1 Introduction
Room-temperature ionic liquids (RTILs) consist of organic cation and anion which exist in liquid state at room temperature or below 100 °C. They are widely used in various fields of electrochemistry and chemistry because of their unique properties such as nonvolatility and nonflammability. RTILs are promising as solvents for synthetic, catalyticl and electrochemical applications in laboratory and in industry [1,2]. The appropriate combination of the anion–cation can obtain many kinds of RTILs possessing characteristic properties, such as miscibility with water and other solvents, dissolving ability, polarity, viscosity or density. Liquid structures of RTILs have been investigated using X-ray diffraction, neutron diffraction, as well as spectroscopic and computational methods [3–6]. Studies on solid-state structure of a salt are indispensable for understanding its liquid structure, especially for local interactions. Because RTILs are liquids at room temperature, it is technically difficult to grow single crystals and to select suitable sample for single, crystal X-ray diffraction at low temperature. So far a few crystal structures have been determined by X-ray diffraction [7–10]. RTILs are typically formed by a combination of large organic cations, such as imidazolium, pyridinium or quaternary ammonium ions with anions, such as common halides or [BF4]−, [PF6]− or [CF3SO3]−. What kind of role do the organic cations which usually have π electron ring play for structure stability? In this research, large cations of 1-butyl-isoquinolinium cation for short (BIQL)+ (structure show in Fig. 1) and anion of GaCl4− were selected to obtain RTILs in the solid state at room temperature and to investigate the action of pi electron rings in the structure. Furthermore, the crystal structure of (BIQL)GaCl4 was analyzed by X-ray diffraction at low temperature.
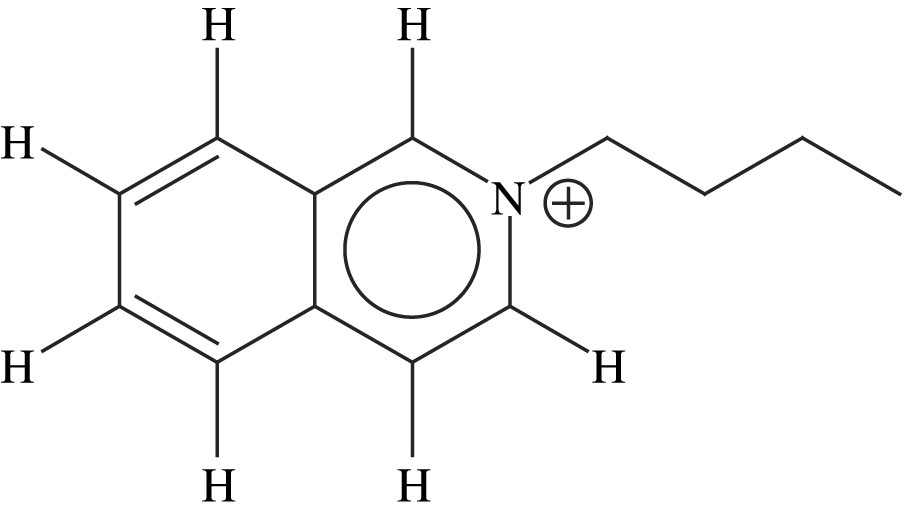
The cation structure of (BIQL)+.
2 Experiment
2.1 Experimental procedure
Moisture unstable samples were handled in a glove box under a dry N2 atmosphere. The anhydrous GaCl3 purity is 99.9% (made in Dalian Industrial, Co. Ltd. China). 1-Butyl-isoquinolinium chloride was synthesized and purified to 99.5% by adding ethyl acetate and recrystallized in acetonitrile solution. Then it was mixed with anhydrous gallium trichloride and 1-butyl-isoquinoline chloride in a 1:1 ratio, leading to the title room-temperature ionic liquid. The solvent evaporation method was used to grow RTILs crystals in ethyl acetate solvent at room temperature. The product is a colorless single crystal which is air and moisture stable (m.p. 59–60 °C). Infrared spectra was obtained on a Fourier-transform instrument (Nicolet Nexus FT-IR 470) with KBr pellets at room temperature. The elemental analysis (Model 240 PerkinElmer elemental analyzer) results of crystals are as follows (wt%): C at 39.60, H at 3.94 and N at 3.70, closely equal to the calculation based on the chemical formula found (wt%): C at 39.25, H at 4.05 and N at 3.52.
