1 Introduction
Kimberlite magmas bring to the Earth’s surface xenoliths sampled from the lithospheric mantle underlying the cratonic areas worldwide. Such xenoliths suites are generally dominated by peridotites, but exceptionally (Roberts Victor and Bellsbank in South Africa, Mbuji-Mayi in DRC, Udachnaya in Russia, Koidu Complex in Sierra Leone [9,14,15,17,26]), eclogites are the dominant variety. Eclogitic xenoliths occasionally occur in kimberlites emplaced in off-craton or craton margin settings, and differ from their cratonic counterparts not only by their common association with lower crustal xenoliths such as granulites [12,23], but especially by their lack of diamond and other high-pressure minerals (coesite), which seems to indicate a shallower origin for these off-craton xenoliths. Along the southwestern margin of the Kaapvaal craton in South Africa, emplacement of Jurassic to Cretaceous kimberlites has sampled eclogites and other mantle derived xenoliths (clinopyroxenites, websterites) together with abundant crustal material [25]. This study focuses on the petrography and mineral geochemistry (major and rare earth elements), as well as the geothermobarometry of various mantle xenoliths sampled from five of these off-craton kimberlitic pipes.
2 Geological setting
The Kaapvaal craton is a complex assemblage of early Archean (3.0–3.5 Ga) granites greenstone terranes and older tonalitic gneisses (ca. 3.6–3.7 Ga) that covers approximately 1.2 million square kilometres [6] and that formed and stabilised between 3.7 and 2.6 Ga years ago [7,22]. It is bounded in its northern part by the Limpopo belt that represents the collisional terrane formed when the Kaapvaal and the Zimbabwe cratons collided during late Archean time, 2.7 Ga ago [7,27]. To the south and to the west, the Kaapvaal craton is flanked by two major Proterozoic mobile belts, i.e. the Namaqua-Natal belt and the Kheis belt, respectively (Fig. 1). The Namaqua-Natal belt is a complex assemblage of high-grade terranes and was formed during a Mesoproterozoic (1.2–1.0 Ga; [24]) episode of intense orogenesis during which occurred the collision, accretion and overthrusting of crustal material onto the Archean cratonic crust [27]. Kimberlitic magmatism affected the Kaapvaal craton during the Jurassic-Cretaceous period, both in the core of the craton (“on-craton”) and along its margins (“off-craton”). The nature and abundance of xenoliths brought to the surface by the kimberlites are highly variable, but we will focus here on eclogites and pyroxenites sampled by kimberlites emplaced along the south-western margin of the Kaapvaal craton, through the Namaqua-Natal mobile belt.
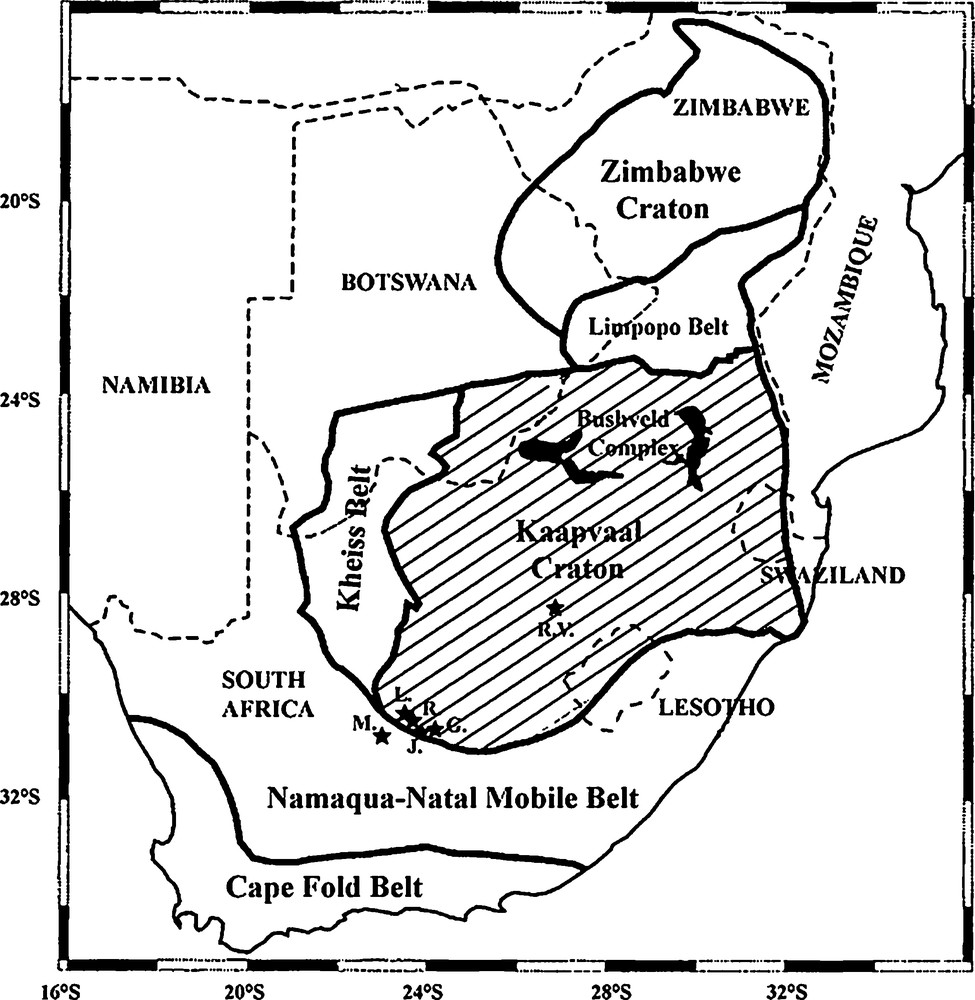
Location of the off-craton kimberlite pipes (L: Lovedale; R: Roodekraal; J: Jachtfontein; G: Goedehoop; M: Markt) as well as the on-craton Roberts Victor pipe (RV) hosting the eclogitic and pyroxenite xenoliths analyzed in this study (modified after [2,4,16]).
