1. Introduction
Methanethiol is an effective material in the synthesis of methionine. It is used as an intermediate in petrochemical and agricultural industries [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12]. The industrial production of methanethiol takes place through the reaction between methanol and hydrogen sulfide. However, recent research has focused on its synthesis from syngas and hydrogen sulfide [13, 14, 15]. Although the industrial production of methanethiol has a history of about a century, research on corresponding catalysts and processes is still ongoing. K2WO4/alumina is the most well-known industrial catalyst for methanol thiolation. In this reaction, adjusting acid–base properties is one of the most important parameters in the design of this catalyst. The reaction between methanol and hydrogen sulfide produces methanethiol or dimethyl sulfide, but the conversion path depends on the acid–base properties of the catalyst [16]. It is well known that the acid–base properties of catalysts are very important in methanol thiolation [13, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26], and suitable acid–base properties can control reaction conversion and direct the selectivity of the reaction toward some desired products. The aim of this paper is to review the effects of acid–base properties on the design of methanol thiolation catalysts.
Reports of the design of this catalyst dating back to 1910 have been reviewed in this paper, and the routes for the design of future catalysts have been specified. Different catalysts including metal oxides and zeolites with different promoters, including alkali metals, are currently designed and synthesized. A general trend has been observed showing that reducing the concentration and the strength of Lewis acid sites while increasing basic properties enhances methanethiol selectivity at the cost of a lower rate of methanol conversion. New catalysts are designed to boost the dispersion of impregnated metals [5].
2. Methanol thiolation reaction network
Methanol thiolation is the reaction between methanol and hydrogen sulfide, where methanol is converted into methanethiol in a fixed bed reactor and in the presence of a heterogeneous catalyst. The main reaction is as follows:
| (1) |
Reviewed catalysts (1910–2019)
| Ref. | Catalyst | Year |
|---|---|---|
| [29] | Thoria | 1910 |
| [30] | Thoria on pumice | 1920 |
| [31] | Thorium/pumice | 1954 |
| [32] | Thorium/pumice | 1954 |
| [33] | Activated alumina and activated gel-type alumina | 1958 |
| [34] | K2WO4/activated alumina | 1958 |
| [35] | Alumina + KOH/NaOH | 1961 |
| [27] | K2WO4/alumina | 1962 |
| [36] | Alumina | 1966 |
| [37] | KW/Al2O3 | 1976 |
| [38] | Zeolites | 1985 |
| [39] | K, W, V/SiO2, Al2O3, AlSi | 1987 |
| [40] | NaxWyOz/alumina, K2WO4/alumina, K2WO4/SiO2 | 1988 |
| [41] | Alumina | 1988 |
| [42] | Alumina | 1989 |
| [43] | K, Na/W/Al2O3 | 1989 |
| [17] | Pure metal oxides (MgO, TiO2, ZrO2, CeO2, Al2O3) | 1993 |
| [22] | K2CO3/Al2O3 | 1998 |
| [8] | CsW/Al2O3 | 1998 |
| [44] | Na or Mo/zirconia or alumina | 1998 |
| [45] | Zeolites | 1998 |
| [9] | KW/Al2O3 | 1999 |
| [46] | Alkali/alumina, alkali/niobia, alkali/silica | 2006 |
| [25] | KW/ammonium salt/Al2O3 | 2007 |
| [10] | CsW with halide | 2008 |
| [47] | Zeolite, metal oxides | 2009 |
| [11] | KW/ammonium, phosphate, sulfide, sulfate salt/Al2O3 | 2010 |
| [12] | KxWOy | 2012 |
| [48] | Si/K2WO4/Al2O3 | 2012 |
| [49] | K2WO4/γ-Al2O3 | 2015 |
| [1] | K/alumina, Rb/alumina, Cs/alumina | 2017 |
| [2] | CsW/Al2O3 | 2017 |
| [3] | CsW/Al2O3 | 2019 |
| [4] | K2WO4/Al2O3 | 2019 |
| [5] | Cs/alumina, Cs/TiO2 (anatase), Cs/ZrO2 | 2019 |
Moreover, there are some side reactions as follows [27, 28]:
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
A reaction network has been recently developed by Pashigreva et al. with six reactions as shown in Scheme 1 [2].
