1 Introduction
Over the last several decades, much of population of Bangladesh and West Bengal switched their water supply from surface water to groundwater. As many as 10 million new domestic wells were installed, providing drinking water for over 100 million people. This large-scale transition to groundwater as the source for domestic water was motivated by the necessity of providing water free of pathogens – diarrheal diseases such as cholera were infecting millions of people. This transition to well water was readily adopted because of the convenience of having a water supply in close proximity to homes and the ease of drilling in the region's high-yielding aquifers. At the same time that drinking wells were drilled, irrigation wells were also installed across the country. Groundwater pumping for irrigation greatly increased food production enabling Bangladesh to become self-sufficient in food, even though the population nearly tripled over the last four decades. Irrigation is necessary for the newly introduced dry-season rice called ‘Boro’ that now provides more yield than the traditional rice grown during the wet season, and Boro rice cultivation and irrigation increased together from 1970 to 2002 (Fig. 1). Thus, issues of groundwater quality and quantity have become vital for both the supply of drinking water and the production of food in Bangladesh.

Cultivation of high yielding Boro rice has greatly expanded over the last several decades to cover approximately 20% of Bangladesh, or approximately 45% of the cultivatable area. Most Boro is irrigated by groundwater so extraction has also risen. Data taken from [10].
La culture de riz Boro à haut rendement s'est fortement étendue sur les dernières décennies, pour couvrir 20% de la surface du Bangladesh ou 45% de la surface cultivable. La majorité des terres à riz Boro est irriguée par de l'eau souterraine, dont l'extraction a donc aussi augmenté. D'après [10].
Tragically, much of the region's groundwater is dangerously contaminated by naturally occurring arsenic, and consumption of this water has already created severe health effects. About half of the wells in the country have concentrations greater than 10 μg l−1, now a common standard. Much of our understanding of the distribution of arsenic across Bangladesh's groundwater comes from the comprehensive work of the British Geological Survey (BGS) [6,12]. Their work shows that high arsenic concentrations may be found throughout the flood plains and delta of the Ganges Brahmaputra and Megnha Rivers, but the Delta region of the southern half of the country is the most contaminated. Yu et al. [20] combine the BGS's database with dose-response models to estimate that, if consumption of contaminated water continues, the prevalence of arsenicosis and skin cancer in Bangladesh will be approximately 2 000 000 and 100 000 cases per year, respectively, and the incidence of death from cancer induced by arsenic will be approximately 3000 cases per year. Because detailed health records are not kept in Bangladesh, these estimates were made using dose-response curves from the literature. How accurately these dose-response relationships apply to the broad population of Bangladesh remains an open question and existing epidemiological surveys show a wide spread of arsenicosis prevalence estimates [20].
2 Biogeochemistry and arsenic mobility
Researchers largely agree that dissolved arsenic in the groundwater of Bangladesh originates from the sediments. However, there is no evidence of widespread, unusually high, levels of solid phase arsenic in the aquifer material – concentrations are typically less than 10 ppm in sandy sediment and less than 100 ppm in clays and peats [14–17]. High solid-phase concentrations have been reported in the soils of irrigated fields, but these could be the result of arsenic input from groundwater irrigation and sorption to the soils [1,13]. Thus, it appears that high dissolved-arsenic concentrations are the result of particular hydrologic and biogeochemical conditions that partition arsenic from the solid to aqueous phase, but have not yet flushed dissolved arsenic from the subsurface.
