1 Introduction
2-Amino-2-chromene derivatives represent an important class of chemicals being the main components of many natural products, and are widely employed as pigments, cosmetics, photoactive materials, and potential biodegradable agrochemicals [1–3]. These compounds are generally prepared through the multicomponent reactions of aromatic aldehyde, malononitrile and activated phenol in the presence of a basic catalyst in organic solvent. Recently, various modified procedures have been reported to construct this heterocyclic system, such as tetrabutylammonium bromide (TBAB) [4], γ-alumina [5], hexadecyltrimethylammonium bromide (HTMAB) [6], nanosized magnesium oxide [7], diazabicyclo[2.2.2]octane (DABCO) [8], 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) [9], nanostructured diphosphate Na2CaP2O7 [10], disodium hydrogen phosphate (Na2HPO4) [11], benzyltrimethylammonium hydroxide (triton B) [12], thiourea dioxide [13], nanozeolite clinoptilolite [14], hydroxyapatite and sodium-modified hydroxyapatite [15], amberlyst A21 [16], [Cu(2,2′-bipyridine 1,1′-dioxide)2·2H2O]2+-supported SBA-15 [17], guanidine supported on magnetic nanoparticle Fe3O4 [18], amino-functionalized MCM-41 [19], potassium phthalimide [20], 4-dimethylaminopyridine functionalized polyacrylonitrile fiber (PANDMAPF) [21], potassium sodium tartrate [22], and silica-supported piperazine [23]. At the same time, some methods including the use of microwave irradiation [24–25], ultrasonic irradiation [26] and grinding [27] have also been developed to promote this reaction. However, some of the reported methods require prolonged reaction time, volatile organic solvents, special apparatus and laborious workup procedure.
Poly(ethylene glycols) (PEGs) [28–30] and ionic liquids (ILs) [31–33] have attracted considerable attention in the last decade, due to their intrinsic chemical and physical properties, such as good thermal and chemical stability, low or null vapor pressure, the possibility of recycling, and the ability to act as efficient solvents or supports for reagent and catalyst immobilization. The functionalized ionic liquids which incorporated poly(ethylene glycol) moieties into cationic units make the link between the two distinct but very similar fluids, generating an attractive group of compounds that find applications across a range of disciplines, including extractions, biphasic systems, gas separations, carbon capture, carbohydrate dissolution, polymer chemistry, organic synthesis and catalysis [34–39]. Recent studies have demonstrated that ether-functionalized ionic liquids tend to reduce toxicity than their aliphatic-substituted counterparts [40]. Some functionalized ionic liquids, such as 1-butyl-3-methyl imidazolium hydroxide ([Bmim][OH]) [41], N,N-dimethylaminoethylbenzyldimethyl-ammonium chloride ([PhCH2Me2NCH2CH2NMe2][Cl]) [42], 1-(2-aminoethyl)-3-methylimidazolium imidazolide ([2-aemim][im]) [43], 2-hydroxyethanaminium formate, 3-hydroxypropanaminium formate, 2-hydroxyethanaminium acetate and 3-hydroxypropanaminium acetate [44], have been successfully used as catalysts for the synthesis of 2-amino-2-chromenes.
Due to the wide-ranging applications of these heterocyclic compounds and the advantages of poly(ethylene glycol)-based ionic liquids, the further development of catalysts that are recyclable easily and efficiently is a desirable goal. In continuation of our work about green synthesis in the presence of the poly(ethylene glycol)-grafted functionalized dicationic ionic liquid [45–53], herein we designed and prepared a novel poly(ethylene glycol) grafted triethylamine functionalized dicationic ionic liquid ([TEA-PEG800-DIL][Cl]) and applied it as an efficient and recyclable catalyst for the synthesis of 2-amino-2-chromenes in aqueous medium.