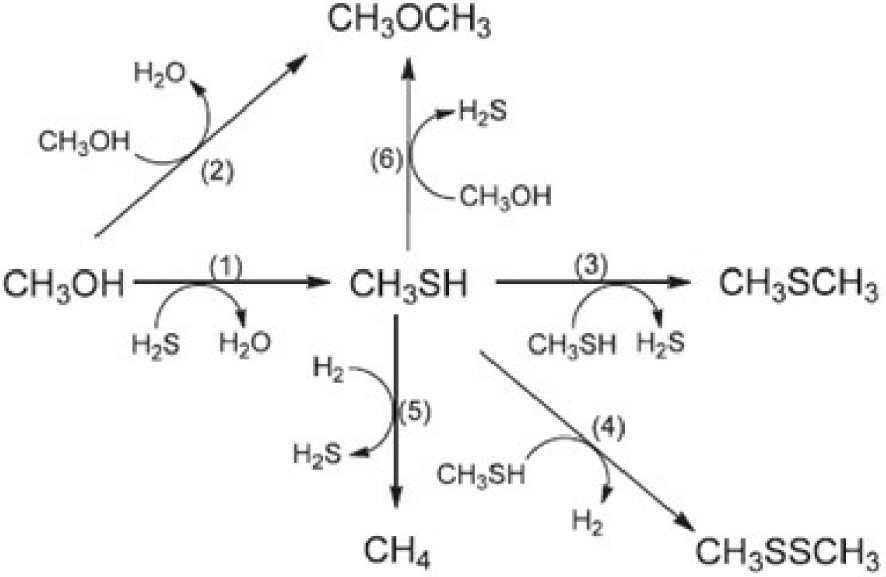
Network for the reaction of methanol with H2S (with permission of Ref. [2]).
Besides the main reaction, five side reactions are shown in Scheme 1. In this scheme, methanethiol itself is the core for four side reactions. Different side products can be produced under different process conditions (e.g. CO and H2 are produced at high temperatures).
3. Review of catalysts
Different catalysts including thoria, metal oxides, zeolites, alumina silicates, and metal oxides promoted by alkali metals and transition metals have been manufactured and evaluated for the methanol thiolation process over the past 110 years. Table 1 reports the data on these catalysts from 35 references.
The historical trend in the type of catalysts shows that alumina was the most frequently used base material from 1958 to 2019. A review of the publication dates of the papers shows that the publication rate has been higher recently, which reflects the importance of optimization for the methanol thiolation process.
4. Acid–base properties
Charge transfer through an electron transfer or a proton transfer takes place in most surface reactions. The ability of an oxide catalyst to donate or accept electrons/protons is related to its acid or base properties, and it can be defined by the Lewis or the Brønsted acid/base concept. According to the definition mentioned in the work of Somoraja [50], a Lewis acid site can receive a pair of electrons from the adsorbate, while a Lewis base site can transfer them to it. In addition, a Brønsted acid site can lose a proton to the adsorbate, but a Brønsted base site can accept a proton from it.
Although the first paper regarding a methanol thiolation catalyst was published in 1910, the first paper on the acid–base properties of a catalyst was released only 70 years later [39]. The main studies regarding the effect of acid–base properties on catalyst design based on the catalyst type have been organized into three categories such as zeolites, metal oxides, and supported catalysts.
4.1. Zeolites
Acid–base properties of different zeolites including HZSM-5, faujasite, SAPO-18, and AlPO-18 were studied in the reaction between methanol and hydrogen sulfide [45, 47, 51, 52]. The results showed that acidic zeolites tend to form DMS and hydrocarbons, where reducing the acidity of zeolites increases the selectivity toward methanethiol. Comparing HZSM-5 with HNaY, NaX, and NaY shows that the activity drastically diminishes with decrease in acidity and increase in alkalinity in the following order:
More alkali metals impregnated in faujasite zeolites were investigated by Ziolek et al. [45]. Both methanethiol and DMS can form on acidic zeolites, where methanol may convert to a hydrocarbon on them. The basic property of zeolites strongly affects the yield of formation of methanethiol (Table 2).

