1 Introduction
Algeria's municipal sewage treatment plants generate around 1,000,000 m3 of sewage sludge annually. The waste sludge formed during wastewater treatment contains constituents collected at different stages of the wastewater treatment process resulting in different compounds of agricultural value (including organic matter, nitrogen, phosphorus and potassium). Moreover, organic pollutants, pathogens and concentrated metals (e.g., aluminum and iron) may also be present in excess [1,2] due to the use of metal salts such as Al2(SO4)3 as coagulants during water purification processing. In many countries, high treatment costs further increase the likelihood of ineffective or incomplete treatment, which poses direct and immediate risks to the environment and human health.
For example, extensive use of aluminum-rich sludge in agriculture may contaminate the soil and nearby waterways. The form in which aluminum is present in solution is crucial to its impact on the environment. The inorganic ionic forms are more reactive and more toxic with respect to the fauna and flora. In humans, abnormally high aluminum levels have been linked to important pathologies [3]. Therefore, metal removal from sludge is a desirable pre-treatment prior to its use as a fertilizer or soil conditioner.
The electrokinetic (EK) technique , also referred to as “electro-remediation”, “electro-reclamation” or “electrochemical remediation”, is widely used to separate and extract charged contaminants from soils [4–6] and other polluted areas [7,8] and has been shown to successfully decontaminate sludges containing a variety of contaminants [9,10]. The significance of this technique is its low operational cost and potential applicability to a wide range of contaminant types [11,12]. The electrokinetic technique needs a low-level direct current between electrodes to remove contaminants. The process induces physicochemical changes in the applied media, leading to species transport by coupled mechanisms, such as electromigration, electroosmosis, and electrolysis of water [13].
While electromigration and electroosmosis are the most important mechanisms in the electrokinetic removal of contaminants from soils and sludges. The electrolysis of water also plays a part as it produces hydrogen ions in the anode compartment, which causes an acid front to migrate through the porous media. This in turn causes contaminants to be desorbed and/or dissociated and results in an initiation of electromigration (i.e. the transport of ions and polar molecules under the influence of an applied electric field). Thus, the applied electrical potential gradient contributes to electroosmosis (i.e. the flow of an ionic liquid under the action of an applied electric field relative to a charged surface).
As mentioned above, while electromigration has been commonly applied to extract metals from sludges, especially heavy metals [9,10], few have investigated aluminum removal. The topic is a critical one given aluminum's well known toxicity. Initial work by Cherifi et al. [14,15] has investigated the influence of some parameters such as sludge pH on the electrokinetic removal of aluminium from a sludge formed during water potabilization treatment. In the present work, the effects of prolonging the treatment time in conjunction with controlling the cathode's solutions are investigated.
2 Materials and methods
2.1 Water potabilization treatment sludge
The sludge used in the present work was collected from a drinking water treatment plant in Annaba, Algeria immediately after the stage of raw water clarification with sulfate aluminum as the coagulant. The recovered sludge was dried in an oven at 105 °C for 24 h before EK and analysis.
2.2 Chemical and physical analysis
Chemical and physical analysis of dewatered sludge was conducted. The chemical analyses have been reported extensively elsewhere [14]. Metal cations contained in the sludge were analyzed by atomic absorption (Perkin Elmer 3110), and the anions were assayed by ion chromatography (ICS 3000) using an Ion pack AS 18column. Concentrations of the main inorganic species contained in the dry sludge have also been previously reported [15]. The morphological structure and qualitative elemental composition of the sludge were analyzed by using scanning electron microscopes (Jeol.Fine Coat.ion sputter JFC 1100) and (Jeol, JSM-330A).
2.3 Electrokinetic experiments
The EK experiments were conducted in a 14 × 4 × 5 cm glass cell (Fig. 1) consisting of three compartments: a cathode reservoir (2 cm in length), an anode reservoir (2 cm in length) and a sludge specimen chamber (10 cm in length). Fiber glass filter paper of about 500 μm was used to enhance the transport of ions toward the electrode compartments and to prevent sludge particles from flowing into the electrode compartments. Two sets of graphite rod electrodes were installed at each side of the sludge specimen immediately behind the filters to prevent the electrode–electrolysis reactions. A constant-voltage gradient was used to maintain the net rate of the electrolysis reaction at a constant level and to minimize complicated current boundary conditions during the experiment.