Localisation des pipes kimberlitiques off-craton (L. : Lovedale ; R. : Roodekraal ; J. : Jachtfontein ; G. : Goedehoop ; M. : Markt), ainsi que du « pipe » « on-craton » de Roberts Victor (R.V.) contenant les xénolites éclogitiques analysées dans ce travail (d’après[2,4,16]).
3 Petrographic description of the xenoliths
Major element compositions of clinopyroxene, garnet, orthopyroxene and accessory minerals for 44 samples from five off-craton kimberlitic pipes (Lovedale, Roodekraal, Markt, Jachtfontein and Goedehoop) were determined at the University of Toulouse III using a Camebax SX50 and at the University of Cape Town using a Cameca-Camebax electron microprobe. The concentrations of rare earth elements (REE) were determined in situ with a Perkin-Elmer ELAN 6000 ICP-MS instrument coupled to a Cetac LSX-200 laser ablation module both at the University of Cape Town and the University of Toulouse III. The nature of the studied xenoliths is highly variable: among the 44 samples in total, we identified 30 eclogites with omphacite and garnet (eclogite sensu stricto; [3]), four kyanite eclogites, one orthopyroxene eclogite, one orthopyroxene-bearing garnet clinopyroxenite, five garnet clinopyroxenites and four garnet websterites. The classification and the geographical distribution of the different samples as well as their petrographic and mineralogical characteristics are shown in Table 1.
Nature, mineralogy and texture of the different xenoliths from this study (cpx = clinopyroxene, gnt = garnet, opx = orthopyroxene, ky = kyanite)
Nature, minéralogie et texture des différents xénolites de cette étude (cpx = clinopyroxène, gnt = grenat, opx = orthopyroxène, ky = disthène)
| Classification | Locality | Modal mineralogy | Texture | Accessory minerals (< 5%) |
| Bimineralic eclogites | ||||
| 10063 | Markt | cpx 53 gnt 43 | Medium granuloblastic | Alkali feldspars, rutile |
| JJG2294 | Markt | cpx 52 gnt 46 | Medium granuloblastic | Alkali feldspars, rutile |
| MKT-01 | Markt | cpx 80 gnt 19 | Coarse granular | Rutile, phlogopite |
| 10093 | Markt | cpx 60 gnt 35 | Coarse granuloblastic | Rutile, phlogopite |
| 10053 | Markt | cpx 80 gnt 20 | Coarse granuloblastic | |
| 42163 | Roodekraal | gnt 60 cpx 39 | Coarse granuloblastic | Rutile, phlogopite |
| 42143 | Roodekraal | cpx 52 gnt 47 | Coarse granuloblastic | Rutile |
| 42233 | Roodekraal | cpx 54 gnt 46 | Coarse granuloblastic | |
| 42223 | Roodekraal | gnt 57 cpx 42 | Coarse granuloblastic | Rutile, phlogopite |
| 42153 | Roodekraal | cpx 52 gnt 45 | Coarse granuloblastic | Rutile, phlogopite |
| JJG4541 | Roodekraal | cpx 51 gnt 47 | Coarse granuloblastic | Rutile, phlogopite |
| 02083 | Lovedale | cpx 63 gnt 35 | Coarse granuloblastic | Phlogopite |
| 02/100 | Lovedale | cpx 55 gnt 45 | Coarse granuloblastic | |
| 02/200 | Lovedale | cpx 55 gnt 45 | Coarse granuloblastic | |
| 02500 | Lovedale | cpx 65 gnt 32 | Coarse granuloblastic | Rutile |
| 2/1 | Lovedale | gnt 61 cpx 38 | Coarse granuloblastic | Phlogopite |
| 026203L | Lovedale | gnt 69 cpx 30 | Coarse granuloblastic | Rutile |
| 026203N | Lovedale | gnt 75 cpx 24 | Coarse granuloblastic | Rutile |
| 026203I | Lovedale | gnt 52 cpx 46 | Coarse granuloblastic | Phlogopite, rutile |
| 026203J | Lovedale | cpx 54 gnt 46 | Coarse granuloblastic | |
| 026203B | Lovedale | cpx 50 gnt 50 | Coarse granuloblastic | |
| 026203K | Lovedale | cpx 61 gnt 37 | Coarse granuloblastic | Rutile |
| 02G30 | Lovedale | cpx 50 gnt 50 | Coarse granuloblastic | Quartz, apatite |
| HGX23 | Goedehoop | cpx 51 gnt 49 | Medium granuloblastic | Corundum, rutile |
| 20700 | Jachtfontein | cpx 51 gnt 49 | Coarse granuloblastic | Rutile |
| 20083 | Jachtfontein | cpx 57 gnt 40 | Coarse granuloblastic | Rutile, phlogopite |
| 20263 | Jachtfontein | gnt 55 cpx 45 | Medium granuloblastic | Rutile, phlogopite |
| 20293 | Jachtfontein | cpx 55 gnt 40 | Coarse granuloblastic | Rutile, phlogopite |
| 20163 | Jachtfontein | cpx 66 gnt 32 | Coarse granuloblastic | Rutile, phlogopite |
| 20253 | Jachtfontein | cpx 49 gnt 48 | Coarse granuloblastic | Rutile, phlogopite |
| Kyanite eclogites | ||||
| 42123 | Roodekraal | gt 48 cpx 47 ky 4 | Coarse granuloblastic | Rutile, phlogopite |
| JAR02213 | Lovedale | cpx 75 ky 15 gnt 10 | Coarse granular | |
| 026103 | Lovedale | cpx 62 gnt 35 ky 3 | Coarse granuloblastic | |
| 02G10 | Lovedale | cpx 50 gnt 43 ky 7 | Coarse granuloblastic | |