2.2 X-ray diffraction analysis
The single crystal of 0.26 × 0.24 × 0.20 mm was mounted on a Bruker SMART CCD X-ray diffractometer equipped with a graphite-monochromated Mo Kα radiation (λ = 0.071073 nm). The data were collected at 113 K by using a scan technique in the range of 1.89° < θ < 28.28° with index ranges as follows: −9 ≤ h ≤ 9, −18 ≤ k ≤ 19, −21 ≤ l ≤ 21. A total of 15,871 reflections were collected, of which 4068 were unique (Rint = 0.037) and equivalent reflections were merged. The data obtained were processed using the CRYSTALCLEAR program [11]. The structures were solved and refined using the SHELX-97 suite of programs [12]. All the non-hydrogen atoms were determined via direct method and difference Fourier synthesis by introducing anisotropic displacement parameters, whereas all the hydrogen atoms were refined using appropriate riding models. The final R = 0.0266, wR = 0.0341, calc w = 1/[σ2(F02 + (0.0321P)2 + 0.0000P], where P = (Max(F02,0) + 2Fc2)/3, σ = 0.994, (Δρ)max = 0.38 e/Å3, (Δρ)min = −0.45 e/Å3 and (Δρ/σ)max = 0.001. The crystal structure of the ionic liquid (BIQL)GaCl4 and the molecule arrangement in a unit cell are shown in Figs. 2 and 3. The unit cell parameters and refinement statistics for (BIQL)GaCl4 are given in Table 1, and the atomic coordinates and displacement parameters are given in Table 2. Their selected bond lengths and angles are listed in Tables 3 and 4, respectively. The distances beyond the asymmetric unit out to 3.60 Å involving hydrogen bond geometries are summarized in Table 5.

ORTEP diagram of (BIQL)GaCl4 structure with an atom numbering scheme.

The packing diagram of a unit cell for (BIQL)GaCl4 as viewed along a-axis and b-axis.
Crystal data and refinement results for (BIQL)GaCl4
| Empirical formula | C13H16NCl4Ga |
| Formula weight | 397.81 |
| Temperature (K) | 113(2) |
| Wavelength (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P21/c (No. 14) |
| Unit cell dimensions | |
| a (Å) | 7.1438(12) |
| b (Å) | 14.3490(14) |
| c (Å) | 16.3531(18) |
| β (°) | 94.885(7) |
| Volume (Å3) | 1670.2(4) |
| Z value | 4 |
| Calculated density (g cm−3) | 1.582 |
| Absorption coefficient (mm−1) | 0.124 |
| F(000) | 800 |
| Crystal size (mm3) | 0.26 × 0.24 × 0.20 |
| μ (Mo Kα) (cm−1) | 22.717 |
| θ range for data collection (°) | 1.89–28.28 |
| Index ranges | −9 ≤ h ≤ 9, −18 ≤ k ≤ 19, −21 ≤ l ≤ 21 |
| Reflections collected | 15,871 |
| Independent reflections | 4068 |
| Completeness to θmax (%) | 28.28 (98%) |
| Max. and min. transmission | 0.6350 and 0.559 |
| Data/restraints/parameters | 4068/0/175 |
| Goodness-of-fit on F2 | 0.994 |
| Final R indices [I > 2σ(I)] | R1 = 0.0266, wR2 = 0.0606 |
| R indices (all data) | R = 0.0341, wR = 0.0586 |
| Largest difference peak and hole (e/Å3) | 2.745 and −1.097 |
| Max shift/error in final cycle | 0.001 |
| Absorption correction | Semi-empirical from equivalents |
| Refinement method | Full-matrix least-squares on F2 |
Atomic coordinates (×104) and displacement parameters (Å2 × 103) of (BIQL)GaCl4
| Atom | x | y | z | Ueq | Atom | x | y | z | Ueq |
| Ga(1) | 7044(3) | 2130(10) | 3340(10) | 1496(5) | C(5) | 2335(2) | 1380(12) | −463(10) | 1370(2) |