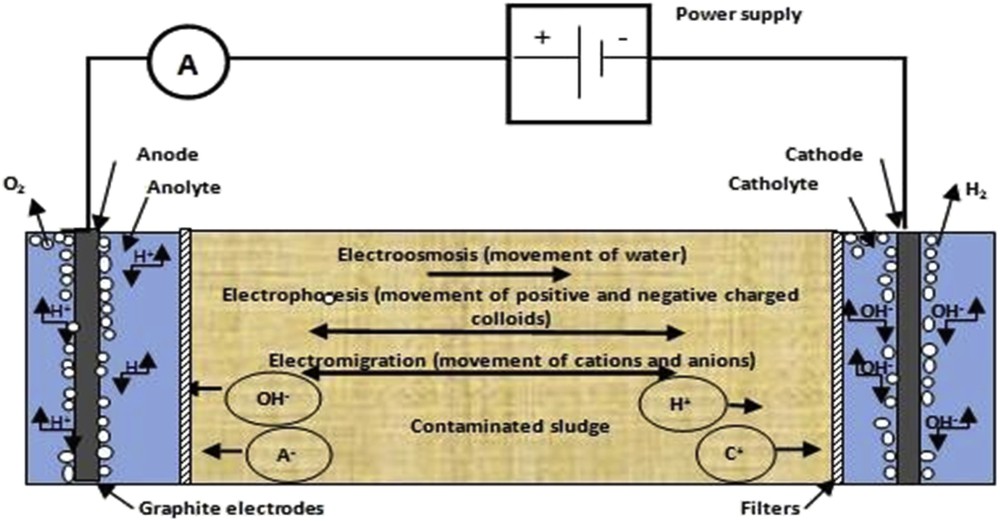
Schematic representation of the experimental device.
About 75 g of the prepared sludge was packed into the electrokinetic cell for each experiment. The anode and cathode electrolyte solutions were placed into the electrode compartments. The level of the processing fluid in the anode and cathode chambers was kept the same to prevent hydraulic gradient across the sludge in the cell. During the experiments, the overall current that drops across the EK cell was measured, and the pH variation of both the electrode solutions and sludge bed was also measured.
After the EK treatment, the central compartment was cut into five equal sections. The sampling and analysis were performed for each section separately. The concentration of aluminum in the sludge and both electrode solutions was measured.
2.4 Acidification of sludge
For the first electrokinetic test EK1, 75 g of dewatered sludge was diluted with 100 mL of tap water for 10 days; the two electrolytic compartments also contained tap water at the same level of sludge of the bed. The effect of sludge pH was investigated in EK2 by mixing 75 g of dewatered sludge with 100 mL of acetic acid solution 3 M, and the cathode compartment was also conditioned with the same solution, while the anode compartment contained tap water for a period of 10 days of run time. In an attempt at optimizing the electromigration process, EK3 was monitored under the same conditions of EK2 for a more extended period of 30 days. The study was undertaken based on the hypothesis that prolonging the running time would offer more opportunities for charged contaminants to join their electrodes. All tests were monitored at 25 °C and under a 10 V current.
3 Results and discussion
3.1 Properties of water potabilization treatment sludge
To preserve the environment from pollution of sewage sludge which may contain heavy metals, organic compounds and other toxic pollutants, different jurisdictions have selected profoundly distinctive levels. The aluminum concentration of 105 g kg−1 in our studied sludge exceeds 2.5 times its content level in the Quebec standards [16]. While the content of heavy metals is below the French norms set by the technical requirements for speeding sludge on agricultural land (Decree No. 97-1133 of 08/12/97), the high aluminum content reported is related to the use of aluminum salt during the raw water coagulation step. Scanning Electron Microscopy (SEM) images in Figs. 2 and 3 provide information about the morphology and qualitative elemental composition of the sludge generated by the potable water treatment process.

SEM picture of the dewatered sludge at magnifications of: a) 5 μm and b) 200 μm.