Reactions of methanol on acid–base sites of zeolites. BAS: Brønsted acid sites with different strengths:
Activity and yield of products in the hydrosulfurization of methanol (with permission of Ref. [45])
| Catalyst | LiNaY | NaY | KNaY | RbNaY | CsNaY | HNaX∗ | LiNaX | NaX | KNaX | CsNaX |
|---|---|---|---|---|---|---|---|---|---|---|
| Methanol conv. (%) | 26 | 27 | 22 | 19 | 41 | 90 | 36 | 55 | 80 | 72 |
| Yield (%) | ||||||||||
| CH3SH | 17 | 20 | 13 | 12 | 18 | 2 | 30 | 50 | 71 | 65 |
| (CH3)2S | 2 | 2 | 0.5 | 0.5 | 0 | 18 | 3 | 3 | 5 | 4 |
| (CH3)2O | 6 | 4 | 7 | 5 | 21.5 | |||||
| C2–C4 | 1 | 1 | 1.5 | 1 | 1.5 | |||||
*50% of Na exchange on NH4+; 90% of crystallinity in the dehydrated sample; this sample is active in the formation of hydrocarbons.
The acidity of zeolites decreases in the following order:
| (9) |
| (10) |
A reaction mechanism on zeolites was proposed according to Scheme 2. Methanol can be adsorbed on both Brønsted basic site and a pair of Lewis acid–base site in two different pathways. The presence of strong Brønsted acidic sites can convert the methanethiol and dimethyl ether (DME) products to DMS and hydrocarbon.
SAPO-18 and AlPO-18 were studied for the production of dimethyl sulfide and methanethiol from methanol and H2S. The number of acid sites decreases in the following order:
4.2. Metal oxides
The activity and selectivity of some metal oxides including SiO2, BeO2, MgO, ZrO2, ZnO, TiO2,
Activities and selectivities of metal oxides in the reaction of methanol with hydrogen sulfide (360 °C, H2S/MeOH = 1.6, conversion = 50%–60%) extracted from the data in Ref. [53]
| Catalyst (ionization potential, eV) | W1 | Selec. (%) of CH3SH | Yield (%) of CH3SH (average) |
|---|---|---|---|
| SiO2 (45.13)2 | 0.006 | 22 | 9 (max.) |
| BeO2 (18.21)2 | 0.04 | 47 | 19 (max.) |
| MgO (15.03)2 | 0.05 | 65 | 26 (max.) |
| ZrO2 (33.97) | 0.06 | 85 | 46 |
| ZnO (17.96) | 0.10 | 0 | 0 |
| TiO2 (43.24) | 1.2 | 56 | 30 |
| γ-Al2O3 (28.44) | 1.9 | 37 | 20 |
| η-Al2O3 (28.44) | 3.2 | 35 | 19 |
| WO3 (56) | 32 | 73 | 40 |
| V2O5 (65.2) | 44 | 85 | 46 |
1 Reaction rate for methanethiol (mmol⋅m−2⋅h−1). 2 Conversion less than 40%.
Al2O3 is the main pure metal oxide utilized for methanol thiolation reaction at all times. It has the highest activity among other pure metal oxides (Table 4). However, its weak basic sites cause reactions to form dimethyl sulfide with high selectivity. MgO shows minimal activity (about 3% as methanol conversion) but 100% selectivity for methanethiol due to very strong basic sites. According to the data from Table 4, ZrO2 with a yield of 69.1% for methanethiol formation at a H2S to CH3OH ratio of 2 is located at the top. The selectivity for dimethyl sulfide was observed to be inversely proportional to the number of basic sites. Metal oxides with very strong Lewis acid (and moderately basic) sites were reported to be suitable for the synthesis of dimethyl sulfide [17].