2 Results and discussion
The synthetic route of the poly(ethylene glycol) grafted triethylamine functionalized dicationic ionic liquid is illustrated in Scheme 1. Two methods for preparing [TEA-PEG800-DIL][Cl] were investigated. The first method was the classical approach with the quaternization of PEG-800 bridged di-imidazolium compound (2) and 2-chloro-N,N-diethylethan-1-amine hydrochloride. However, this method suffered from tedious workup and it was impossible to purify the ionic liquid. Alternatively, the other method was found to be much better. 2-Chloro-N,N-diethylethan-1-amine hydrochloride reacted with imidazole and NaOH to afford N,N-diethyl-2-(1H-imidazol-1-yl)ethanamine (3) firstly, and then quaternized with PEG-800 dichloride (1); the target ionic liquid (4) could be obtained in high purity. According to the thermal gravimetric analysis (TGA), [TEA-PEG800-DIL][Cl] is quite stable since it does not decompose below 250 °C (Fig. 1). In addition, the solubility of [TEA-PEG800-DIL][Cl] was determined at room temperature. In general, it is soluble in dichloromethane, ethyl acetate, acetonitrile, ethanol, N,N-dimethylformamide (DMF) and water, while insoluble in hexane, cyclohexane, petroleum ether and diethyl ether.
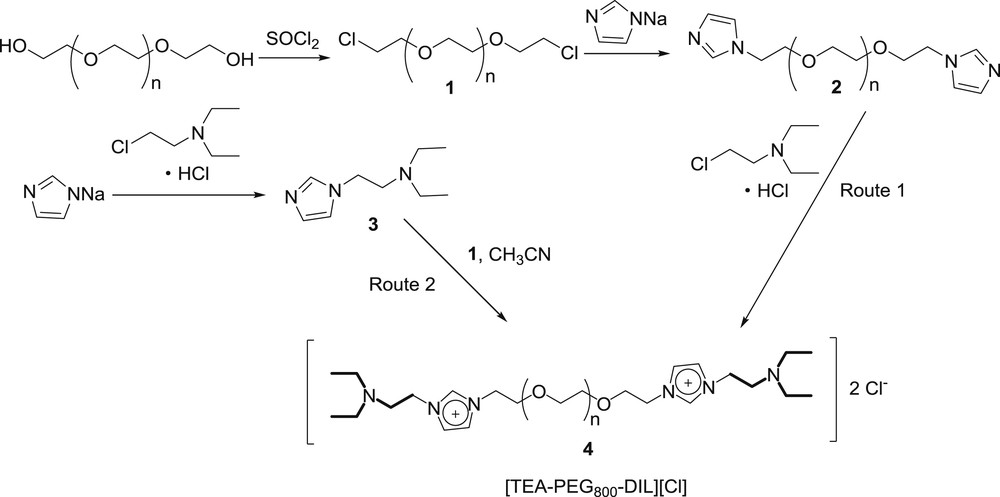
The synthesis of [TEA-PEG800-DIL][Cl].

The TGA spectrum of [TEA-PEG800-DIL][Cl].
In order to evaluate the catalytic activity of [TEA-PEG800-DIL][Cl], the reaction of 4-methylbenzaldehyde, malononitrile and 1-naphthnol was selected as a model reaction, and selected results from our screening experiments are listed in Table 1. When [TEA-PEG800-DIL][Cl] was used as both catalyst and solvent, only 64% yield was obtained, which might result from the inefficient mass transfer due to the high viscosity of the ionic liquid (Table 1, entry 1). However, none of the desired product was detected when the reaction was carried out in water under reflux for 25 min without any catalyst (Table 1, entry 2). Further optimization suggested that the amount of catalyst had a strong effect on this process. The yield was increased with the addition of [TEA-PEG800-DIL][Cl] and the optimal amount of catalyst was 5 mol% (Table 1, entries 3–7). Furthermore, low temperatures decelerated the reaction and led to lower yields (Table 1, entries 8–9).