The reducing conditions of almost all groundwater in Bangladesh (demonstrated by high levels of dissolved ferrous iron and methane, and low measurements of Eh), and the weak but statistically-significant positive correlation of dissolved arsenic to iron and bicarbonate, suggest that most arsenic is liberated by dissolution of iron (oxi)hydroxides, or perhaps desorption of arsenic after reduction from arsenate to arsenite [6,8,16]. The low concentrations of sulfate (and in some areas the negative correlation between arsenic and sulfate) as well as the generally reducing conditions indicate that arsenic has not been directly mobilized from sulfide minerals (e.g., [9]). However, the arsenic in the Ganges Delta sediments likely originated from sulfide minerals that weathered out of the granitic and metamorphic source rock of the Himalayas, and it remains a possibility that at the land surface, where oxygen is introduced as the water table rises and falls, sulfide minerals could be oxidized and dissolved thereby liberating arsenic. The long-term implications of such cyclical near-surface processes in a rapidly accreting
Several research teams [6,8,15,19] describe two distinct types of aquifer sediment: brown (or orange to yellow) sediment presumably containing iron (oxi)hydroxides where dissolved arsenic concentrations are low, and grey sediments where dissolved arsenic concentrations may be high. The brown sediments are found at depth in the older Pleistocene aquifers such as the Dupi Tilla formation, where low-arsenic water is obtained, as well as near the surface. Dissolved arsenic is presumably low in these sediments because of the capacity of iron (oxi)hydroxides to adsorb arsenic. Islam et al. [11] showed that arsenic is liberated from sediments collected in West Bengal by the addition of organic carbon. They do not report the in-situ arsenic concentration in the pore-water, but the sample contains iron (oxi)hydroxides and is described as coming from a transition zone between a region of oxidizing conditions and a region with reducing conditions. The role played by iron (oxi)hydroxides within the contaminated grey sediments of the Holocene aquifer, where most wells withdraw water, is much more enigmatic. Iron (oxi)hydroxides must exist, or have existed very recently, according to the theory that arsenic is released from iron (oxi)hydroxides in local sediments by organic carbon oxidation. However these iron (oxi)hydroxides have not been definitively demonstrated in the grey sediment and high concentrations of methane and hydrogen [8] in strongly reducing water indicate that geochemical conditions are not conducive to stability of iron (oxi)hydroxides. On the other hand, Swartz et al. [17] show that only small quantities of iron (oxi)hydroxides would be required to explain current geochemical conditions, and McArthur et al. [15] provides a geologic explanation for why the Ganges Delta sediment would have been deposited with relatively little iron (oxi)hydroxides. Thus, it is conceivable that slow reductive dissolution within aquifer sediments could be responsible for high dissolved arsenic concentrations, but only if the geochemical system happens to be in a state where iron (oxi)hydroxides have released almost all of their sorbed arsenic. In other words, the aquifer sediments must be poised in a geochemical state where the inventory of iron (oxi)hydroxides is nearly (or recently) exhausted, yet arsenic has not been flushed away by flowing groundwater. Other explanations, which we explore below, are that both the physical flow system and the biogeochemical system have recently been perturbed, and that dissolved arsenic originates from near-surface sediments above the aquifer.
Dissolved arsenic concentrations are maintained in grey sediment because several geochemical factors conspire to prevent arsenic that has been dissolved from sorbing back onto this aquifer sediment. First, the paucity of ferric (oxi)hydroxides means there are few adsorption sites. Second, high concentrations of other anions, such as silicate and phosphate, which compete with arsenic for surface sorption sites, are prevalent in groundwater throughout most of the arsenic-affected areas. (However, there is no convincing correlation between these anions and arsenic to indicate that these anions explain the spatial pattern of dissolved arsenic.) Appelo et al. [5] have also suggested that competition by bicarbonate, which correlates better with arsenic over the country, might explain the distribution of dissolved arsenic. By this scenario, oxidation of organic carbon liberates arsenic indirectly through desorption caused by its byproduct, bicarbonate, rather than directly by reduction of iron oxides or arsenate. However, equilibrium chemical modeling using the parameters measured at our site indicates that the effect of bicarbonate on arsenic sorption is less than that of silicate and no more than phosphate [17]. These conceptual geochemical models are further complicated by the fact that arsenic likely adsorbs to surfaces of many solid phases other than oxihydroxides, such as magnetite, green rust, and potentially siderite and apatite. Arsenic is known to sorb readily to magnetite [7] and the results of our density and magnetic separations show that the magnetite fraction has the highest arsenic concentration by weight [17].
Several research groups postulate that irrigation pumping may flush arsenic from aquifers [8,15]. Harvey et al. [8,9] support this contention by comparing concentrations sampled from irrigation wells to concentrations from drinking water wells to show that irrigation wells, which flush much greater quantities of water, have significantly lower arsenic concentrations. At a national scale, Ali [4] estimated, using 1996 irrigation data, that each year, groundwater irrigation removes from aquifers, and then applies to fields, 1 kg of arsenic per hectare of irrigated area. Thus, in 2001 (Fig. 1), irrigation pumping would have extracted much more than a million kilograms of arsenic per year from the aquifers and moved it into rice fields. On the other hand, some evidence suggests that arsenic concentrations may rise after pumping commences. Kinniburgh et al. [12], van Geen et al. [19], and McArthur et al. [15] all provide strong statistical evidence that arsenic concentrations in domestic well water correlate to the age of the well, suggesting that arsenic concentrations may rise after a well is installed, perhaps because irrigation wells, which have much greater effects on the local groundwater system, are installed in the region at the same time as the domestic wells where arsenic is measured. Can these apparently contradictory suggestions of both falling and rising arsenic concentrations be reconciled? Clearly pumping removes some arsenic from the aquifer. (In fact, irrigation pumping can be viewed as analogous to ‘pump-and-treat’ groundwater remediation methods employed in North America and Europe, but without the ‘treat’ and with extraction rates that are actually higher than at many sites!) However, increased flushing may be concurrent with increased arsenic input either by simple transport or release caused by input of organic carbon from the surface sources, or potentially drawn from peat layers. This concurrent enhancement of both sinks and sources of arsenic to the groundwater system by human perturbation could potentially create very complex temporal and spatial behavior of dissolved arsenic.