| Cl(1) | 5701(6) | 3428(3) | 2918(3) | 2091(8) | C(6) | 1597(2) | 1034(12) | −1240(10) | 1760(3) |
| Cl(2) | 7862(6) | 2230(3) | 4653(2) | 1782(8) | C(7) | 1338(2) | 99(12) | −1345(11) | 1940(3) |
| Cl(3) | 9513(6) | 1895(3) | 2675(3) | 2298(9) | C(8) | 1807(2) | −528(12) | −691(11) | 1950(3) |
| Cl(4) | 5049(6) | 985(3) | 3153(3) | 2494(9) | C(9) | 2504(2) | −212(12) | 66(11) | 1840(3) |
| N(1) | 3364(19) | 2661(10) | 388(8) | 1560(2) | C(10) | 3691(2) | 3686(12) | 492(11) | 1800(3) |
| C(1) | 2678(2) | 2337(12) | −335 (10) | 1550(2) | C(11) | 1871(2) | 4206(11) | 592(10) | 1590(2) |
| C(2) | 3751(2) | 2073(12) | 1043(11) | 1990(3) | C(12) | 924(2) | 3936(12) | 1360(10) | 2010(3) |
| C(3) | 3477(2) | 1141 (12) | 961(10) | 2010(3) | C(13) | −894(2) | 4467(12) | 1438(11) | 2310(3) |
| C(4) | 2773(2) | 755(12) | 198(10) | 1510(2) |
Selected bond lengths (Å) for (BIQL)GaCl4 (at 113 K)
| Bond | Dist | Bond | Dist | Bond | Dist |
| Ga(1)–Cl(1) | 2.1791(4) | C(1)–C(5) | 1.407(2) | C(7)–C(8) | 1.416(2) |
| Ga(1)–Cl(3) | 2.1760(5) | C(2)–C(3) | 1.355(2) | C(8)–C(9) | 1.371(2) |
| Ga(1)–Cl(2) | 2.1833(3) | C(3)–C(4) | 1.418(2) | C(10)–C(11) | 1.520(2) |
| Ga(1)–Cl(4) | 2.1793(4) | C(4)–C(5) | 1.419(2) | C(11)–C(12) | 1.527(2) |
| N(1)–C(1) | 1.325(2) | C(4)–C(9) | 1.414(2) | C(12)–C(13) | 1.521(2) |
| N(1)–C(2) | 1.374(2) | C(5)–C(6) | 1.422(2) | ||
| N(1)–C(10) | 1.497(2) | C(6)–C(7) | 1.364(2) |
Selected bond angles (deg) for (BIQL)GaCl4 (at 113 K)
| Angle | (°) | Angle | (°) | Angle | (°) |
| Cl(1)–Ga(1)–Cl(2) | 109.201(18) | N(1)–C(1)–C(5) | 121.48(15) | C(5)–C(6)–C(7) | 119.45(15) |
| Cl(1)–Ga(1)–Cl(3) | 109.156(18) | N(1)–C(2)–C(3) | 120.76(15) | C(6)–C(7)–C(8) | 120.63(15) |
| Cl(1)–Ga(1)–Cl(4) | 109.588(18) | C(2)–C(3)–C(4) | 120.69(15) | C(7)–C(8)–C(9) | 121.06(15) |
| Cl(2)–Ga(1)–Cl(4) | 107.710(18) | C(3)–C(4)–C(5) | 117.46(15) | C(4)–C(9)–C(8) | 119.72(15) |
| Cl(2)–Ga(1)–Cl(3) | 109.968(18) | C(3)–C(4)–C(9) | 123.44(15) | N(1)–C(10)–C(11) | 111.55(13) |
| Cl(3)–Ga(1)–Cl(4) | 111.187(18) | C(5)–C(4)–C(9) | 119.09(14) | C(10)–C(11)–C(12) | 114.02(13) |
| C(1)–N(1)–C(2) | 120.91(14) | C(1)–C(5)–C(4) | 118.66(14) | C(11)–C(12)–C(13) | 112.55(14) |
| C(1)–N(1)–C(10) | 119.30(13) | C(1)–C(5)–C(6) | 121.30(15) | ||
| C(2)–N(1)–C(10) | 119.78(13) | C(4)–C(5)–C(6) | 120.03(15) |
Distances beyond the asymmetric unit out to 3.60 Å involving hydrogens for (BIQL)GaCl4
| Atom⋯atom | Dist | Atom⋯atom | Dist | Atom⋯atom | Dist |
| Ga(1)⋯H(2) | 3.411 | Cl(2)⋯H(10A)(2) | 3.154 | Cl(4)⋯H(1)(2) | 3.228 |
| Cl(1)⋯H(2) | 2.862 | Cl(2)⋯H(11A)(4) | 2.966 | Cl(4)⋯H(2) | 3.250 |
| Cl(1)⋯H(3)(1) | 3.506 | Cl(2)⋯H(11B)(5) | 3.395 | Cl(4)⋯H(3) | 2.926 |
| Cl(1)⋯H(6)(2) | 3.279 | Cl(2)⋯H(12A)(4) | 3.343 | Cl(4)⋯H(10A)(2) | 3.131 |
| Cl(1)⋯H(9)(1) | 3.024 | Cl(2)⋯H(13C)(4) | 3.089 | Cl(4)⋯H(10B)(5) | 3.447 |
| Cl(1)⋯H(10B) | 3.245 | Cl(3)⋯H(1)(4) | 3.170 | Cl(4)⋯H(11B)(5) | 3.154 |
| Cl(1)⋯H(12B) | 3.288 | Cl(3)⋯H(6)(4) | 2.848 | Cl(4)⋯H(12B)(5) | 3.563 |
| Cl(1)⋯H(13B)(3) | 2.966 | Cl(3)⋯H(7)(6) | 2.857 | Cl(4)⋯H(13A)(7) | 3.491 |
| Cl(2)⋯H(1)(4) | 3.385 | Cl(3)⋯H(8)(6) | 3.305 | Cl(4)⋯H(13B)(7) | 3.551 |