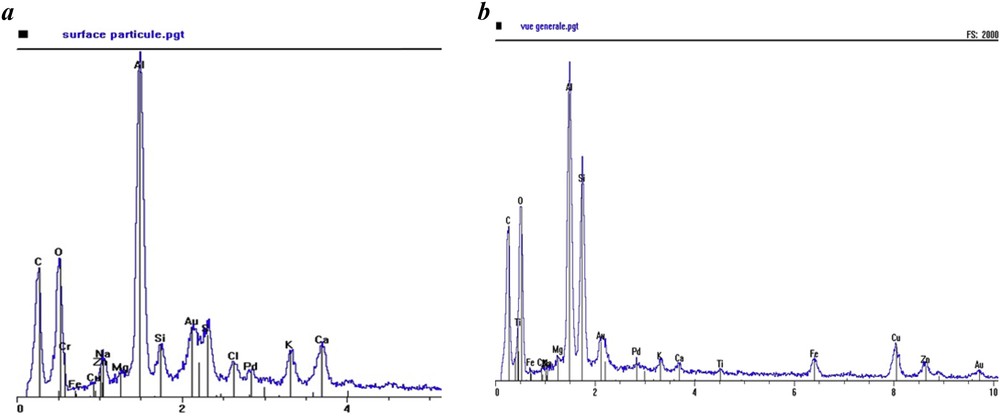
Spectrum analysis by SEM of the studied sludge: a) particle surface and b) general view.
Atomized sludge has different particle sizes and its constituents accumulate in the form of aggregates. The existence of metals and especially the strong presence of aluminum are confirmed by the SEM micrograph. The images clearly show that aluminum is the dominant component in the dewatered sludge. However, the properties of such sludge are highly variable and dependent upon both the type of raw water and the chemical composition of coagulant [17].
3.2 Electrokinetic experiments
3.2.1 Current profile during electrokinetic treatment
Fig. 4 shows the changes in the electrical current that occurred during the electrokinetic experiments. The current values generally reached the peak at the start of testing. However, the current gradually declined. This has been observed by others [4,18]. A higher electrical current was observed in the test monitored with the acetic acid enhancement. Higher current were obtained at about 52 mA (EK2) and 60 mA (EK3) and then gradually decreased to lower values after a number of days: 7 days for EK2 and 10 days for EK3. In EK1, the current dropped below 10.37 mA within a few hours. The initial increase of current was probably due to the dissolution of salts and minerals, and the greater solubility of the cationic contaminants at a lower pH, which produced a pore solution with a high ionic strength. Initially, when the voltage gradient was first applied, the current is low, because it takes time for the solution to migrate into the sludge from the electrode reservoirs and for the salts and/or contaminants to dissolve. This generally occurs within a few hours. The initial voltage gradient reaches a peak value due to the strong ionic concentration and then, the current gradually decreases, because the cations and anions electromigrated toward their respective electrodes. In addition, the products of the electrolysis reactions or other chemical species may reduce the current by neutralizing the migrating ions. For instance, H+ ions migrating towards the cathode could be neutralized by OH− ions migrating towards the anode, thereby forming water and diluting the number of ions in solution.