At our site in Munshiganj, processes appear to be competing to both increase and decrease arsenic concentrations: arsenic is being extracted from the system and radiocarbon dating of dissolved carbon indicates that arsenic has been mobilized recently [8]. The radiocarbon data show that detrital organic carbon has not driven recent biogeochemical reactions. The byproducts of microbial activity, both inorganic carbon and methane, have much younger dates than the dissolved organic carbon or the sediment, and the concentration of this inorganic carbon is much larger than that of the older organic carbon. In fact, at 20-m depth, the inorganic carbon has levels of carbon-14 higher than 100% modern. This is carbon from bomb testing, so it entered the aquifer in the last 50 years. At three 30-m wells, dissolved inorganic carbon (DIC) radiocarbon ages are 462, 770, and 823 years, much younger than the local sediment and the radiocarbon age of dissolved organic carbon (DOC) (3636, 1538, and 1890 years). Tree roots and burrowing animals are unlikely to penetrate below 10 m because the aquifer remains saturated all year. (Later, for the hydrologic model, we will consider whether roots in villages may penetrate through 6 m of clay to reach the aquifer.) Thus, dissolved carbon with a radiocarbon age younger than the sediment age was transported downward, and laterally, by flowing groundwater. The presence of young DIC and old DOC in the same water does not appear to result from the mixing of young, DIC-containing water and old, DOC-containing water. The concentrations correlate strongly (i.e. water high in young DIC is also high in old DOC); they do not follow a mixing-line that would have a negative correlation. Thus it appears that the older DOC was mobilized from the sediment concurrently with the production or inflow of young DIC. McArthur et al. [15] argue that buried peat deposits have provided the organic carbon that drives reduction at our field site, but they do not attempt to reconcile the different radiocarbon ages of dissolved organic carbon and inorganic carbon.
3 Geochemical profiles with depth
In this section, we consider how geochemical characteristics vary with depth in aquifers, and hence how chemical conditions relate to flow paths and groundwater age. Fig. 2 compares depth profiles of solute concentrations measured at our field site in Munshiganj with averaged values from the BGS and DPHE [6] dataset. Our site in the Munshiganj district (Fig. 4) is located 30 km south of Dhaka and 7 km north of the Ganges. It contains a small intensive-study area (100 m2) with 25 sampling wells that extract water from depths ranging between 5 and 165 m below the land surface. We also monitor water levels at 87 other locations in the surrounding 16-km2 region. We describe some similarities between results at our single site and the averaged national dataset that suggest some general characteristics of geochemical evolution and transport across the region.
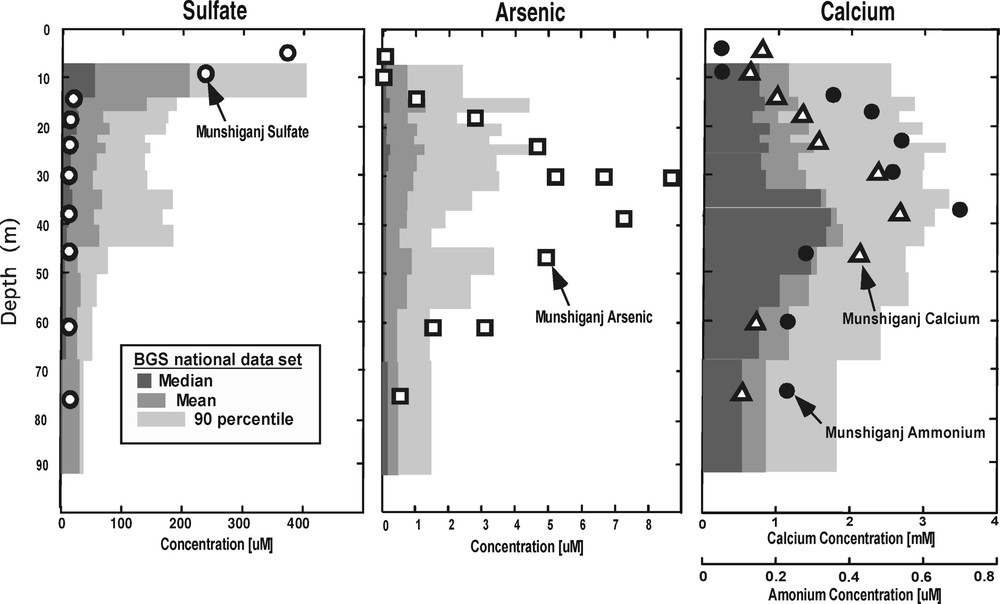
Vertical profiles of groundwater chemical characteristics at our intensive study site in Munshiganj (yellow triangle in Fig. 4) compared to the mean, median and 90-percentile of the shallowest 2848 samples from the country-wide BGS dataset [6], binned into depth intervals of ∼ 200 samples each.