| Cl(2)⋯H(8)(1) | 2.935 | Cl(3)⋯H(12A)(3) | 3.087 | Cl(2)⋯C(1)(2) | 3.495 |
| Cl(2)⋯H(9)(1) | 3.099 | Cl(3)⋯H(13A)(5) | 2.977 | Cl(1)⋯C(6)(1) | 3.431 |
3 Results and discussion
3.1 IR spectrum
The infrared absorption spectrum of (BIQL)GaCl4 between 4000 and 400 cm−1 was obtained. In the FT-IR, the peak at 3087 cm−1 is the characteristic adsorption stretching vibration of the ArC–H group, shifting to lower frequency at 3090 cm−1 corresponding to the isoquinoline ring. The absorption peaks at 831 and 878 cm−1, assigned to bending vibrations out of the plane of (C–H) isoquinoline, shift to higher frequency than those of free isoquinoline (827 and 860 cm−1). It is indicated that there exists some weak interaction between the C–H and GaCl4−.
3.2 Crystal structure of (BIQL)GaCl4
The single crystal structure analysis reveals that (BIQL)GaCl4 contains a pair of ions, one cation (BIQL)+ and one GaCl4−anion. It has a monoclinic lattice within its asymmetric unit. The ORTEP diagram of the asymmetric unit is shown in Fig. 2. The expected intra-molecular bond lengths and angles in (BIQL)GaCl4 show an almost tetrahedral geometry with Ga–Cl bond lengths in the range of 2.1760–2.1793 Å and angular [Cl(2)–Ga(1)–Cl(4) 107.710(18)°, Cl(3)–Ga(1)–Cl(4) 111.187(18)°] distortions of less than 1.7567°. The crystal lattice consists of one-dimensional pillars along the a-axis (Fig. 3). The BIQL cation forms a pillar with the butyl group sticking out of the isoquinoline ring plane torsion angle [C(1)–N(1)–C(10) 119.30(13)°, and C(2)–N(1)–C(10) 119.78(13)°]. The lengths of Cl(4)⋯H(3) and Cl(1)⋯H(2) are 2.926 and 2.862 Å, respectively, beyond hydrogen bond distance, the chlorine atoms Cl(4) and Cl(1) of (BIQL)GaCl4 are almost in the plane of isoquinoline, and distances of Cl(2)⋯H(8)(1) of the border upon layer molecular and Cl(3)⋯H(7)(6) of the molecular border upon the same layer are 2.935 and 2.857 Å, respectively [(1) −X + 1, Y + 1/2, −Z + 1/2; (6) −X + 1,−Y,−Z]. It is indicated that π electron rings of isoquinoline interleave stack in layer and the layer distance is 3.431 Å (Fig. 3, b-axis). The cation and anion of (BIQL)GaCl4 arrange orderly in pair and the results indicate that coulombic attraction is more dominant than that in hydrogen bonding, although some local weak hydrogen bonds exist in the molecules in the same layer and/or border layer.
4 Conclusion
The room-temperature ionic liquid of (BIQL)GaCl4 is of lamellar structure as mainly π electron plane of isoquinoline interleave and its layer distance is 3.431 Å, connected with GaCl4− structure size. The π electron ring is stacked interleaving between two layers. The results of cation and anion of (BIQL)GaCl4 arranged orderly in pair indicate that coulombic attraction is more dominant. Simultaneously, the weak local hydrogen bonds C–H⋯Cl exist in the molecules in same layer and/or border layer.
Supplementary material: The supplementary material has been sent to the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK, as supplementary materials No. CCDC: (for (BIQL)GaCl4).
Acknowledgement
The project was sponsored by SRF for ROCS, SEM (No. [2004]176).



Vous devez vous connecter pour continuer.
S'authentifier