Activity and selectivity of catalysts (with permission of Ref. [17])
| Conversion selectivity (%) | Catalyst | |||||||
|---|---|---|---|---|---|---|---|---|
| PO | MgO | MgAl2O4 | TiO2 (R) | ZrO2 | TiO2 (A) | CeO2 | γ-Al2O3 | |
| CH3OH conversion (%) | ||||||||
| H2S:CH3OH = 2:1 | 0 | 2 | 32 | 45 | 72 | 91 | 68 | 99 |
| 1:1 | 0 | 3 | 15 | 25 | 34 | 52 | 59 | 99 |
| 0.5:1 | 1 | 2 | 14 | 15 | 16 | 32 | 42 | 43 |
| CH3SH selec. (%) | ||||||||
| H2S:CH3OH = 2:1 | 0 | 100 | 91 | 95 | 96 | 57 | 80 | 46 |
| 1:1 | 0 | 100 | 87 | 91 | 90 | 36 | 50 | 15 |
| 0.5:1 | 0 | 100 | 96 | 85 | 100 | 31 | 36 | 2 |
| (CH3)2S selec. (%) | ||||||||
| H2S:CH3OH = 2:1 | 0 | 0 | 8 | 3 | 3 | 41 | 1 | 53 |
| 1:1 | 0 | 0 | 1 | 1 | 9 | 57 | 7 | 84 |
| 0.5:1 | 0 | 0 | 4 | 3 | 0 | 52 | 1 | 93 |
| CH4 selec. (%) | ||||||||
| H2S:CH3OH = 2:1 | 0 | 0 | 0 | 2 | 1 | 2 | 19 | 0 |
| 1:1 | 0 | 0 | 0 | 8 | 1 | 7 | 43 | 1 |
| 0.5:1 | 0 | 0 | 0 | 12 | 0 | 17 | 63 | 5 |
4.3. Supported catalysts
The principal research regarding the effect of acid–base properties of supported catalysts on the methanol thiolation reaction has been conducted during the past 30 years. The promotion of different supports has been tested including γ-Al2O3, SiO2, ZrO2, Nb2O5, and TiO2 by WO3, alkali metals and hydroxide or carbonate of alkali metals [1, 2, 4, 5, 39, 43, 44, 46, 47].
Catalysts and their properties extracted from the data in Ref. [39]
| Catalyst | Sspec (m2/g) | W (mmol/g⋅h) | Selec. (%) | Properties of L-sites (N, μmol/g) (Q, kJ/mol) | Properties of base sites | ||
|---|---|---|---|---|---|---|---|
| MM | DMS | First type XN XX(PA) | Second type XN XX(PA) | ||||
| SiO2 | 350 | 0.14 | 0.03 | 30 | 0 | 0 | 0 |
| 7% WO3–SiO2 | 290 | 9.7 | 4.6 | 46 | - | - | - |
| 10% K2WO4–SiO2 | 290 | 2.7 | 0.01 | 90 | 0 | 47 (910) | 214 (805) |
| AlSi | 430 | 0.83 | 4.5 | 6 | 22 (53) | 80 (910) | 40 (800) |
| 7% WO3–AlSi | 406 | 68 | 13 | 56 | 120 (56) | 0 | 40 (800) |
| 10% K2WO4–AlSi | 430 | 15.0 | 3.7 | 65 | 0 | 40 (915) | 670 (800) |
| γ-Al2O3 | 300 | 236 | 118 | 37 | 690 (34) | 96 (900) | 546 (810–840) |
| 7% WO3–Al2O3 | 260 | 280 | 100 | 51 | 381 (35.5) | 29 (915) | 325 (800) |
| 10% K2WO4–Al2O3 | 260 | 12.1 | 0.05 | 87 | 214 (31.5) | 137 (910–940) | 425 (810) |
| 15% K2WO4/Al2O3 | 270 | 9.5 | 0.02 | 93 | 135 (31.5) | 330 (900–925) | 670 (810) |
| 10% Na2WO4/Al2O3 | 230 | 12.4 | 0.2 | 85 | 250 (32.5) | 260 (900–930) | 556 (810) |
Sspec (m2/g, catalyst specific area); W (mmol/g⋅h, catalytic activity); Selec. (%, selectivity toward methanethiol); N (μmol/g, number of Lewis sites); Q (kJ/mol, strength of Lewis sites); XN (μmol/g, number of base sites); XX(PA) (kJ/mol, the energy of proton addition, PA stands for proton affinity).
Adding WO3 to γ-Al2O3 reduces the number of Lewis acid sites and basic sites, but this increases the production rate of methanethiol and lowers the production rate of dimethyl sulfide [47]. The addition of K2WO4 to γ-Al2O3 was found to cause more reduction in the number of Lewis acid sites and basic sites. This resulted in a near-zero production yield for dimethyl sulfide, enhancing the selectivity for methanethiol. Such behavior has also been observed for SiO2 and AlSi (Table 5). It was concluded that acidic catalysts have higher activities, but their production rate for methanethiol and dimethyl sulfide is 50–50. Catalysts with strong Lewis acid sites have a higher tendency to produce dimethyl sulfide, and for the selective production of methanethiol, it is necessary to use catalysts with strong basic sites on the surface [39].