Profils verticaux des caractéristiques chimiques de l'eau souterraine à notre site d'études intensives de Munshiganj (triangle jaune sur la Fig. 4) comparés à la moyenne, à la médiane et au quantile 0,9 de l'échantillon 2848 le plus superficiel de la base de données BGS à l'échelle du pays [6], regroupées en classes de profondeur d'environ 200 échantillons par classe.
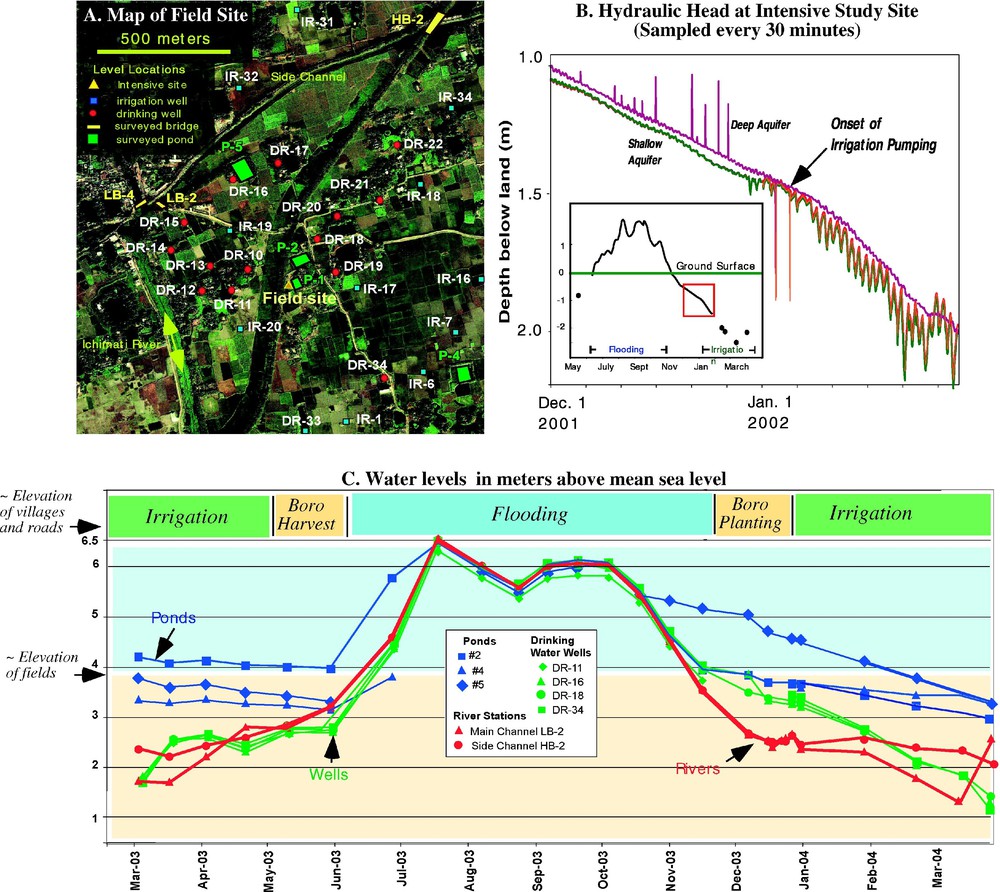
(A) Map of the water level recording locations for the data shown in (B) and (C). (B) Pressure transducer data from the intensive field area during 2001–2002, with the data from December and January expanded to show the effects of irrigation pumping. (C) Hand-measured water levels in drinking water wells, ponds, and rivers at locations shown in (A).
(A) Carte de la position des enregistreurs de niveau d'eau pour les données de (B) et (C). (B) Enregistrements des capteurs de pression du site d'études intensives en 2001–2002, avec les enregistrements de décembre et janvier dilatés pour mettre en évidence les effets des prélèvements pour irrigation. (C) Mesures manuelles des niveaux d'eau dans les puits d'eau potable, les mares et les rivières aux emplacements donnés en (A).