| Orthopyroxene eclogite | ||||
| 42103 | Roodekraal | cpx 42 opx 38 gnt 19 | Medium granular | Rutile |
| Garnet clinopyroxenites | ||||
| 20023 | Jachtfontein | gnt 60 cpx 40 | Coarse granuloblastic | Spinel, phlogopite |
| 20213 | Jachtfontein | cpx 60 gnt 40 | Medium granuloblastic | Rutile |
| JJG2275 | Markt | cpx 65 gnt 33 | Medium granuloblastic | Rutile, phlogopite |
| GHGG01 | Goedehoop | cpx 70 gnt 28 | Medium (mosaic) granuloblastic | Rutile |
| RDK-1 | Roodekraal | cpx 48 gnt 48 | Coarse mosaic granuloblastic | opx, rutile |
| Garnet websterites | ||||
| 20770 | Jachtfontein | cpx 68 gnt 17 opx 15 | Coarse mosaic granuloblastic | Spinel, phlogopite |
| 20063 | Jachtfontein | cpx 55 opx 27 gnt 18 | Medium (mosaic) granuloblastic | Spinel |
| 20043 | Jachtfontein | cpx 69 gnt 19 opx 10 | Coarse mosaic granuloblastic | Rutile, phlogopite |
| 20123 | Jachtfontein | cpx 66 gnt 29 opx 5 | Medium mosaic granuloblastic | Spinel |
All samples are medium to coarse-grained and are characterised by a predominant granuloblastic texture with a few exceptions showing a granular texture. Banding appears in one eclogite s.s. (026203J), while a slight foliation is visible in another eclogite s.s. (42153). Garnets and clinopyroxenes define wide ranges in size, from 0.2 to 5 mm (biggest crystals in sample 10093), and from 0.2 to 4 mm, respectively. Most of the eclogites studied are equigranular, but a few samples show a bimodal distribution in the grain size. Clinopyroxenes are always larger (0.5–3 mm) than garnets (0.2–1 mm) in the garnet websterites, two garnet clinopyroxenites (GHGG01 and RDK-1) and the orthopyroxene eclogite (42103). Garnets are usually subrounded to subangular, but irregular shapes are also frequently observed (sample JAR02213 is an exception, with garnet appearing as a major mass of poikiloblastic grains). Most of the garnets are clear to slightly fractured, although some highly fractured garnets occur in the most altered samples (JJG4541, 2/1) or in the rare alteration zones of the fresh samples. Garnets are all unzoned. Subrounded inclusions of clinopyroxene are very common, as well as irregular to subrounded rutile inclusions. In a few samples (026203L, 026203N, 026203K), thin exsolutions of clinopyroxene in garnet were observed. Coronas of kelyphite around the garnets are frequent. This kelyphitic material, interpreted as a decompression reaction product of the garnet during the ascent to the surface, can totally replace the smallest grains of garnet. In the garnet websterites, two garnet clinopyroxenites (GHGG01 and RDK-1) and the orthopyroxene eclogite (42103), most of the garnets occur associated with the 120° triple junctions defined by the clinopyroxenes. These garnets are subrounded to amoeboid, slightly fractured and kelyphitised. In samples where orthopyroxene is relatively common, it occurs as subhedral crystals, varying from 0.5 to 1 mm in length, and sometimes showing fractures and inclusions of clinopyroxene. Clinopyroxenes are subangular to subrounded, with frequent irregular shapes due to alteration of their rims (“spongy rims”), but abundant 120° triple junctions occur in some samples. Fractures are common and alteration has developed along them. A slight optical zonation towards the rims of grains was observed in a few samples, but chemical analyses revealed the insignificant character on a chemical level of these zonations, and average values were then calculated. In samples where a bimodal grain size distribution is present, the smallest clinopyroxenes are entirely altered. Subrounded inclusions of garnet in clinopyroxene are common (less frequent in kyanite eclogites), whereas inclusions of orthopyroxene and rutile are scarce. Clinopyroxenes frequently show mineral exsolution phenomena: orthopyroxene in many samples, spinel in samples 20123, 20063 and 20770. Triple junctions are common between the minerals, reflecting textural equilibrium of the samples. Accessory minerals include rutile, spinel, kyanite, quartz, apatite, orthopyroxene, corundum (sample HGX23) and alkali feldspars (samples 10063 and JJG2294). Alteration minerals include greenish amphibole, sulphides, chlorite, vesicles of carbonates (attributed to decompression melting during ascent) and phlogopite. The latter commonly occurs in coronas of kelyphite where it is associated to euhedral dark green spinels, in veins and along grain boundaries.