In a similar study, some γ-Al2O3 based catalysts were promoted by K2WO4, K2CO3, KOH, and NaOH. K2WO4 led to the formation of relatively weaker Lewis acid sites and moderate basic sites, but the other three catalysts had strong Lewis acid sites and basic sites. As shown in Table 6, K2WO4 created more selectivity for methanethiol, but it exhibited low activity. For example, at 360 °C, the selectivity values for methanethiol were 96%, 95%, 92%, and 90%, but the methanol conversion values were 47%, 56%, 55%, and 53% for K2WO4, K2CO3, KOH, and NaOH, respectively. The other three catalysts, on the other hand, were more active and less selective. It was concluded that strong acidic and basic sites are more active but less selective for producing methanethiol [43].
Catalytic activities of catalysts extracted from the data in Ref. [43]
| Catalyst | T (°C) | MeOH concentration (%) | Rate (mol/g) × 104 | Selectivity toward MM (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| MeOH | MM | DMS | DME | CH4 | CO2 | ||||
| K2WO4/Al2O3 | 360 | 47 | 46 | 44 | 0.5 | 1.2 | 0.0 | 0.0 | 96 |
| K2CO3/Al2O3 | 360 | 56 | 64 | 61 | 2.0 | 0.3 | 0.0 | 0.0 | 95 |
| KOH/Al2O3 | 360 | 55 | 59 | 54 | 2.4 | 2.1 | 0.0 | 0.0 | 92 |
| NaOH/Al2O3 | 360 | 53 | 58 | 52 | 2.5 | 2.1 | 0.0 | 0.0 | 90 |
| K2WO4/Al2O3 | 400 | 68 | 62 | 59 | 1.5 | 0.7 | 0.4 | 0.2 | 95 |
| K2CO3/Al2O3 | 400 | 75 | 84 | 76 | 5.3 | 0.0 | 0.8 | 0.7 | 90 |
| KOH/Al2O3 | 400 | 77 | 69 | 62 | 5.0 | 0.4 | 0.7 | 0.5 | 90 |
| NaOH/Al2O3 | 400 | 75 | 65 | 57 | 5.0 | 0.3 | 0.6 | 0.5 | 88 |
| K2WO4/Al2O3 | 500 | 89 | 225 | 175 | 7.0 | 0.0 | 21 | 9.0 | 78 |
| K2CO3/Al2O3 | 500 | 86 | 303 | 201 | 18 | 0.0 | 34 | 19 | 66 |
| KOH/Al2O3 | 500 | 86 | 256 | 175 | 19 | 0.0 | 29 | 18 | 68 |
| NaOH/Al2O3 | 500 | 90 | 313 | 221 | 22 | 0.0 | 24 | 23 | 71 |
The use of metals as promoters for methanol thiolation reaction has been taken into consideration for the past 20 years. The addition of Na to γ-Al2O3 and ZrO2 enhances methanethiol selectivity, but it reduces catalyst activity (Tables 7 and 8). Na was loaded on γ-Al2O3 from 0.25% up to 4.5%. Therefore, the selectivity increased from 28% to 100%, and at the same time, methanol conversion decreased from 91% to 11%. The addition of Mo to ZrO2 enhances the activity, yet this reduces the selectivity for methanethiol (Table 9). Moreover, Mo increases the acidity of the catalyst [44].
