Recently, we described the synthesis of a 2:1 cyclodextrin–fullerene conjugate 1a [1], designed to be highly soluble in water for biological and biophysical applications [2]. It was hoped that solvent-dependent equilibria between conformers such as A, B and C (Fig. 1) could occur.
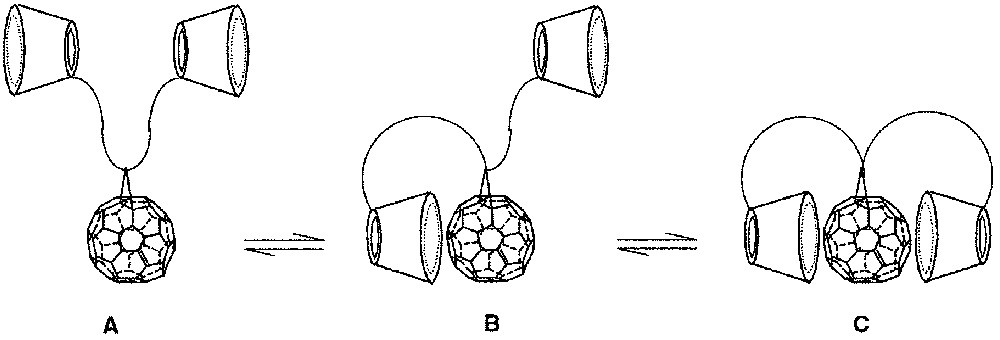
Possible equilibria between conformers A, B and C of 1a.
In water, A and B could form micelle-like aggregates, while if the cyclodextrins (CD) insure a sufficient hydrophilic protection, C could exist as a non-associated species. It was found on the basis of UV and NMR spectra that aggregates were present in water solution, and this has since been confirmed by photophysical measurements [3]. In order to study the influence of the linker on solubility and/or aggregation, we have prepared the other conjugates 1b and 1c (Fig. 2 ).
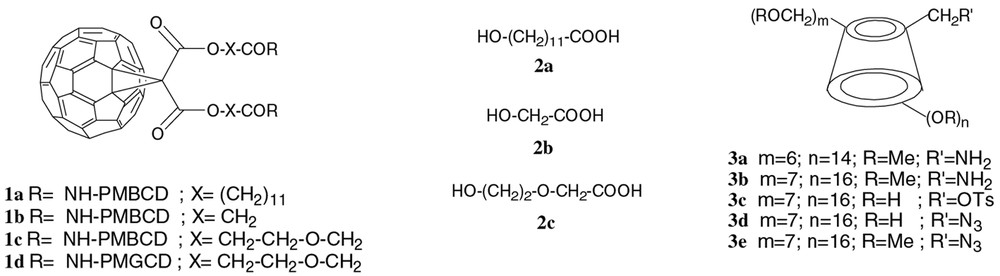
Compounds 1a–d, 2a–c, 3a–e.
In 1a, a rather long and hydrophobic linker (2a) connected the methanofullerene moiety to the two β-CD derivatives. Two different linkers, 2b and 2c, have now been used: 2b is less hydrophobic than 2a and too small to be compatible with internal complexation (conformation C), while 2c, longer and more hydrophilic, could allow both internal complexation and a better solubility in water. These two conjugates were prepared (Fig. 3) from 4a and 4b, obtained by standard methods (starting from 2-benzyloxy(ethanol) [4] for 4b). Satisfactory MS, 1H and13C NMR have been obtained for these new compounds.
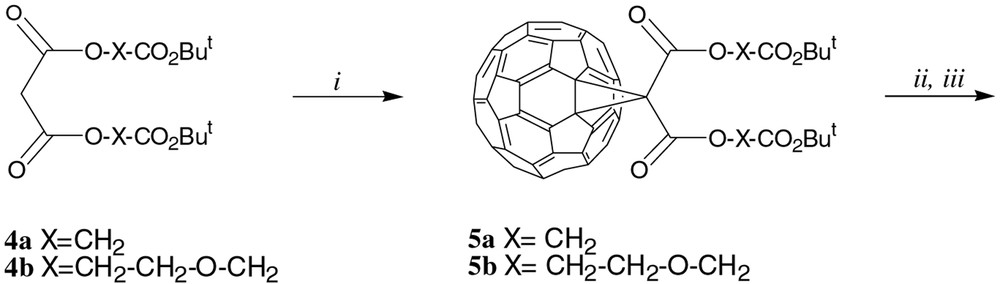
Reagents and conditions. (i) C60, I2, DBU, toluene (5a: 43%; 5b: 27%); (ii) TFA, CH2Cl2; (iii) 3a (or 3b for 1d), 1-hydroxybenzotriazole (HOBt), DCC, CH2Cl2, rt, 48 h (1b : 79%; 1c : 94%; 1d : 59%).
1b and 1c are very soluble in dichloromethane and in chloroform and have a very high solubility in water at 20 °C, greater than 250 mg ml–1 for 1b and 300 mg ml–1 for 1c. Clear solutions were obtained after dissolving 1b (25 mg) in water (100 μl), and 1c (90 mg) in water (300 μl). Contrary to the negative solubility coefficient previously found for 1a, no precipitation occurred when the temperature of these water solutions was raised.