4 Major element mineral composition
Clinopyroxenes found in the kyanite eclogites (Q53.71–74.41Jd25.59–46.27Ae0–0.020) and eclogites sensu stricto (Q49.55–78.60Jd14.34–50.43Ae0–10.54) are omphacites, whereas those from garnet clinopyroxenites (En43.38–49.22Fs1.17–8.4Wo46.64–49.61), orthopyroxene-bearing garnet clinopyroxenite (En45.14Fs6.31Wo48.55) and garnet websterites (En43.09–49.31Fs1.34–9.80Wo47.12–49.66) are diopsides. Although sample 42103 could be classified as a garnet websterite due to its high modal proportion of orthopyroxene (38%; Table 1), the fact that it contains omphacites (Q70.78Jd20.05Ae9.171; Fig. 2) allows it to be classified as an orthopyroxene eclogite. Omphacites are richer in Al2O3 (5.36–14.13 wt.%) and Na2O (2.60–7.15 wt.%) than diopsides (Al2O3: 0.78–5.35 wt.% and Na2O: 0.25–2.85 wt.%, respectively), whereas the latter display higher MgO contents (13.15–17.72 wt.%) than omphacites (6.55–13.13 wt.%). Nevertheless, some overlap does exist between omphacites and diopsides, and diopsides of the garnet clinopyroxenites appear intermediate in composition between the omphacites from the eclogites and the diopsides from the garnet websterites (Figs. 3 and 4). For similar contents in Al2O3 and MgO, omphacites from sample HGX-23 (corundum-bearing eclogite) have lower contents in Na2O than those of the other omphacites (Figs. 3 and 4). Both omphacites and diopsides are very low in Cr2O3 (< 0.53 wt.%). Mg# (100* atomic Mg / [Mg + Fetotal]) values range from 66.1 to 91.3 for omphacites and from 78.1 to 94.2 for diopsides. The pyroxene-rich mantle xenoliths from the on-craton locality of Roberts Victor [14], shown for comparison in Figs. 3 and 4, have been similarly subdivided into a diopside-bearing group, rich in MgO and poor in both Na2O and Al2O3 (garnet clinopyroxenites and garnet websterites) and an omphacite-bearing group, poorer in MgO, but richer in both Al2O3 and Na2O (kyanite eclogites, eclogites and one garnet websterite, after Hatton’s nomenclature). Within each group, clinopyroxenes from off-craton xenoliths (this study) have similar compositions in Al2O3 and Na2O than clinopyroxenes from on-craton eclogites from Roberts Victor. However, clinopyroxenes in the off-craton kyanite eclogites have lower Al2O3 content compared to clinopyroxenes from on-craton kyanite eclogites, for a similar sodium content (Fig. 3). In a MgO versus Na2O diagram (Fig. 4), it is evident that for a similar Na2O content, clinopyroxenes from off-craton xenoliths have 1 to 3 wt.% less MgO than their on-craton counterparts. Clinopyroxenes from off-craton kyanite eclogites are an exception, being slightly richer in MgO, at a given sodium content, than clinopyroxenes from kyanite eclogites from Roberts Victor.

Classification of clinopyroxenes as diopsides (targets: garnet websterites; striped circles: garnet clinopyroxenites) and omphacites (empty squares: eclogites; empty circles: kyanite eclogites; stars: orthopyroxene eclogite) after Morimoto [19].
Classification des diopsides (cibles : webstérites à grenat ; cercles rayés : clinopyroxénites à grenat) et omphacites (carrés blancs : éclogites biminérales ; cercles blancs : éclogites à disthène ; étoiles : éclogite à orthopyroxène), d’après Morimoto [19] .
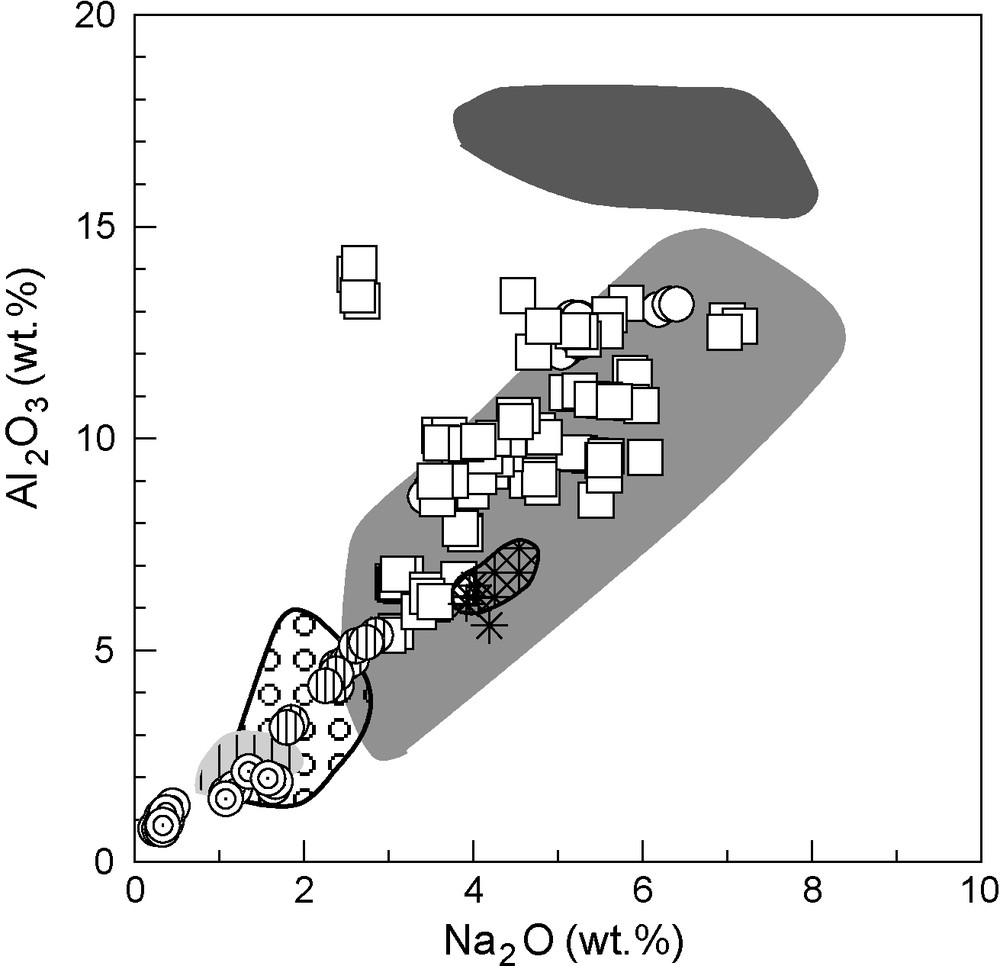
Al2O3-Na2O binary diagram (in wt.%) for clinopyroxenes. Symbols as in Fig. 2. Composition fields for Roberts Victor (after Hatton, [14]) for comparison: dark grey field for kyanite eclogites, intermediate grey field for eclogites, light grey field vertically striped for garnet clinopyroxenites, dotted field for garnet websterites and black striped field for the unique sample HRV110 (“type 1” garnet websterite where clinopyroxene is omphacite).