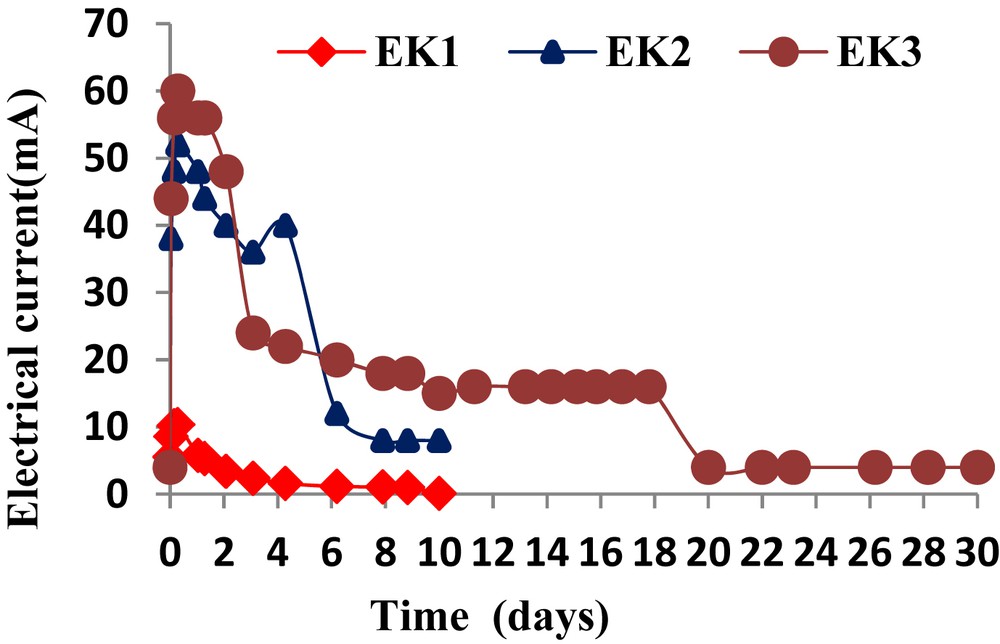
Evolution of the electrical current with time.
3.2.2 pH profiles during and after EK treatment
During the experiment, variations of pH in the anode and cathode electrolyte solutions were monitored (Fig. 5), and at the end of the experiment the pH in the different sludge sections was also measured (Fig. 6). The electrolysis of water in the anode compartment generated H+ ions with the liberation of O2 (Eq. (1)). For this reason, the overall pH of the anode electrolyte solution was low over the period of the experiments. On the other hand, at the cathode the water reduction occurs, and hydroxyl ions (OH−) are formed (Eq. (2)) with the liberation of H2, which justifies the high pH values reached at the cathode during the experiment EK1
| 2H2O → O2 + 4H+ + 4e− | (1) |
| 2H2O + 2e− → H2 + 2OH− | (2) |
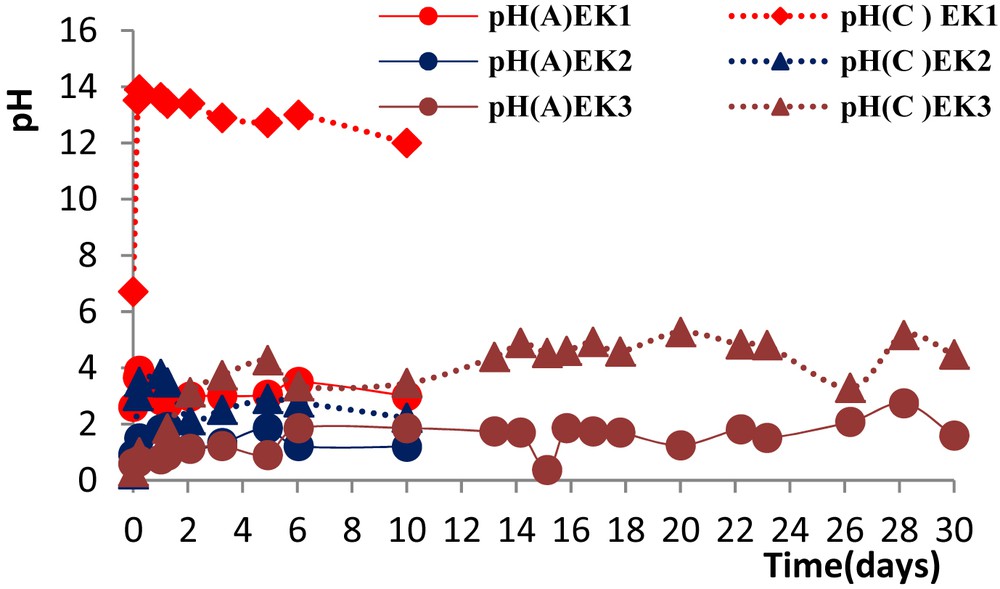
pH of anode and cathode electrolyte reservoirs versus time during the EK removal experiments.