Optimization of the reaction conditions.a
| Entry | Amount of catalyst | Temperature/°C | Solvent | Time/min | Yield/%b |
| 1 | 10% | 100 | – | 25 | 70 |
| 2 | – | 100 | H2O | 25 | – |
| 3 | 1% | 100 | H2O | 25 | 38 |
| 4 | 3% | 100 | H2O | 25 | 74 |
| 5 | 5% | 100 | H2O | 25 | 92 |
| 6 | 7% | 100 | H2O | 25 | 92 |
| 7 | 10% | 100 | H2O | 25 | 93 |
| 8 | 5% | 80 | H2O | 35 | 78 |
| 9 | 5% | 90 | H2O | 25 | 86 |
a Reaction conditions: 4-methylbenzaldehyde (2 mmol), malononitrile (2 mmol), 1-naphthol (2 mmol), water (2 mL).
b Isolated yield.
To explore the application of this method, the scope of the substrates was evaluated with a variety of aromatic aldehydes under the optimal condition. The results are summarized in Table 2. It is found that the substituents of the aromatic aldehyde and the position of the hydroxyl on the naphthol dramatically influence the reaction. Aromatic aldehydes bearing electron-donating groups (such as methyl, hydroxy, methoxy) (Table 2, entries 1–4) required longer reaction times and provided lower yields than those bearing electron-withdrawing groups (such as halide and nitro) (Table 2, entries 6–11). It is worth noting that ortho and meta substituents did not significantly hamper the reaction (Table 2, entries 9–11). The heterocyclic aldehyde (such as furyl aldedyde) was also demonstrated to be an efficient reagent for this reaction (Table 2, entry 12). Furthermore, the activity of 2-naphthol was lower than that of 1-naphthol because of the electronic effect (Table 2, entries 13–15).
Synthesis of 2-amino-2-chromenes by [TEA-PEG800-DIL][Cl] in water.a
| Entry | R | Naphthol | Time/min | Product | Yield/%b | Mp/°C | Lit Mp/°C |
| 1 | 4-CH3 | 1-Naphthol | 25 | 8a | 92 | 205–206 | 206–207 [41] |
| 2 | 4-OH | 1-Naphthol | 25 | 8b | 91 | 250–251 | 249–251 [4] |
| 3 | 4-CH3O | 1-Naphthol | 30 | 8c | 91 | 191–192 | 190–192 [42] |
| 4 | 3-CH3O-4-OH | 1-Naphthol | 35 | 8d | 89 | 136–137 | 136–138 [42] |
| 5 | H | 1-Naphthol | 20 | 8e | 93 | 216–217 | 215–217 [8] |
| 6 | 4-Cl | 1-Naphthol | 15 | 8f | 95 | 246–247 | 245–248 [8] |
| 7 | 4-Br | 1-Naphthol | 15 | 8g | 94 | 240–242 | 241–243 [8] |
| 8 | 4-F | 1-Naphthol | 12 | 8h | 96 | 232–234 | 232–233 [41] |
| 9 | 2-NO2 | 1-Naphthol | 15 | 8i | 95 | 241–242 | 241–242 [8] |
| 10 | 3-NO2 | 1-Naphthol | 10 | 8j | 95 | 218–220 | 217–219 [8] |
| 11 | 4-NO2 | 1-Naphthol | 10 | 8k | 96 | 233–235 | 233–235 [14] |
| 12 | 2-Furyl | 1-Naphthol | 25 | 8l | 92 | 168–170 | 169–171 [42] |
| 13 | H | 2-Naphthol | 25 | 8m | 90 | 286–288 | 287–288 [41] |
| 14 | 4-CH3 | 2-Naphthol | 30 | 8n | 87 | 271–273 | 270–272 [27] |
| 15 | 4-Cl | 2-Naphthol | 15 | 8o | 92 | 223–225 | 222–224 [27] |
a Reaction conditions: aromatic aldehyde (2 mmol), malononitrile (2 mmol), naphthol (2 mmol), [TEA-PEG800-DIL][Cl] (0.1 mmol), water (2 mL), 100 °C.
b Isolated yield.