Diagramme de variation Al2O3-Na2O des clinopyroxènes (en % poids d’oxyde). Symboles d’après laFig. 2. Champs de composition de Roberts Victor (d’après Hatton,[14]) pour comparaison : champ gris foncé pour les éclogites à disthène, champ gris intermédiaire pour les éclogites, champ gris clair rayé verticalement pour les clinopyroxénites à grenat, champ à pois pour les webstérites à grenat et champ hachuré noir pour l’échantillon unique HRV110 (« type 1 » webstérite à grenat où le clinopyroxène est de l’omphacite).

MgO-Na2O binary diagram (in wt.%) for clinopyroxenes. Symbols as in Fig. 2. Composition fields for Roberts Victor (after Hatton, [14]) for comparison (as in Fig. 3).
Diagramme de variation MgO-Na2O des clinopyroxènes (en % poids d’oxyde). Symboles d’après laFig. 2. Champs de composition de Roberts Victor (d’après Hatton,[14]) pour comparaison (d’après laFig. 3).
Compositions of garnets that have equilibrated with diopsides (Py38.0–58.6 Alm24.4–47.7 Gr5.9–14.3) are similar to those equilibrated with omphacites (Py27.3–59.3Alm2.3–51.3Gr7.5–30.8), although the latter cover a wider range in pyrope and especially in grossular compositions. FeO* (all Fe calculated as Fe2+) contents are similar between the two groups of garnets (11.3–25.8 wt.% when in equilibrium with omphacite and 12.4–23.6 wt.% when in equilibrium with diopside), with kyanite eclogites showing a more restricted range (12.7–16.5 wt.%). All garnets display a large variation in MgO contents (7.2–16.2 wt.% and 10.0–16.2 wt.% when in equilibrium with omphacite and diopside, respectively), leading to a large variation in Mg# values for both groups (35.51–69.73 and 43.91–69.83, respectively). CaO content defines a low and restricted range for garnets that have equilibrated with diopsides (4.13–5.79 wt.%), whereas garnets from eclogites, kyanite eclogites and the orthopyroxene eclogite (sample 42103) have CaO contents spanning from 4.21 wt.% (42103) to 12.94 wt.% (HGX-23). Finally, Cr2O3 contents are low for garnets coexisting with omphacite (0–0.18 wt.%) and those from garnet clinopyroxenites (0.03–0.28 wt.%), but reach higher values for garnets that equilibrated with significant amounts of orthopyroxene (mean content of 0.39 wt.% Cr2O3 for the orthopyroxene eclogite and range of 0.21–2.14 wt.% Cr2O3 for garnet websterites). All garnets have low amounts of Na2O (< 0.21 wt.%). When compared group by group to garnets from Roberts Victor in a Ca-Mg-Fe ternary diagram (Fig. 5), garnets from off-craton xenoliths show similar contents in Ca, but are slightly enriched in Fe or/and depleted in Mg relative garnets from on-craton xenoliths.

Ca-Mg-Fe ternary diagram for garnets. Symbols as in Fig. 2 and fields as in Fig. 3.
Diagramme ternaire Ca-Mg-Fe pour les grenats. Symboles d’après la Fig. 2 et champs d’après la Fig. 3 .
5 Geothermometry–Geobarometry
Temperatures were estimated by using the gnt-cpx Mg-Fe2+ geothermometer calibrations of Ellis and Green (TEG; [10]) and Krogh (TK; [18]) for all the samples, and the two-pyroxene geothermometer of Brey and Köhler (TBK; [2]) for samples containing orthopyroxene (i.e. garnet websterites, the orthopyroxene eclogite and the orthopyroxene-bearing garnet clinopyroxenite). Ferric iron contents were estimated using the stoichiometric calculations of Droop [8]. For the samples in which clinopyroxenes may exhibit exsolutions, only not exsolved clinopyroxenes were used for the temperatures calculations. An assumed pressure of 30 kb (pressure at which many geothermometers are calibrated) was used in the calculations for off-craton xenoliths. Unfortunately, no suitable geobarometer exists for the orthopyroxene-free eclogitic assemblages, but the equilibration pressures for the orthopyroxene-bearing samples could be estimated using the Al-in-opx barometer of Brey and Köhler (PBK, [2]).