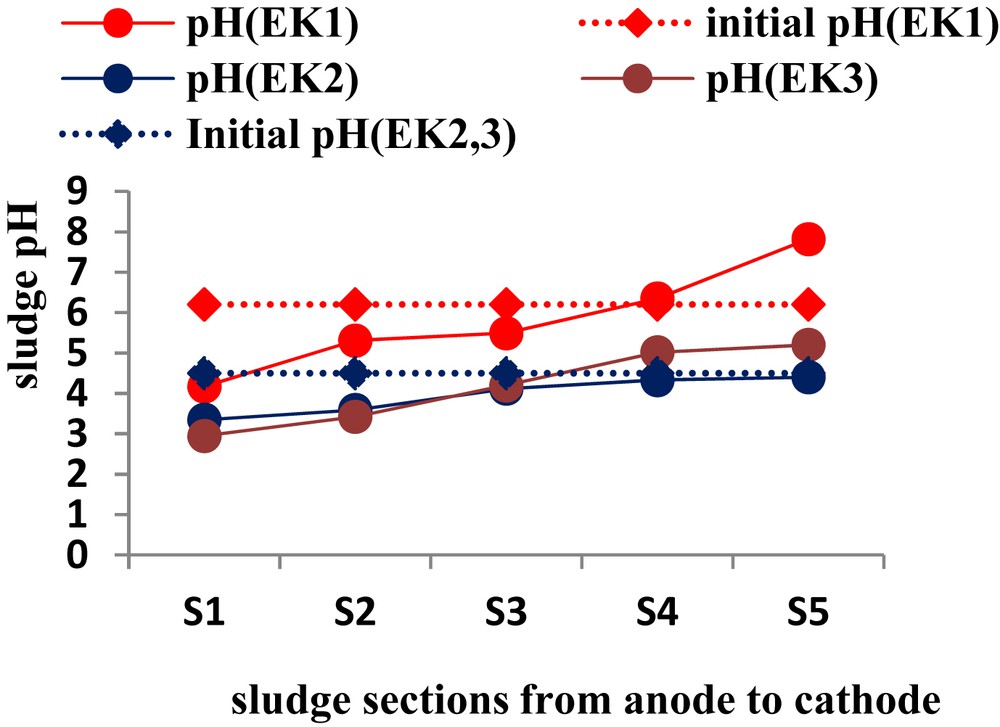
pH profile in drinking water treatment sludge.
The opposing production of hydroxide ions at the cathode (EK2, and EK3) was neutralized by the addition of acetic acid. pH in the cathode did not exceed 4.78 for EK2 and 5.16 for EK3.
Fig. 6 shows the normalized distance from the anode versus the sludge pH for all the tests; it can be seen that in the EK1 without acidification the overall pH in the sludge bed did not decrease sufficiently during the treatment. It varied from 4.17 to 7.82 by the end of the test. This illustrates that an acid front of solution, which was generated by the electrolysis reaction at the anode, migrated from the anode towards the cathode, and this solution significantly lowered the pH through the first sludge section from the anode. Conversely, it is also evident from this figure that an alkaline solution, generated by the electrolysis reaction at the cathode, migrated towards the anode and increased the pH in the sludge region nearest to the cathode. However, in EK2 and EK3, the initial mixture of the sludge and the addition of CH3COOH (3 M) to the cathode for a prolonged period [10 and 30 days (EK2 and EK3)] aimed to recover aluminum in the locker to ensure the passage of current. This was done to also control the neutralization acid–base reactions generated by the electrolysis reactions keeping the acidity of the sludge to a pH of 4.4 and 5.2 for EK2 and EK3. At these pH values, the solubility of aluminum is maximum, thereby facilitating their mobility and subsequent recovery in the cathode compartment. This is not valid for EK1 given the basic pH values on the cathode side where the possibility of strong adsorption/precipitation of metal studied.