In view of green chemistry, the catalyst was further explored for the reusability by the model reaction of 4-methylbenzaldehyde, malononitrile and 1-naphthnol. Upon the completion of the reaction, the product was isolated by filtration while the filtrate containing the catalyst was reused in subsequent reactions without further treatment. As shown in Fig. 2, the reaction medium could be recycled at least five times without considerable decrease of activity.
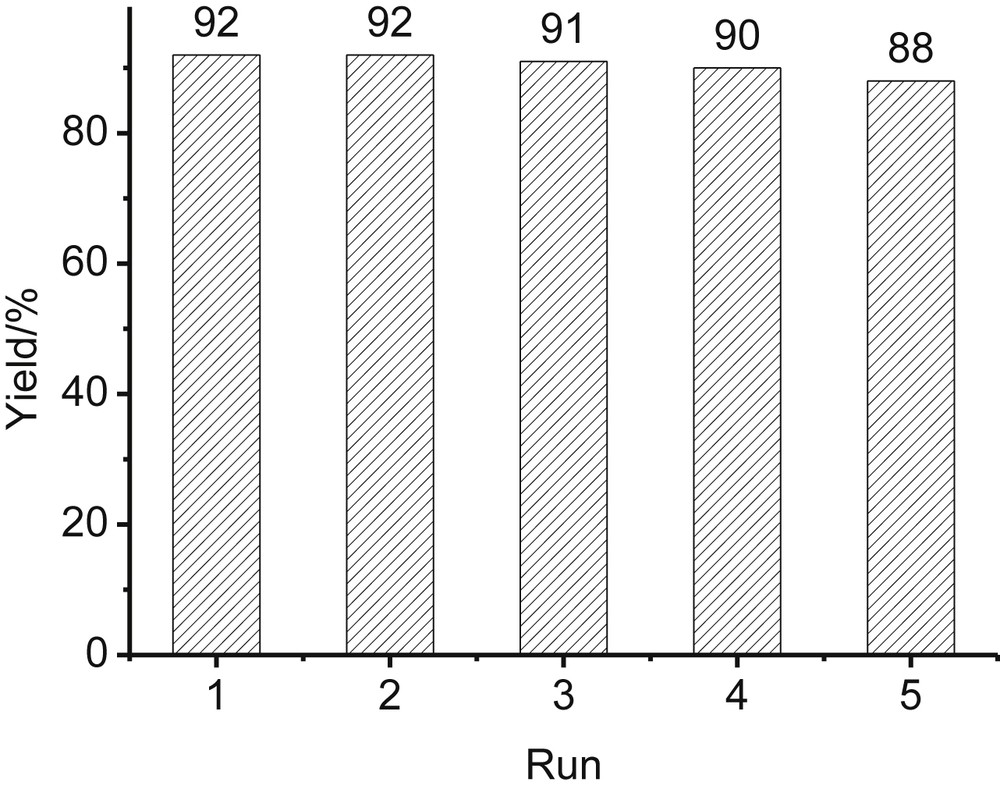
The recycling of the [TEA-PEG800-DIL][Cl]/H2O system.
The efficiency of our newly synthesized catalyst for the synthesis of 2-amino-2-chromenes was further evaluated by comparing its performance with previously reported methods in the literatures. These comparative experiments were based on the synthesis of compounds 8a from 4-methylbenzaldehyde, malononitrile and 1-naphthnol, and the results are listed in Table 3. The results show that this method is superior to some of the earlier methods in terms of yield and reaction time.
