenomenon was evidenced by equilibrium adsorption studies in acidic conditions (pH =3) whatever the solvent (pure water or mixture water/methanol) or at pH =7 (in water/methanol mixture) [44, 49] in agreement with the previous results of other authors [50]. In fact, the adsorption of ibuprofen is in general exothermic and the apparent endothermic character is explained by the hydrophobic interactions of ibuprofen with the solvent (i.e., water) requiring to overcome an energy barrier for the adsorption. Using such argument, Iovino et al. [51] have established a model taking into account the increase of ibuprofen solubility during heating, which could explain the positive apparent measured enthalpy.
3.3.1. Adsorption isotherms of ibuprofen in water medium
The ibuprofen adsorption uptake (Figure 6b) is the lowest in water medium for AC sonicated in H2O2 at 20 kHz, which is also the most oxidized sample, possessing the highest amount of carbonyl groups (Section 3.3.2). This could be explained by the hydrophilic nature of this sample in which the adsorption competition between ibuprofen and water occurs. Except for AC[35%H2O2]20 kHz–33 °C, the adsorption uptake of ibuprofen in water of each sonicated material is higher than the one of AC. In water medium, an increase in the adsorption capacity was found after sonication at 500 kHz (in concentrated formic acid or in water or in H2O2) or at 20 kHz–60 °C in formic acid.
The profile of the isotherms is typically “Langmuirian” showing a plateau at the high concentrations and a well-defined knee except for the AC isotherm for which the plateau is not attained at the highest studied concentration (about 30 mg⋅L−1). This reflects a higher interaction of ibuprofen with the ultrasonically treated samples compared to raw AC.
Among the different treatments, whatever the solution, the sonication at 500 kHz appears to be more efficient at increasing the adsorption uptake of ibuprofen measured in water medium. The highest increase in the adsorption capacity observed after mid-oxidation of AC by sonication at 500 kHz in H2O2 suggests a contribution of the surface groups having high pKa values such as phenols in the adsorption of ibuprofen.
However, this could not be the only reason for such an increase in the adsorption capacity. A textural modification has also operated through sonication which reflects in the increase in external surface (Table 4) related to particle size reduction. The reduction in the particle size has been found to be very important after 20 kHz sonication but has not been observed after 500 kHz ultrasound irradiation (Figure 5). The lower adsorption capacity of ibuprofen after 20 kHz than after 500 kHz sonication indicates that the reduction in the particle size has created new adsorption sites which were for the most part inefficient at adsorbing ibuprofen mainly because of the nature of the oxygen surface groups on these sites. The 500 kHz sonication has led to a small increase in the external surface without any particle size reduction and only a slight oxidation exclusively in hydrogen peroxide. Thus, it could have promoted the ibuprofen molecule adsorption through hydrophobic–hydrophobic and van der Waals bonds interactions on non-oxidized sites or hydrogen bonding on phenol groups.
3.3.2. Adsorption isotherms of ibuprofen in water/ methanol mixture
The adsorption of ibuprofen on the pristine AC is quite different in a mixture of water and methanol (Figure 6a). The ibuprofen solubility rise in the presence of methanol (10% vol.) reduces the adsorption uptake on AC by a factor of two compared to the one in pure water medium. In the presence of methanol as co-solvent, the increase in the adsorption capacity of the most oxidized sample (sonicated at 20 kHz in hydrogen peroxide) compared to raw AC is attributed to the particular surface chemistry of this sample containing carbonyl groups as previously observed [17]. By contrast, in pure water medium, the adsorption uptake of this most oxidized sample is smaller than in AC.
Except the case of the AC sonicated at 20 kHz, the same trend of the adsorption uptakes has been globally observed both in water medium and in water/methanol mixture. The 500 kHz sonicated materials show the highest uptakes (increase of 75% compared to AC uptake) while the 20 kHz sonicated carbons display intermediate uptakes (increase in the range of 40% compared to AC). Thus, the sonication at 500 kHz has led to textural modification under controlled surface chemistry (without strong oxidation) favourable toward adsorbing ibuprofen.
4. Conclusion
A commercial microporous/mesoporous activated carbon was submitted to ultrasound irradiation at 20 kHz and 500 kHz in concentrated formic acid, in hydrogen peroxide solution and in water. The microporosity (pore diameter <2 nm) of the carbon material was almost not modified by the ultrasonic treatment but a slight increase in the external surface area (i.e. the mesoporosity) has been observed whatever the sonication frequencies. For the sonication at 20 kHz, this slight increase in the porous volume is accompanied by a reduction of the particle size from ∼400 μm to micrometric and submicrometric size range depending on the solution. The size reduction was more effective and led to the smallest particles (mode at 3–8 μm) in non-oxidant solvents such as water or concentrated formic acid. The particle size distribution was almost not affected by the sonication at 500 kHz.
Almost no changes were observed in the surface chemistry after sonication in water or formic acid at 500 kHz whereas the ultrasonication at 20 kHz led to an oxidation of the surface chemistry more or less intense depending on the solution. The proposed mechanisms of this oxidation is firstly the C−C bond breaking of the aromatic planes through the mechanical effects of ultrasounds and cavitation, and further the oxidation at the edge of the layers by reaction with the solvent (for example, water), or the OH° radicals emitted through the water sonolysis or hydrogen peroxide decomposition.
The most oxidized samples have been obtained by sonication in hydrogen peroxide or in formic acid at 20 kHz–60 °C. The sonication in hydrogen peroxide at 20 kHz led to an enhanced oxidation due to the oxidizing power of the solvent. The carbons sonicated at 20 kHz either in water or concentrated formic acid (at about 30 °C) have been only slightly oxidized. The sonication in concentrated formic acid at 20 kHz–60 °C led to the modification of the surface chemistry of the activated carbon, leading to slightly basic modified carbons containing either phenol or ether groups. The formation mechanism of this particular surface chemistry involving evolved gas and radicals has not been elucidated yet.
The activated carbons derived from sonication showed an increased ibuprofen adsorption uptake at pH 3 either in pure water medium or in methanol co-solvent (10% vol.) in comparison with the pristine activated carbons. The sonication at 500 kHz of the activated carbon in different solutions (water, formic acid or hydrogen peroxide) has led to slight textural modification under controlled surface chemistry (without strong oxidation) which has proved to be more effective at increasing the ibuprofen adsorption uptake (increase of 25% in water and 75% in water/methanol mixture) compared to sonication at 20 kHz (increase of 15% in water and 40% in water/methanol mixture). The increase in the adsorption capacity after a sonication at 500 kHz of the activated carbon powder can be explained on one hand by surface chemistry modification promoting hydrophobic–hydrophobic interaction of ibuprofen and the carbon surface, and on the other hand by an increase in the transfer of adsorbate because of surface structure modification without any decrease in the particle size leading to a slight increase in the external surface and an enhanced access to the pores. The sonication treatment at 500 kHz prior to adsorption is assumed to unblock the mesopores at the carbon surface thus promoting an easy diffusion of the adsorbate toward the smallest pores (micropores) possibly by a decrease of the tortuosity. In terms of ibuprofen capacity of adsorption at pH 3, we have found high values of the sonicated activated carbons of about 175 mg/g in pure water and 250 mg/g in water/methanol mixture (10 vol. % of methanol), and higher than the highest uptake of 138 mg/g mentioned for powdered activated carbons in the comparative report of Guedidi et al. [44].
Supplementary data
Supporting information for this article is available on the journal’s website under https://doi.org/10.5802/crchim.3 or from the author.



 CC-BY 4.0
CC-BY 4.0