Temperatures obtained for the garnet websterites are relatively uniform and are the lowest among all studied samples, with both Ellis and Green (742–781 °C) and Krogh (669–697 °C) thermometers, although temperatures obtained with TEG are always higher (by 72 to 86 °C) than those obtained with TK. TBK results (739–820 °C) are more in agreement with the temperatures obtained with TEG than with TK. Sample 20063 yields the lowest temperatures with all calibrations (TEG: 655 °C, TK: 575 °C, TBK: 642 °C). Garnet clinopyroxenites also yield higher temperatures with TEG (715–830 °C, mean value of 754 °C) than with TK (635–748 °C, mean value of 670 °C). They appear to have equilibrated at slightly higher temperatures than the garnet websterites. It must be noted that sample 20023 plots apart from the rest of the garnet clinopyroxenites (Fig. 6) with lower equilibration temperatures for both thermometers (TK: 501 °C and TEG: 589 °C, respectively), being more similar to the garnet websterites. Although the temperatures obtained for some samples appear to be outside the ranges of calibration for both geothermometers, they are in agreement with the temperatures obtained with Ai geothermometer [1], calibrated for a wide range of conditions of equilibration (1 to 6 GPa and 600 to 1500 °C). In a matter of clarity and because Ellis and Green geothermometer and Krogh geothermometer are the most widely used in eclogite literature, the authors only reported the results for these last two calibrations in this paper.
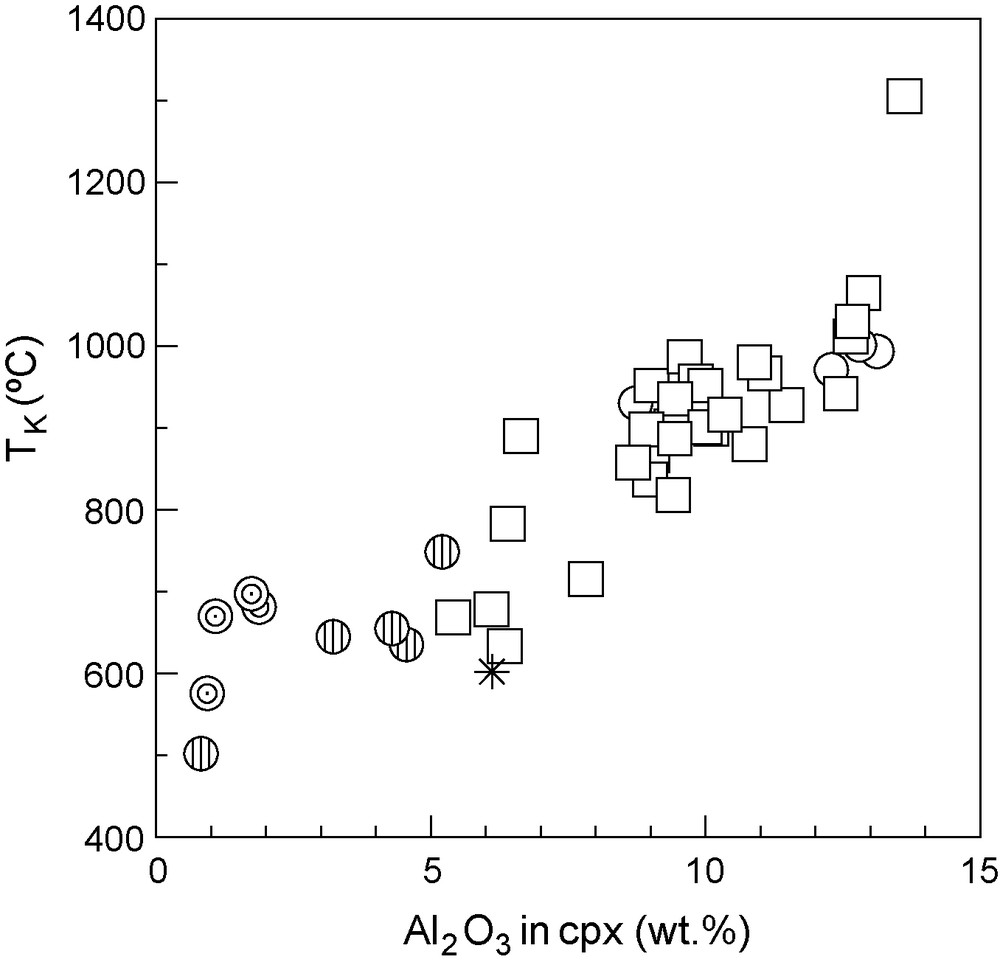
Diagram showing TK (in °C) vs Al2O3 in clinopyroxenes. Symbols as in Fig. 2.
Diagramme T K (en °C) vs Al 2 O 3 dans les clinopyroxènes. Symboles d’après la Fig. 2 .
Ranges in temperature obtained for the eclogite xenoliths are wide, with TEG = 707 to 1056 °C (mean 913 °C) and TK = 633 to 1064 °C (mean 887 °C). Once again, temperatures obtained with TEG are higher (between 2 and 75 °C higher) than those obtained with TK. Both thermometers yield similar results for the kyanite eclogites (in this case TEG are only 10 °C higher than TK) indicating equilibration at significantly higher temperatures: TEG varies from 940 to 1010 °C (mean value of 984 ± 44 °C) and TK from 930 to 1001 °C (mean value of 974 ± 44 °C). Sample HGX-23, the corundum-bearing eclogite, displays higher temperatures of equilibration than the rest of the eclogites: 1258 °C after TEG and 1305 °C after TK. This sample also plots off the trend defined by the rest of the eclogites in terms of temperature variation with Al2O3 content in clinopyroxene (Fig. 6), suggesting different conditions of equilibration. Finally, the orthopyroxene eclogite (sample 42103) shows results corresponding to the lower limits of the temperature range obtained for eclogites s.s., with a TEG of 707 °C and a TK of 602 °C.