Comparison of different catalysts for the synthesis of 8a.
| Entry | Catalyst | Conditions | Time (min) | Yield (%) |
| 1 | [TEA-PEG800-DIL][Cl] | 100 °C, H2O | 25 | 92 (present work) |
| 2 | [Bmim][OH] | 100 °C, H2O | 30 | 91 [41] |
| 3 | Silica-supported piperazine | Reflux, CHCl3 | 1860 | 63 [23] |
| 4 | Nanozeolite clinoptilolite | Reflux, H2O | 30 | 88 [14] |
| 5 | Sodium-modified hydroxyapatite | Reflux, H2O | 180 | 79 [15] |
| 6 | Nanostructured diphosphate Na2CaP2O7 | Reflux, H2O | 300 | 72 [10] |
A probable mechanism for the synthesis of 2-amino-2-chromene derivatives is also outlined in Scheme 2. Only one side is displayed because the [TEA-PEG800-DIL][Cl] is symmetrical. We assume that [TEA-PEG800-DIL][Cl] acts as the Brønsted proton scavenger in the reaction. The first step is the formation of the olefin catalyzed by [TEA-PEG800-DIL][Cl], which is formed in situ by Knoevenagel condensation of aromatic aldehyde and malononitrile. [TEA-PEG800-DIL][Cl] also catalyzes the generation of the naphtholate anion, which reacts with the dicyanoolefin, followed by cyclization to give the iminium ion. After hydrolysis, the final product could be obtained. Thus, the existence of [TEA-PEG800-DIL][Cl] is essential for both steps.
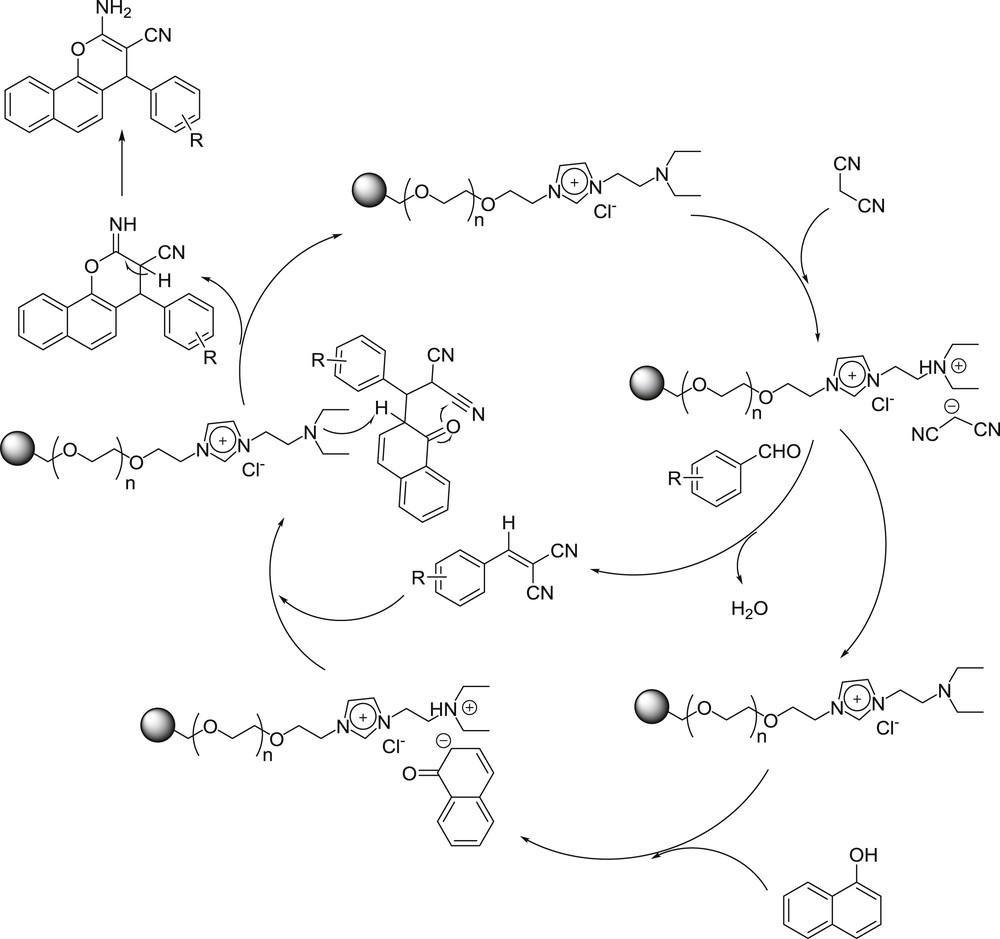
The proposed mechanism for the synthesis of 2-amino-2-chromene derivatives by [TEA-PEG800-DIL][Cl].