3.1 Arsenic as a function of depth
At the site in Munshiganj, dissolved arsenic has a distinct peak at approximately 30 m depth, but we find no chemical characteristic of the solid sediment to explain this pattern [8,17]. Dissolved components in the groundwater indicate an arsenic source that is hydrologically upgradient. Furthermore, several types of data, when taken together, suggest a relation between the arsenic peak and groundwater flow patterns. The hydraulic conductivity data (Fig. 3A) indicate variations less than an order of magnitude, but suggest that a lower conductivity layer at 22 m may work to separate horizontal flow paths. The head data (Fig. 3B) show that, at least during some times of year, there is convergent vertical flow that mixes water from above and below 30 m, thus horizontal flow must accelerate to conserve mass at this depth. Evidence for groundwater mixing at 30 m is supported by the 18O profile (Fig. 3C); the range of isotope ratios at 30 m is consistent with mixing of lighter water from above and heavier water from below. The heavier water below 30 m could represent infiltrated pond, river or rice-field water that has been subject to relatively more evaporation. Measurable tritium values are found to a 60-m depth [9], indicating the presence of at least a component of water that is less than 40 years old throughout this depth interval. Furthermore, the tritium values show a sharp decrease to less than 1 T.U. below 24 m, so that the peak of high dissolved arsenic corresponds with the depth where older water mixes with younger recharge.
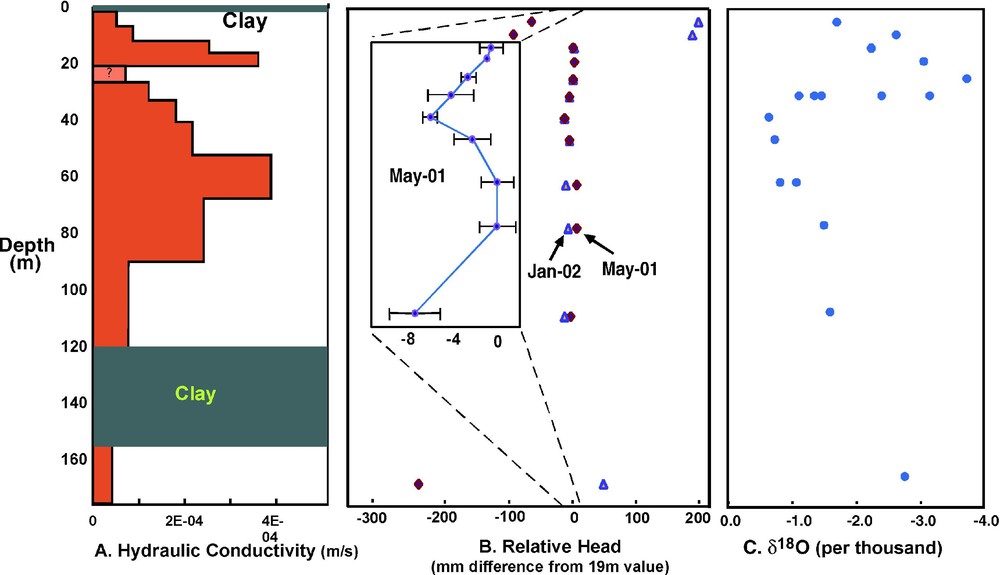
(A) Horizontal hydraulic conductivity as estimated from pump test at the intensive study site. The conductivity at 22 m is uncertain because of well silting. (B) Hydraulic heads relative to the 20-m piezometer during the dry season, early irrigation (January) and late irrigation (May). The inset represents heads in May, measured with a manometer that obtains relative differences to within 1 mm. (C) Oxygen-18 isotope ratios relative to SMOW.
(A) Conductivité hydraulique horizontale estimée à partir des essais de débit au site d'études intensives. La conductivité à 22 m est incertaine par suite de l'ensablement du puits. (B) Charges hydrauliques dans le piézomètre de 20 m pendant la saison sèche, le début de l'irrigation (janvier) et la fin de l'irrigation (mai). L'encart représente la charge en mai, mesurée avec un manomètre qui donne une précision relative de 1 mm. (C) Rapports isotopiques de l'oxygène 18 par rapport au standard SMOW. Masquer
(A) Conductivité hydraulique horizontale estimée à partir des essais de débit au site d'études intensives. La conductivité à 22 m est incertaine par suite de l'ensablement du puits. (B) Charges hydrauliques dans le piézomètre de 20 m pendant la saison ... Lire la suite
Kinniburgh et al. [12] and McAthur et al. [15] both describe typical depth profiles of arsenic concentrations as ‘bell shaped’. Although this pattern is not obviously evident from the national dataset (Fig. 2), it is found at a variety of study sites [8,15]. Depth trends can also be considered within different geologic regions, and Yu et al. [20] tabulate the geologic regions of Bangladesh where there is a statistically significant trend of decreasing arsenic with depth. (they did not consider non-monotonic trends). Their geostatistical analysis shows that the trend of decreasing arsenic concentrations with depth explains much of the differences in arsenic concentrations between nearby wells – neighboring wells often have different arsenic levels because one withdraws water from deeper in the aquifer where arsenic concentrations are lower.
3.2 Sulfate, calcium and ammonium as a function of depth
At the Munshiganj site, the inverse relation of dissolved sulfate with As (Fig. 2), and the presence of acid volatile sulfide (AVS) in the sediments near the dissolved As peak, suggest that arsenic has not been released directly by sulfide oxidation. Instead, low dissolved sulfur levels appear to limit the precipitation of sulfides near the arsenic peak.