Activity and yield of product, Na on alumina (with permission of Ref. [44])
| Al2O3 modified with | CH3OH conv. (%) | Yield (%) | |||
|---|---|---|---|---|---|
| (H2S/CH3OH = 1/1) | CH4 | (CH3)2O | CH3SH | (CH3)2S | |
| 0% Na | 99 | - | - | 11 | 88 |
| 0.2% Na | 91 | - | 7 | 26 | 58 |
| 0.5% Na | 83 | - | 25 | 45.2 | 12.5 |
| 1% Na | 59 | 3 | 16.5 | 26.5 | 13 |
| 2.2% Na | 13 | - | - | 13 | - |
| 4.5% Na | 11 | - | - | 11 | - |
Activity and yield of product, Na on ZrO2 (with permission of Ref. [44])
| ZrO2 modified with | CH3OH conv. (%) | Yield (%) | CH3OH conv. (%) | ||
|---|---|---|---|---|---|
| (H2S/CH3OH =1/1) | (CH3)2O | CH3SH | (CH3)2S | (H2S/CH3OH = 2/1) | |
| 0% Na | 60 | 2 | 54 | 4 | 95 |
| 0.1% Na | 20 | Trace | 20 | 20 | |
| 0.2% Na | 9 | - | 9 | - | |
| 0.5% Na | 5 | - | 5 | 5 | |
| 1% Na | 5 | - | 5 | 5 | |
Activity and yield of product, Mo on ZrO2 (with permission of Ref. [44])
| ZrO2 modified with | CH3OH conv. (%) | Yield (%) | |||
|---|---|---|---|---|---|
| (H2S/CH3OH = 1/1) | CH4 | (CH3)2O | CH3SH | (CH3)2S | |
| 0% Mo | 60 | - | 1.8 | 54 | 4.2 |
| 0.25% Mo | 72 | 3.6 | 1.4 | 57 | 10 |
| 0.5% Mo | 81 | 10.8 | 0.8 | 53 | 16 |
| 1% Mo | 93 | 21.6 | 0.4 | 47 | 24 |
The addition of alkali metals to niobia leads to the formation of acid–base pairs, thereby increasing the selectivity of methanethiol and the basic sites on alumina and silica. When a support is impregnated with alkali metals, the Brønsted centers are destroyed [19], where the strength of Lewis acid sites decreases together with increase in the basic property [54]. The selectivity for methanethiol strongly depends on the nature of active sites. Table 10 reports higher methanethiol production by modified niobia, which can be attributed to the presence of acid–base pairs on its surface [46].
Although catalysts have been available for the methanol thiolation process since about 1910, the research in this area is still ongoing. Vast and comprehensive studies have been conducted on methanol thiolation since 2017 in Technische Universität München (TUM) and Institute for Integrated Catalysis by impregnating alkali metals (K, Rb, and Cs) on supports of γ-Al2O3 and TiO2 [1, 2, 4, 5]. They claim that tuning the acid–base properties of the catalyst by adjusting alkali metal properties is an approach to preparing novel catalysts.
Alkali metals have two important roles in the final catalyst: lowering the acidic strength and creating suitable sites for the adsorption of reactants (H2S and CH3OH). Cs has lower electronegativity than those of Rb and K, which increases the electron density on the neighboring anions and enhances their Lewis basic strength [55, 56, 57]. Adding alkali cations to gamma-alumina blocks Lewis acid sites and prevents the formation of DME. Indeed, very strong Lewis acid sites are substituted with the weaker ones by adding alkali cations to gamma-alumina, which makes the neighboring sulfur oxyanions stronger Lewis basic sites through increasing their electric charge. As such, some acid–base pairs are formed, which help in dissociative adsorption of H2S. H2S is dissociated into H+ and SH- as illustrated in Figure 1. Furthermore, SH- generates nucleophilic attacks on the methoxy species, which leads to the formation of methanethiol [1].
The size and loading of cations on gamma-alumina affect the rate of formation of methanethiol. At lower loadings, the rate of methanethiol formation in Cs+ is less than that of Rb+ and K+ due to better dispersion of smaller cations (Rb and K). However, at higher cation loadings, Cs+ provides better results in the formation of methanethiol due to higher electronegativity [1] as shown in Table 11.