3 Conclusions
In conclusion, we have synthesized a novel poly(ethylene glycol) grafted triethylamine functionalized dicationic ionic liquid from the commercially available starting materials, and successfully used as a catalyst for the synthesis of substituted 2-amino-2-chromenes through one-pot three-component condensations of aromatic aldehydes, malononitrile, and naphthol in aqueous media. The attractive features of this method are environmentally friendly, mild reaction conditions, compatible with various functional groups, easy workup and recyclability of reaction medium.
4 Experimental
4.1 General remarks
All of the reagents and solvents were purchased from commercial suppliers and used without further purification. 1H NMR and 13C NMR were recorded on a Bruker (Rheinstetten, Germany) Avance III 500, and tetramethylsilane (TMS) was used as the internal standard. Mass spectra were taken on an Agilent (Santa Clara, CA, USA) liquid chromatography–mass spectrometry (LC–MS) 1100 series instrument in the electrospray ionization (positive electrospray ionization (ESI)) mode. IR spectra were recorded from KBr disks with a Shimadzu (Kyoto, Japan) IRPrestige-21 FT-IR spectrometer. The thermo gravimetric analysis (TGA) was performed on a TGA/SDTA851e thermal analyzer (Switzerland-Mettler Toledo). Samples were loaded into an aluminum oxide crucible and heated at a rate of 20 °C·min−1 from 50 °C to 600 °C under N2. All melting points were determined on a digital melting-point apparatus.
4.2 Synthesis of the poly(ethylene glycol) grafted triethylamine functionalized dicationic ionic liquid
4.2.1 Synthesis of PEG-800 dichloride (1)
A stirred solution of PEG-800 (20.0 g, 25 mmol) and pyridine (4.0 mL, 50 mmol) in CH2Cl2 (120 mL) was cooled in an ice bath to 0 °C, and SOCl2 (4.0 mL, 55 mmol) was added dropwise within 30 min, and then the ice bath was removed and stirring was continued at room temperature for 12 h. After completion, the solvent was removed under vacuum, the residue was then dissolved in ethyl acetate to allow the pyridine hydrochloride to be precipitated and filtered off. Evaporation of the solvent under reduced pressure yielded PEG-800 dichloride (18.6 g, 89% yield). 1H NMR (500 MHz, CDCl3) δ: 3.75-3.73 (t, J = 5.0 Hz, 4H), 3.67–3.60 (m, 65H)
4.2.2 Synthesis of the PEG-800 bridged di-imidazolium compound (2)
Imidazole (3.7 g, 2.4 equiv) was melted at around 100 °C and NaOH (1.8 g, 2.0 equiv) was added while stirring. After the solid NaOH disappeared, toluene (50 mL) was added for azeotropic removal of water for 3 h. Then, the toluene was evaporated under reduced pressure. Subsequently, PEG-800 dichloride (18.6 g) and CH3CN (80 mL) were added. The mixture was stirred under reflux for another 12 h. After completion, the solvent was removed by distillation. The residue was dissolved in CH2Cl2 and washed with water several times to remove unreacted materials. After removal of solvent, the intermediate PEG-800 bridged di-imidazolium compound was obtained. (17.2 g, 86% yield). 1H NMR (500 MHz, CDCl3, ppm) δ: 7.49 (s, 2H), 6.97 (d, J = 13.4 Hz, 4H), 4.06 (t, J = 5.2 Hz, 4H), 3.70 (t, J = 5.2 Hz, 4H), 3.66–3.48 (m, 71H).