Estimations of temperatures of equilibration by Harte and Kirkley [13] on eclogites from Roberts Victor at an assumed pressure of 50 kb, corresponding to the diamond/coesite-bearing lower parts of the South-African cratonic lithosphere [5,13,14], give TEG values ranging from 947 to 1285 °C. Using a similar pressure for the off-craton eclogites yields TEG values in the range 762 to 1122 °C, which on average are still lower than those obtained for eclogites from the on-craton Roberts Victor eclogites. Equilibration pressures obtained for the orthopyroxene-bearing samples are quite heterogeneous, ranging from 16 kb to 33 kb for the garnet websterites (pressure of 9 kb obtained for sample 20063), and values of 23 kb and 18 kb for the orthopyroxene eclogite (42103) and the orthopyroxene-bearing garnet clinopyroxenite (RDK-1), respectively.
6 Trace element mineral composition
Most of the diopsides and omphacites in pyroxene-rich mantle xenoliths show similar convex-upward chondrite-normalized REE patterns (Fig. 7). The enrichment in LREE is variable, being stronger in omphacites (LaN = 1.40–81.4) than in diopsides (LaN = 1.65–21.8). Most of the samples show a positive slope from La to Nd, with (La/Nd)N ratios ranging from 0.16 to 0.75. Enrichment in MREE is variable, with SmN contents ranging between 5.12 and 39.6 for omphacites, with a maximum value of 86.1 for sample 10063 (eclogite s.s.), and between 8.88 and 50.5 for diopsides, reaching a maximum value of 94.9 for sample 20043 (garnet websterite). Finally, contents in HREE are low for all the samples (YbN = 0.11–0.71 for omphacites and YbN = 0.14–0.77 for diopsides). Sample 42103 (orthopyroxene-eclogite) is an exception, having clinopyroxene with a higher mean chondrite-normalized Yb content of 1.57.

Chondrite-normalized rare earth elements patterns for off-craton omphacites (stars) and diopsides (white triangles). Grey field shows rare earth elements compositions of clinopyroxenes from Roberts Victor. Normalisation values after Sun and McDonough [25].
Spectres des terres rares normalisés aux chondrites des omphacites (étoiles) et diopsides (triangles blancs) « off-craton ». Champ gris de composition en terres rares des clinopyroxènes de Roberts Victor. Valeurs de normalisation d’après Sun et McDonough [25] .
In detail, five types of clinopyroxenes with different REE patterns (Fig. 8) can be recognised:
- • a group with overall low REE content (eclogites s.s. 20293, 20263), with flat patterns from La (LaN = 0.7–3.3) through Nd (NdN = 0.71–1.54) to Er (ErN = 0.21–0.49) and then Yb (YbN = 0.19–0.54). These clinopyroxenes show slight to significant positive Eu anomalies (Eu/Eu*: 1.19–1.97);
- • a group with “bumpy” patterns (42163, 20253, 42153) from LREE to MREE [(La/Eu)N vary from 0.035 to 0.17, compared to the rest of omphacites: 0.3–4.6] with a peak towards Eu (positive Eu anomalies, with Eu/Eu* varying from 1.40 to 1.98) and decreasing contents from Eu to HREE in a convex downward shape);
- • two samples with similar REE patterns to the majority, but which have a flat shape towards the HREE (026203-N and 02G30), with (Yb/Dy)N ratios ranging from 0.97 to 1.06;
- • two samples (42143, 02100) which have REE patterns decreasing smoothly from LREE towards MREE [(La/Eu)N = 4.48–4.62], with a flat HREE pattern [(Yb/Dy)N = 0.93–1.05)];
- • diopsides from two samples (20770, 20063) and omphacites from samples 20163 have similar LREE patterns to the previous group [(La/Eu)N = 1.33–2.49], but have low HREE contents (YbN < 0.062). Clinopyroxenes from sample 20770 also display a slight positive Eu anomaly (Eu/Eu* = 1.38).
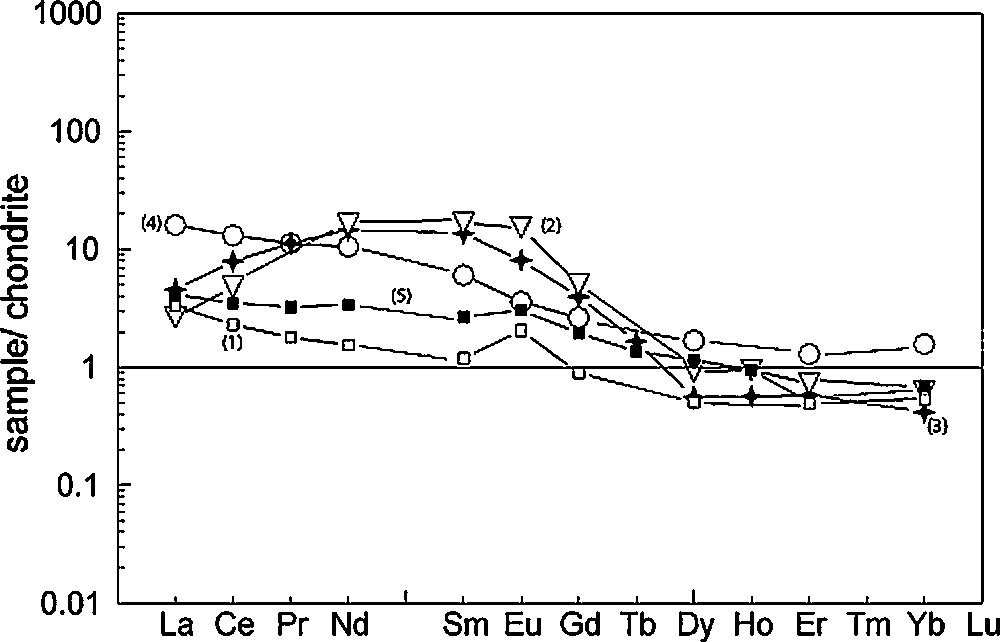
Chondrite-normalized rare earth elements patterns for some “atypical” clinopyroxenes of a few samples. Numbers on patterns refer to their order in the list given in the text.