The BGS and DPHE [6] dataset also shows a distinct, statistically robust, pattern of decreasing sulfate with depth. The rapid decrease of sulfate with depth from the countrywide dataset is consistent with the previously described scenario of cyclical sulfide weathering with water-table oscillations. Furthermore, monthly measurements of sulfate by the BGS and DPHE [6] in very shallow dug wells are also consistent with this scenario, with some showing seasonal time trends and very high concentrations.
At the Munshiganj site, peaks in ammonium and calcium mirror the sharp peak in arsenic (Fig. 2), and these solutes suggest inflow and oxidation of organic carbon, and subsequent mixing of solutes. Ammonium is an oxidation product of natural organic matter, and calcium may be released from solid carbonate after organic carbon oxidation [17]. Furthermore, dissolved organic carbon with radiocarbon ages in accord with sediment ages also shows a bell-shaped profile, indicating that recalcitrant detrital organics have been liberated form sediment concurrent with the processes that liberate arsenic. The BGS and DPHE [6] do not report ammonium for their countrywide dataset, but the profile of calcium with depth appears to show a ‘bell shape’ similar to that found at our site.
3.3 ‘Bell-shaped’ depth profiles
The bell-shaped pattern of solutes (arsenic, ammonium, calcium, and dissolved carbon) with depth is typical of vertical profiles of contaminant plumes that originate from localized surface sources before moving laterally and downward into aquifers at many sites throughout the world. After solute enters an aquifer at a location, a plume migrates laterally away from this source location and is pushed deeper into the aquifer by recharge from above. Thus, the basic hydrologic process of lateral transport creates vertical profiles of contamination with typical “bell” shapes. Such profiles have been characterized on multitudes of groundwater contamination sites in the North America and Europe where release of pollutants at distinct surface locations contaminates groundwater as migrating plumes.
Simple geometric considerations indicate that groundwater fluxes at our site have a large lateral component, consistent with conventional understanding for horizontal alluvial aquifer systems. After water enters the aquifer from the surface, it must move laterally to reach discharge areas. At our site, the lateral velocity component must be relatively large in much of the aquifer because the spacing between discharge areas, irrigation wells and river channels, is as great or greater than the thickness of the aquifer
Thus, the vertical component of flow is important for calculating the chemical flux from the surface down into the aquifer, but the horizontal component of groundwater is important for determining the pattern of solute migration from the source. While one can postulate that the bell-shaped pattern at our site results from a particular local source of organic carbon (i.e. a nearby pond, river or rice field), it remains an open question whether the much less distinct bell-shaped pattern of the national BGS and DPHE [6] data, or similar patterns observed at other small-scale sites, results from the same mechanisms. It is intriguing that the bell-shape pattern of arsenic is mirrored by the distribution of well depths (not shown) in the BGS and DPHE [6] dataset. Most wells withdraw water from depths near 25 m. Similarly, at our site in Munshiganj, nearly all wells (drinking and irrigation) are completed at the same depth where we find the arsenic peak, 30 m. This correspondence may suggest a hydraulic component to the cause of arsenic mobility with depth. Because deeper wells are more expensive, villagers complete wells only to a depth sufficient to provide adequate yield, perhaps below the low conductivity layer we find at 24 m (Fig. 3). Thus, a relationship may exist between the depth at which the aquifer is more conductive to groundwater flow, and the depth of maximum arsenic concentration. However, such speculation has not been confirmed by detailed hydrogeologic studies.
The correspondence of the depth profiles measured at our site to the average BGS and DPHE [6] profiles should not be interpreted as indicating that the same processes are occurring everywhere. There is spatial heterogeneity in aqueous chemical characteristics, much of which is likely induced by the complex mosaic of recharge and discharge areas at the surface, which have variable water chemistry. However, the correspondence does suggest the existence of dominant reactive-transport processes that affect the subsurface biogeochemistry at many locations across the country.
4 The relation of groundwater flow and chemical transport to arsenic concentrations
Much of the existing literature on groundwater flow in Bangladesh focuses on isotopic inference, and not on physical understanding of flow and solute transport. However, several simple lines of reasoning, and some isotope data, indicate that flow and transport play important roles in subsurface arsenic concentrations.
4.1 Irrigation pumping
Fig. 1 indicates that, at a national level, pumping alone drives significant groundwater flow. Boro rice requires ∼1 meter of irrigation annually [10], and roughly 20% of the country cultivates groundwater irrigated Boro. Therefore, assuming a porosity of 20%, this withdrawal cycles 5 m of groundwater flow annually below rice fields, which averaged over the non-irrigated areas amounts to 1 m of vertical groundwater circulation. Thus, pumping-induced groundwater flows reach the depths of 20 or 30 m within two or three decades, on average.