4.2.3 Synthesis of N,N-diethyl-2-(1H-imidazol-1-yl)ethanamine (3)
Imidazole (8.9 g, 130 mmol) was melted at around 100 °C and NaOH (4.8 g, 120 mmol) was added while stirring. After the solid NaOH disappeared, toluene (50 mL) was added for azeotropic removal of water for 3 h. Then, the toluene was evaporated under reduced pressure. Subsequently, 2-chloro-N,N-diethylethan-1-amine hydrochloride (11.1 g, 60 mmol) and CH3CN (80 mL) were added. The mixture was stirred under reflux for another 12 h. After completion, the solvent was removed by distillation. The residue was dissolved in CH2Cl2 and washed with water several times to remove inorganic salts and excess imidazole. After removal of solvent, the desired product N,N-diethyl-2-(1H-imidazol-1-yl)ethanamine was obtained (8.2 g, 81% yield). 1H NMR (500 MHz, CDCl3, ppm) δ: 7.49 (s, 1H), 7.01 (s, 1H), 6.94 (d, J = 1.1 Hz, 1H), 3.95 (t, J = 6.6 Hz, 2H), 2.71 (t, J = 6.6 Hz, 2H), 2.51 (q, J = 7.1 Hz, 4H), 0.96 (t, J = 7.1 Hz, 6H); 13C NMR (126 MHz, CDCl3, ppm) δ: 137.40, 129.07, 119.19, 54.05, 47.40, 46.00, 11.95.
4.2.4 Synthesis of the poly(ethylene glycol) grafted triethylamine functionalized dicationic ionic liquid
A mixture of PEG-800 dichloride (1) (18.6 g) and N,N-diethyl-2-(1H-imidazol-1-yl)ethanamine (3) (8.2 g) in CH3CN (80 mL) was stirred under reflux for 7 days. After completion, the solvent was evaporated in a vacuum. The residue was dissolved in water and washed with CH2Cl2 several times to remove excess unreacted materials. After removal of water, the poly(ethylene glycol) grafted triethylamine functionalized dicationic ionic liquid [TEA-PEG800-DIL][Cl] was finally obtained. (21.9 g, 84% yield). 1H NMR (500 MHz, CDCl3, ppm) δ: 9.94 (s, 2H), 7.60 (s, 2H), 7.52 (s, 2H), 4.53–4.43 (m, 4H), 4.27 (s, 4H), 3.80–3.72 (m, 4H), 3.51 (s, 69H), 2.74 (t, J = 5.2 Hz, 4H), 2.44 (q, J = 7.0 Hz, 8H), 0.82 (t, J = 7.1 Hz, 12H); 13C NMR (126 MHz, CDCl3, ppm) δ: 137.28, 122.74, 122.34, 70.38, 70.28, 70.20, 70.15, 69.12, 52.62, 49.51, 48.03, 46.79, 11.56; IR (cm−1): 3382.00, 2866.97, 1664.08, 1563.61, 1453.93, 1348.39, 1294.61, 1249.31, 1092.35, 945.85, 844.01, 730.66; ESI-MS: 489.36 (M2+/2, n = 16), 511.28 (M2+/2, n = 17), 533.40 (M2+/2, n = 18), 555.36 (M2+/2, n = 19).
4.3 General procedure for the synthesis of 2-amino-2-chromene derivatives and recycling of catalyst
A mixture of aromatic aldehyde (5) (2 mmol), malononitrile (6) (2 mmol), naphthol (7) (2 mmol), H2O (2 mL), and [TEA-PEG800-DIL][Cl] (0.1 mmol) was taken in a round bottom flask. This reaction mixture was stirred at 100 °C for a certain time. The progress and completion of reaction were monitored by TLC. After reaction, the reaction mixture was cooled to room temperature, and the precipitated solid was broken up, filtered off and washed with water. The crude product was purified by recrystallization from methanol to give the pure product (8). All of the products are known and the data are found to be identical to those reported in the literature.
The catalyst that remained in water was reused for the next catalytic cycle without any treatment.
Acknowledgment
The authors gratefully acknowledge the support of this work by the Nanyang Institute of Technology and National Natural Science Foundation of China (No. 21002050).