Conversion and selectivity of alkali modified supports in methanol thiolation reaction (with permission of Ref. [46])
| Conversion (%) | Selec. (%) | ||||
|---|---|---|---|---|---|
| Me2S | MeSH | Me2S2 | Me2O | ||
| Nb2O5⋅nH2O | 74 | 20 | 71 | 4 | 5 |
| Li/Nb2O5⋅nH2O | 14 | 1 | 93 | 4 | 2 |
| Na/Nb2O5⋅nH2O | 18 | 1 | 94 | 3 | 2 |
| K/Nb2O5⋅nH2O | 25 | 1 | 93 | 5 | 1 |
| Rb/Nb2O5⋅nH2O | 17 | 1 | 92 | 5 | 2 |
| Cs/Nb2O5⋅nH2O | 18 | 1 | 94 | 4 | 1 |
| Nb2O5 | 40 | 10 | 79 | 4 | 7 |
| Li/Nb2O5 | 18 | 1 | 92 | 4 | 3 |
| Na/Nb2O5 | 22 | 2 | 90 | 4 | 4 |
| K/Nb2O5 | 17 | - | 96 | 4 | - |
| Rb/Nb2O5 | 18 | - | 96 | 3.5 | 0.5 |
| Cs/Nb2O5 | 10 | 1 | 91 | 5 | 3 |
| Al2O3 | 100 | 63 | 34 | 2 | - |
| Li/Al2O3 | 75 | 30 | 43 | 2 | 25 |
| Na/Al2O3 | 5 | - | 59 | - | 41 |
| K/Al2O3 | 4 | - | 92 | - | 8 |
| Rb/Al2O3 | 23 | 6 | 40 | - | 54 |
| Cs/Al2O3 | 4 | - | 88 | - | 12 |
Methanethiol rates as a function of concentration of alkali cations extracted from the data in Ref. [1]
| Concentration of alkali cations (103 mol/g Al_2O_3) | Rate (105 molCH_3SHs/gcat) | ||
|---|---|---|---|
| K/Al2O3–H2S | Rb/Al2O3–H2S | Cs/Al2O3–H2S | |
| 0.75 | 1.35 | 2.00 | 1.45 |
| 1.15 | 1.12 | 1.80 | 1.80 |
| 1.40 | 1.08 | 1.75 | 2.16 |
| 2.05 | 1.00 | 1.75 | 2.00 |
Due to the good performance of Cs-promoted catalysts, another attempt was made to find how the acid–base properties of the catalyst can affect the reaction route. The acid–base properties of the final catalysts can be manipulated by altering the Cs content. Catalysts (WS2/Al2O3) without Cs produced DME and methanethiol, but Cs-containing catalysts (Cs–WS2/Al2O3) produced methanethiol at a higher yield (Figure 2).
The addition of Cs reduces both the specific area and the acidity of the catalyst. The decrease in acidity is greater than the area reduction. The addition of Cs+ strongly improves the nature of the catalyst and reduces the strength and concentration of Lewis acid sites. In fact, very strong Lewis acid cation sites (Al3+ and W4+) are replaced with the weaker strength Lewis acid sites of Cs+ [2].
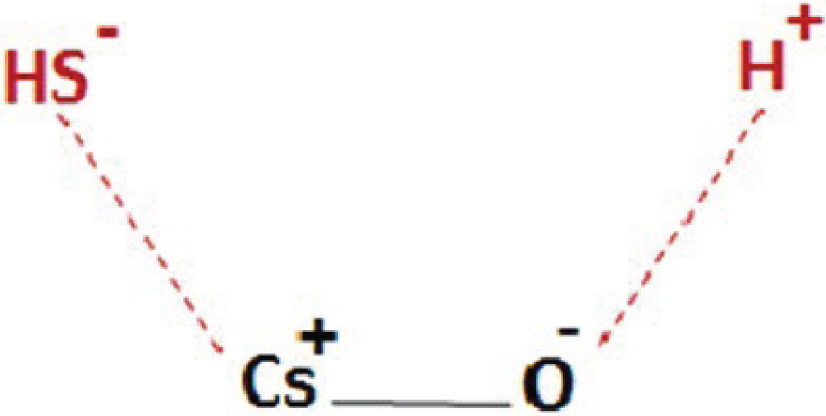
H2S dissociative adsorption on Cs–O.
Cs+ induces very strong basic sites, which increases catalytic activity. The very strong basic sites, which associate with neighboring weak Lewis acid site alkali cations, improve the performance of catalysts. The incorporation of Cs+ lowers the amount of adsorbed methanol but increases the rate of reaction between SH groups and the adsorbed methanol. Cs+ plays a key role in directing methanol conversion to methanethiol selectively. WS2 does not have any role in the methanol thiolation reaction [4].