3.2.3 Aluminum removal
Based on its solubility when it is alone in a simple aluminum solution containing 10−5 M of aluminum, Al (III) has a low solubility and starts precipitating as Al(OH)3 at a pH value of approximately 4.5 [19]. Therefore, the Al(III) ion that was electromigrating towards the higher pH region close to the cathode, might precipitate as Al(OH)3. Fig. 7 shows the recovery of aluminum in each section of sludge and electrode compartments at the end of experiments conducted with and without enhancement. Observable in EK1 was a decrease in the total aluminum concentration in the first three sludge sections from the anode and a considerable increase in the second sludge section from the cathode chamber. Al was not easily removed in the test without enhancement, which is probably due to the insufficient desorption and dissolution of adsorbed and/or complex metal by the acid front, as it migrated from the anode. However, in EK2 and EK3, less than 7.31%–8.13% of aluminum was found in the three sections nearest to the anode, and 32.52%–28.45% was found in the two sections nearest to the cathode. Finally, 28.04%–33.33% of aluminum was recovered in the cathode chamber. The removal of aluminum was more efficient with acidified sludge pH, and a substantial amount of aluminum accumulated in the cathode chamber. Generally, for EK1 and EK2, more than 60% of the initial aluminum present in the sludge was removed from the first 3 sections adjacent to the anode. This shows the potential of the technique to be highly effective at a reasonable cost to reduce its aluminum pollution in land filling and agricultural uses.
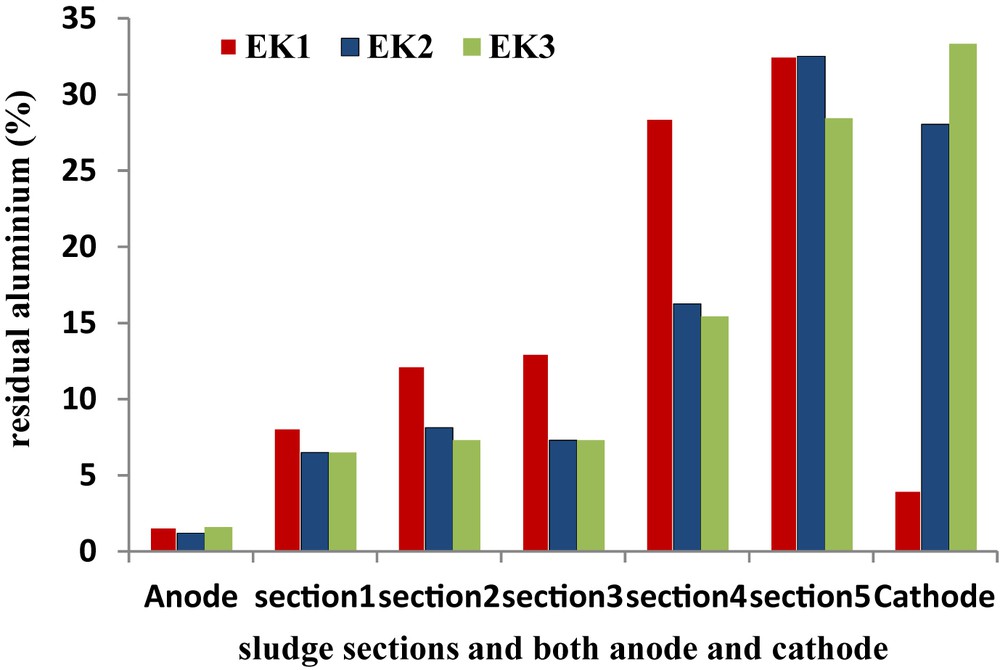
Residual aluminium in sludge and electrolyte compartments after EK treatment.
A relatively higher removal efficiency of Al was found in EK3, which might result from the easier formation of free metal ion and acetic–metal complex ions within the low pH sludge. However, aluminum was less accumulated in the second section near to the cathode, and thus, collected more in the cathode chamber. Comparing the results, the test duration did not had a significant influence on the removal efficiency of aluminum from the sludge, which was expected [20] and is probably due to the use of an open EK cell, which inhibits the displacement of ions. Consequently, under the experimental conditions applied in this study, the sludge pH profile is the most significant factor in predicting the effectiveness of the electrokinetic removal of metals.