Some researchers have suggested that pumping does not greatly change groundwater flow because natural flow is already rapid [3]. Pumping must change the pattern of flow because it introduces spatially distinct sinks (well screens) into the groundwater system, altering the natural 3-D flow paths of groundwater. Because hydraulic heads are described by a 3-dimensional parabolic differential equation, inserting a sink in the system will change heads, and hence flow throughout the domain. Groundwater flow is not like rapid river flow; extracting groundwater changes the gradients everywhere, including up-gradient flow.
However, it is possible that pumping would not greatly change the average residence time of groundwater in the aquifer. Irrigation extraction could be offset by decreased natural discharge to rivers during the dry season. Although pumping must change groundwater flow paths, it would not change the average residence time if there was no return flow (re-infiltration of irrigation water) and total irrigation withdrawals equaled the reduction in discharge to the rivers. Outflow from the system would simply be switched from discharge to rivers to increased evapotranspiration from crops. Such a scenario assumes perfectly efficient irrigation (i.e. no return flow) and an exact tradeoff between discharge to rivers and pumping. However, since there is return flow, there must be an increase in flow through the system (i.e. reduction in average residence time) even if the pumping withdrawal is offset by a decrease in discharge to the river. Current modeling work will address this question directly by estimating fluxes with and without irrigated agriculture.
4.2 Groundwater tritium
Measured tritium values indicate groundwater flow through at least the upper 30 m is often rapid. The largest set of tritium data has been gathered by the IAEA [2] over the last thirty years and their final report concludes that groundwater ages in the upper 100 m are generally less than 100 years. Their recent plot [3] shows a somewhat more complex picture, with tritium values greater than 1 tritium unit (TU) penetrating below 25 m in 1999, but not in 1979 prior to the onset of large-scale irrigation pumping. At our site, we also find tritium values above 0.2 TU to a depth of 60 m indicating a component of water less than 40 years. Clearly, some areas of stagnant water exist, either because of low hydraulic conductivity (e.g., clay layers) or because of local recharge and discharge patterns [18]. However, the common occurrence of measurable tritium in groundwater indicates that groundwater flow is sufficient to rapidly transport arsenic, or solutes that interact with arsenic, through many regions.
4.3 Patchiness of dissolved arsenic
Arsenic concentrations are extremely patchy over small spatial scales. In the vertical dimension, high concentrations can be found within tens of meters of low concentrations. If there is any groundwater flow across these concentration gradients, arsenic will be transported from areas of high concentrations to areas of low concentration, and vice versa. If flow does not cross these arsenic gradients, then the patchy spatial pattern of dissolved arsenic must correspond to the spatial pattern of groundwater flow paths. In either case, understanding the effects of flow and transport is important for understanding the behavior of dissolved arsenic; groundwater is either transporting arsenic, or the spatial pattern of flow paths is related to the spatial pattern of dissolved arsenic.
The complex mosaic of recharge and discharge areas at the surface provides one simple potential explanation for this spatial complexity in the subsurface. Because the topography is essentially flat, local flow cells dominate the flow system. Discharge areas (irrigation wells and rivers) and recharge areas (pond, rice fields, and rivers) are all spaced within 10's and 100's of meters of each other as demonstrated by the satellite image in Fig. 4. This spacing of sinks and sources drives groundwater flow through a complex transient three-dimensional system of flow paths that also must have spatial scales of tens and hundreds of meters.
4.4 Why is dissolved arsenic still in the groundwater?
The short residence time (decades) of contaminated groundwater, as indicated by both tritium concentrations and pumping rates, suggests that arsenic is being flushed from the system. Indeed, estimated groundwater ages, and the rates of pumping-induced circulation described above, combined with the low estimated retardation coefficients for arsenic, raise the question of how can such high concentrations of dissolved arsenic remain in the groundwater? Kinniburgh et al. [12] estimate the retardation factor for arsenic to be as low as two. We also find that, where arsenic is high, the retardation factor is less than ten [8,17]. These values imply a residence time for arsenic of decades to centuries in aquifers that are thousands of years old. So why is the arsenic still there? Three possible explanations are: (1) groundwater flow has not been rapidly flushing aquifers over centuries, but is rather the result of the recent advent of massive irrigation; (2) geochemical conditions have recently shifted to mobilize arsenic; or (3) dissolved arsenic is provided hydrologically upgradient of the sampling wells by near-surface processes. We suspect that aspects of all three explanations are important to varying degrees at different locations, and that their relative importance will only be elucidated when the groundwater flow system is better understood.