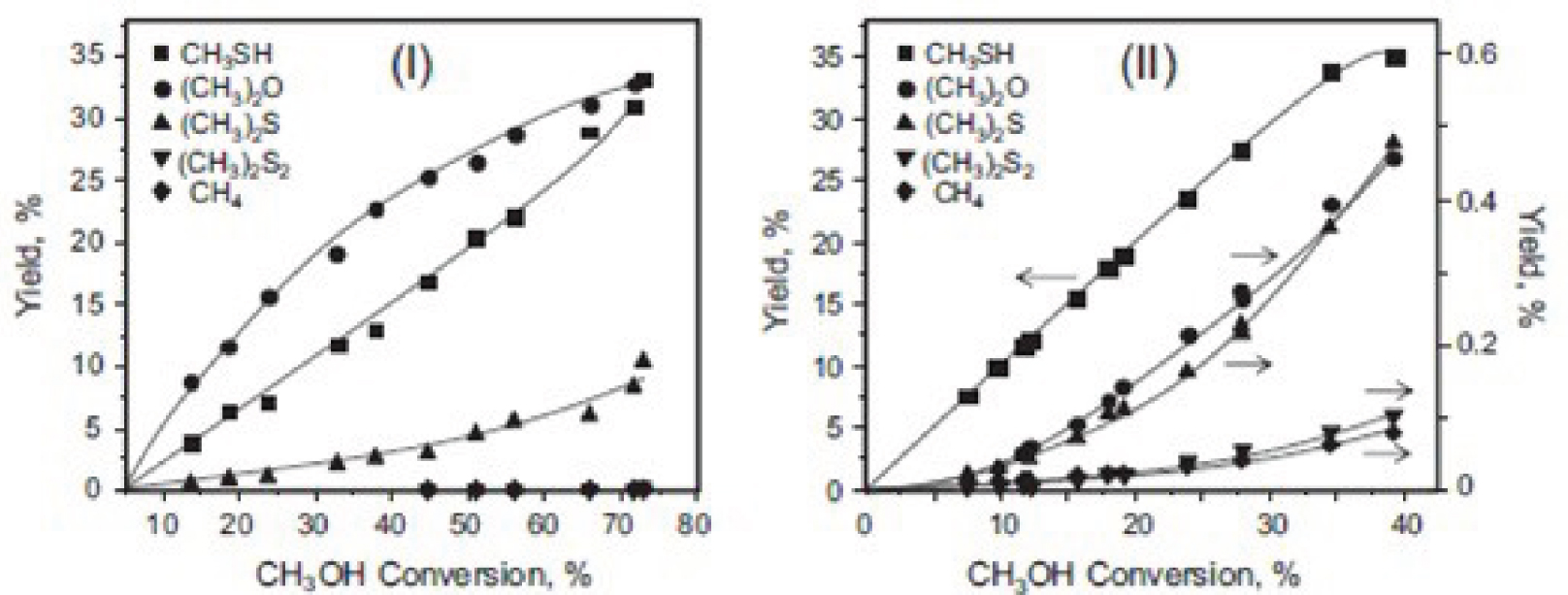
Product yields during the reaction between methanol and H2S over WS2/Al2O3 (I) and Cs–WS2/Al2O3 (II) for varying methanol conversion (360 °C, 9 bar) (with permission of Ref. [2]).
Cs/Al2O3 and CsW/Al2O3 reveal the same reaction rates. The binding of Cs+ and WS2 is strong and makes the catalyst more stable. Two transition metal oxides, TiO2 and ZrO2, were examined for comparison. They were impregnated by Cs+ but without the addition of WS2. TiO2 and ZrO2 provide Lewis acid–base pairs, while gamma-alumina has a combination of weak and strong Lewis acid sites. The highest methanethiol initial rates were observed with TiO2. The results indicated that a methanol thiolation catalyst can be developed without WS2. The main parameter in the design of catalysts is the existence of acid–base pairs, which provide suitable sites for the dissociative adsorption of H2S and CH3OH, where SH generates a nucleophilic attack on the methoxy species. Strong basic anions play the main role in the absence of strong Lewis acid sites [5].
5. Conclusion
A review of historical trend in the development of methanol thiolation catalysts indicates that the primary catalysts used in the thiolation of methanol were metal oxides. The next generation of catalysts was promoted by alkali metals and tungsten. Cs was found to be the best promoter among alkali metals. The inefficiency of tungsten was also proved; the search for a new and more stable catalyst led to Cs-promoted TiO2. The schematic of this trend is illustrated in Scheme 3.

Historical trend in the development of methanol thiolation catalysts.
As the final result, it can be concluded that the best catalyst for methanol thiolation is a catalyst with Lewis acid–base pairs in which weak acid sites are located near strong basic sites.
In general, basicity influences the yield of methanethiol. Strong basic sites help in the dissociative adsorption of H2S, thus increasing the yield of methanethiol. The strength of neighboring acid sites affects the performance of strong basic sites. Although strong neighboring acid sites suppress the effectivity of strong base sites in methanethiol formation, the presence of weak neighboring acid sites has a synergistic effect on enhancing methanethiol production.



 CC-BY 4.0
CC-BY 4.0