As for 1a, aggregates are present in water solutions: the NMR spectra of 1b and 1c are much broader in water than in chloroform. The UV spectra of dichloromethane solutions of 1a and 1b are not distinguishable from those of 1c. In water solution, these three compounds have slightly different UV spectra (Fig. 4): while 1a presents a shoulder at 330 nm, a relative maximum is found for 1c, 1b being intermediate between 1a and 1c ; none of these spectra display the absorption peak at 430 nm observed in dichloromethane solutions. As found for 1a, these NMR [5] and UV [6,7] spectra show the presence of aggregates in water solutions. The UV spectra suggest that at the same total concentration, the proportion of aggregates decreases in the order 1a > 1b > 1c, which is confirmed by photophysical measurements [3].
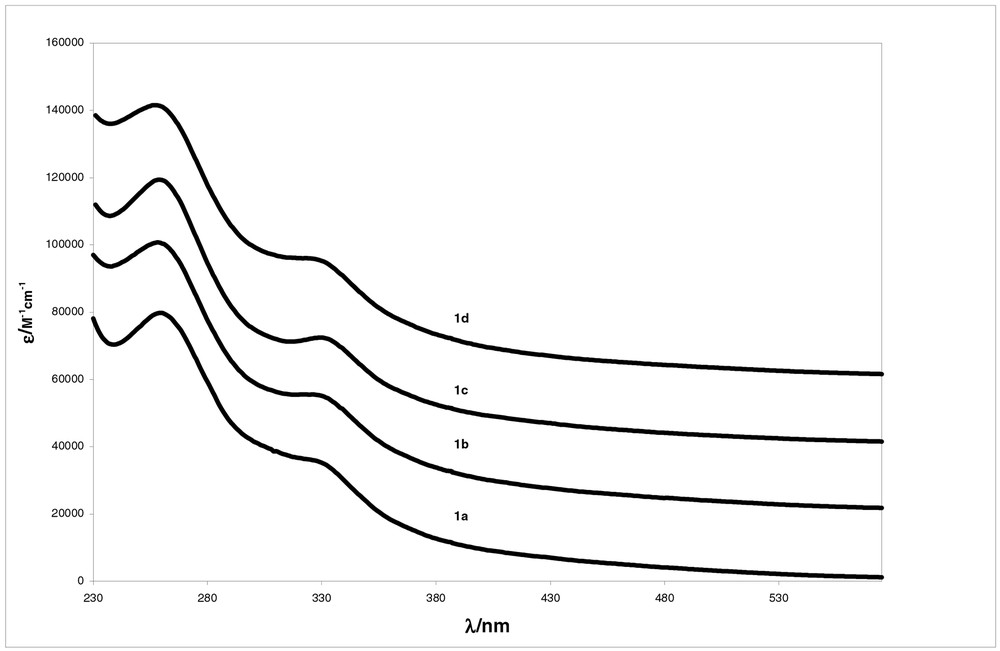
Absorption spectra of water solutions of the CD-fullerene conjugates. The spectra of 1a, 1b, 1c and 1d have been shifted upwards by increments of 2 × 104 M–1 cm–1, in that order.
As again for 1a, water solutions (concentrations 10–4–10–5M) of these conjugates did not show any circular dichroism in the absorption band of C60, between 200 and 700 nm, although induced circular dichroism has been observed for a γ-CD/C60 complex [8].
There is thus no evidence of internal complexation in these three conjugates. Since it is possible that the affinity of β-CD for the fullerene moiety is not sufficient to induce this type of complexation, a γ-CD conjugate 1d was also prepared. NH2–PMGCD 3b was obtained in total 6% yield from γ-CD, through the sequence of reactions (γ-CD → 3c → 3d [9] → 3e → 3b) similar to the one previously used for the preparation of NH2–PMBCD 3a; 3b was converted to 1d as shown in Fig. 3. In the two last steps, we used conditions previously applied to the transformation of the corresponding β-CD derivatives [10] (see also [1]). Here again, a very soluble molecule was obtained, with solubility in water at 20 °C greater than 300 mg ml–1 to a clear solution was obtained after dissolving 1d (8 mg) in water (25 μl). As before, the UV and NMR spectra in water indicated the presence of aggregates, but the peak at 330 nm present in 1c was now replaced by a shoulder, as in 1b, a possible indication of a similar degree of aggregation. Again, no induced circular dichroism was observed in water solutions of 1d.
The CD-fullerene conjugates described in this communication are very soluble. They have the greatest solubility in neutral water ever reported for fullerene derivatives [1,2,11,12]. The UV spectra show that aggregates are present in these solutions, the linewidths suggesting a degree of aggregation decreasing in the order 1a > 1b ~ 1d > 1c. Because the formation of aggregates could be due to the position of the linker and/or to the use of fully methylated cyclodextrins, modified conjugates of general formula Z (Fig. 5) are being studied.
Modified molecules under study: various different linkers are attached to a secondary hydroxyl groups and the β- and γ-CDs are methylated or not.