5 The annual cycle of groundwater flow in central Bangladesh
Discharge and recharge of groundwater follows a dramatic annual cycle, with floodwater returning the aquifer to full conditions every year. After floodwaters recede in the fall, groundwater is lost both by discharge to rivers and by evapotranspiration, which is enhanced by irrigation pumping. Then in the late spring, groundwater is recharged by direct rainfall, and by rising river levels.
5.1 Hydrologic characteristics of the Munshiganj field site
Here we present hydrologic data that characterize the annual cycle of water levels at our field site and demonstrate several features of groundwater flow that are important for arsenic mobilization and transport. Fig. 4A shows a map of a region within our larger study area. The hydrostratigraphy of our site (Fig. 3) is representative of the deltaic depositional environment that is pervasive in Bangladesh and West Bengal. The surface consists of flat fields, composed of organic-rich clay/silt, local ponds, which may be up to several meters deep and generally have low-permeability clay bottoms, and the Ichamati River, which traverses the site and eventually flows into the Ganges approximately 7 km away. The surface overbank clay/silt extends to a depth of approximately 2–3 m below ground surface, and acts as confining or semi-confining layer to the underlying sand aquifer. Villages and roads rise 2–3 m above the level of the fields, and are constructed on clay/silt borrowed to excavate ponds. The approximately 80–100 m thick sand aquifer is fairly homogeneous at our site, although localized peat and silts have been reported in other locations. Groundwater wells are completed in this aquifer, most screened at around 30-m depth.
The time trends of water levels for irrigation wells, domestic wells, ponds and a river are graphed in Fig. 4B and C for the 12 months from May 2003 to April 2004. Some basic features of these water levels are the following: (1) during monsoon flooding, all water levels, and therefore hydraulic heads, are nearly identical so groundwater flow essentially ceases; (2) as the flood recedes, but before irrigation begins, the river level drops more quickly than the groundwater level, which drops more quickly than pond levels; (3) when irrigation begins in January, the decline of the river level slows but groundwater decline accelerates such that the hydraulic head in the aquifer falls below that in the river; (4) when monsoon flooding begins in June, all water levels rise rapidly together as both rain and river flooding inundate the land, and the aquifer is recharged. Our water-level observations allow us to develop a conceptual model of water flow in the vicinity of our field site. Generally, recharge enters the subsurface during flood or irrigation, and flows vertically through the overlying confining unit, pond and river bottoms into the lower aquifer. Undisturbed, the water would then flow laterally out to discharge into the rivers. Indeed, the drop in water levels in the aquifer after flooding recedes in December, but before irrigation pumping, is caused by discharge to the river.
The groundwater levels recorded every hour at our intensive study area (Fig. 4B) show that beginning in January, irrigation pumping greatly changes flow in the system. At the onset of irrigation pumping, the rate at which groundwater heads decline doubles, and dramatic diurnal head oscillations develop as pumps are turned on during the day and off at night. Also in the beginning of January, the river reverses flow direction and no longer discharges to the Ganges, but rather flows, at a relatively low rate, from the Ganges to the field area. While spatial gradients in the aquifer are very small, temporal fluctuations, caused by irregular pumping schedules, are more significant and spatially extensive, consistent with the high transmissivity of the aquifer. Heads recorded in different wells on the same day rarely differ by more than several centimeters, yet the effect of the seasonal trend over a day is more than a centimeter and the daily oscillation from irrigation pumping can be more than 20 cm. From a practical point of view, this makes it very difficult to map groundwater flow directions. In the time required to walk from one well to another, the head in both wells may change by an amount larger than the instantaneous difference in head between the two wells. Thus, the paths by which recharging water enters the aquifer are not readily apparent from the water level data in Fig. 4C and are a focus of current numerical modeling analysis. Characterizing the paths of recharge into the aquifer remains an important research question because this recharge introduces modern organic carbon, which may mobilize arsenic, or oxidants, which may immobilize arsenic, into the aquifer.
In summary, the comparison of data from our field site with the comprehensive national dataset collected by the BGS allows us to identify some general patterns of arsenic mobilization and transport. Results from a variety of research groups show that arsenic mobilization is linked to reductive processes driven by organic carbon, and a broad variety of data indicate that patterns of dissolved arsenic concentrations are affected by patterns of groundwater flow. However, the location of arsenic mobilization, and the paths by which arsenic, organic carbon (that mobilizes arsenic), and oxidants (that immobilize arsenic) move through the aquifers, all remain poorly characterized. Understanding the spatial and temporal patterns of arsenic mobilization will require better hydrogeologic models that describe how groundwater flow carries solutes into the subsurface, and flushes solutes from the subsurface. Hydrologic data from our field site indicates that groundwater flow follows a dramatic seasonal cycle as irrigation pumping removes water, irrigation return flow provides recharge, aquifers exchange with local rivers, ponds recharge aquifers, and groundwater flow nearly halts for half the year when the region is flooded.