3.3 Energy consumption
According to Yuang et al. [20], the overall expense for EK treatment was classified to four parts: 35–40% for electrode construction, 27–32% for electricity and materials, 17% for labor, and 16% for licenses and other fixed costs. Among these, the cost of the electricity and materials were considered as operational costs. Energy expenditure is calculated using the following equation:
| (3) |
In our constant-voltage tests, the energy expenditure is directly related to the processing time. Fig. 8 shows the calculated energy consumption in kWh kg−1 of sludge processed in all of the experiments performed in this study. By comparing EK1, EK2 and EK3, the energy needed in EK1 and EK2 increased when an acid was used as a processing fluid. Additional energy was also needed when the testing duration increased from 10 days to 30 days (EK2 versus EK3). In summary, the required ESSC was directly related to the processing fluid composition and the processing time. These phenomena have been seen by other researchers in related studies (e.g., [20,21]). Higher aluminium removal efficiency was also observed in the test that consumed higher electrical energy.
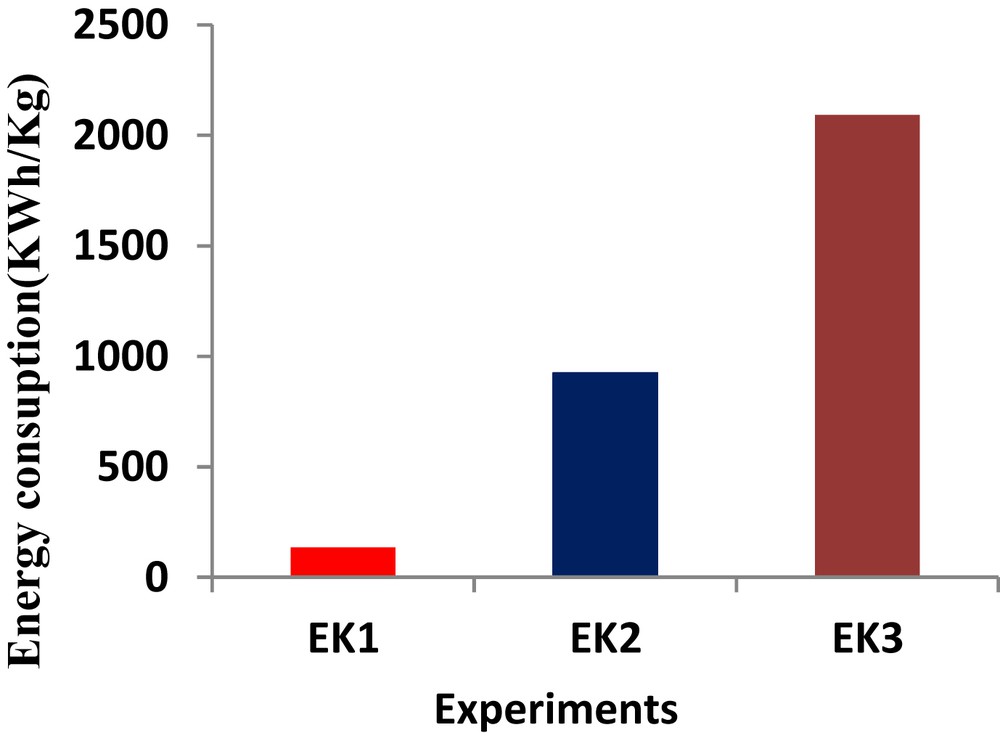
Energy consumption for various EK tests.
4 Conclusions
With the presented laboratory tests on the electrokinetic removal of aluminum from drinking water sludge, the following conclusions can be drawn:
- 1 As the acid front generated by the electrolysis of water at the anode compartment migrated towards the cathode, the complex and/or adsorbed aluminum was dissolved and desorbed, along with the migration of the acid front.
- 2 Little aluminum removal occurred in non-acidified drinking water sludge when tap water was present in both electrode reservoirs. However, significant aluminum removal occurred in the sludge when the pH at the cathode and the anode chambers was controlled, along with acidification of the sludge. Hence, the efficiency of the electrokinetic process may be increased by adjusting the sludge pH using appropriate treatments, such as, chemical preconditioning and conditioning of anode and cathode electrolyte solutions.
- 3 Aluminum removal performance by the EK process can be further improved by choosing an appropriate processing fluid. While prolonging the processing time did not enhance effectively the aluminum removal efficiency under the tested operational conditions, the increase of the potential gradient indicates that further investigation is warranted.