Spectres des terres rares normalisés aux chondrites pour les clinopyroxènes « atypiques » de plusieurs échantillons. Les chiffres sur les spectres se réfèrent à l’ordre des spectres donné dans le texte.
When compared to on-craton clinopyroxenes from Roberts Victor pyroxene-rich mantle xenoliths (Fig. 7), off-craton clinopyroxenes appear enriched in REE, more particularly in MREE (“bumpy” patterns). Taken all together, clinopyroxenes from off-craton xenoliths have higher La (LaN = 1.40–81.4) and Sm (SmN = 5.12–95) contents than clinopyroxenes from Roberts Victor (LaN = 1.08–25.5 and SmN = 1.27–16.6, respectively). Yb contents give a narrower range of values for off-craton clinopyroxenes (YbN = 0.11–0.77, with one sample at 1.57) compared to clinopyroxenes from Roberts Victor eclogites (YbN = 0.05–0.76, with one sample at 1.61).
Chondrite-normalized REE patterns of garnets that equilibrated with diopsides have a slightly different overall shape than those for garnets that have equilibrated with omphacites (Fig. 9): the former group shows a rather steep positive slope from LREE to MREE [(Ce/Nd)N = 0.04–0.6] that flattens down from MREE to HREE (“stretched” REE patterns), whereas the latter group has a strong positive slope from LREE to MREE [(Ce/Nd)N = 0.04–0.17], a “hump” towards Sm and Eu (Sm abundances of 6 to 48 times chondrites) and a slight negative slope from Gd to Yb [(Gd/Yb)N = 0.84–4.5]. Significant positive Eu anomalies in garnets were only observed in eclogites from Roodekraal (except the orthopyroxene-bearing eclogite 42103) and Jachtfontein (Eu/Eu* = 1.24–1.83). None of the garnets in diopside-bearing samples show significant anomalies in Eu. Both groups of garnets are strongly depleted in LREE, with CeN varying from 0.038 to 0.97 (only garnets in sample 026203-B from Lovedale have a high mean value of 1.60). HREE are variably enriched, both between and among the two groups of garnets, with values in YbN ranging from 4.5 to 29 for the garnets that have equilibrated with omphacites and from 8.9 to 41 for the garnets that have equilibrated with diopsides. Values for YbN reach the highest in garnets from samples 42103 (YbN = 92) and 20043 (YbN = 96).
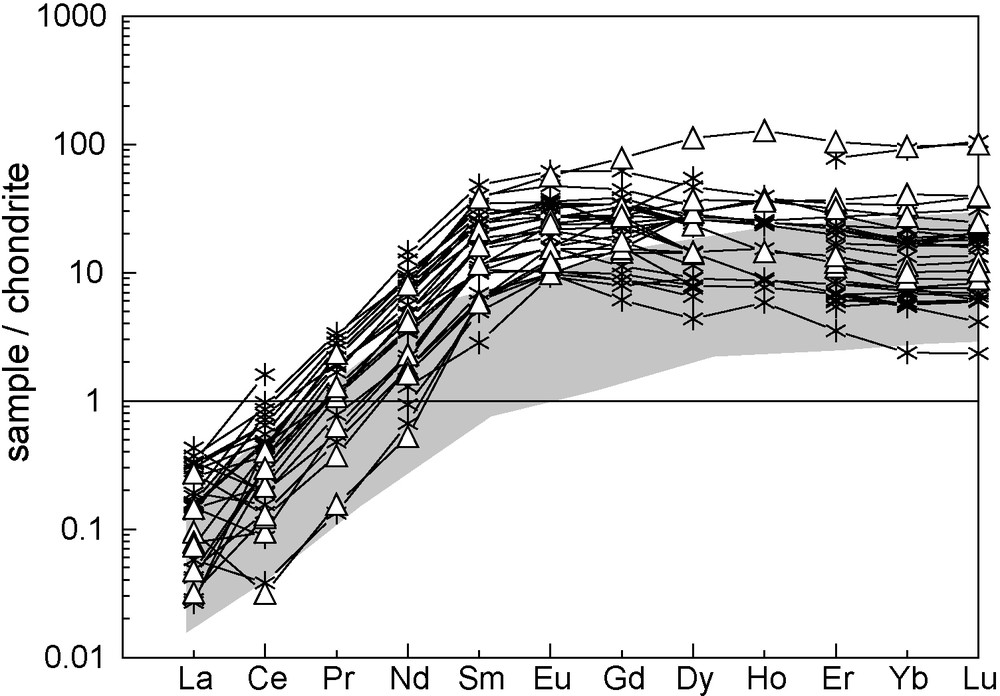
Chondrite-normalized rare earth elements patterns for off-craton garnets that coexist with omphacites (stars) or diopsides (white triangles). Grey field for rare earth elements compositions of garnets from Roberts Victor (this study). Normalisation values after Sun and McDonough [25].
Spectres des terres rares normalisés aux chondrites des grenats off-craton à l’équilibre avec des omphacites (étoiles) ou des diopsides (triangles blancs). Champ gris de composition en terres rares des grenats de Roberts Victor. Valeurs de normalisation d’après Sun et McDonough [25] .
When compared to garnets from Roberts Victor eclogites (Fig. 9), garnets in eclogites from these off-craton localities show similar LREE abundances, but higher MREE abundances (SmN = 6.03–48.2; sample 20770 at 0.58), in comparison to garnets from Roberts Victor eclogites (SmN = 0.24–6.6). HREE abundances are similarly higher in garnets from off-craton eclogites (YbN = 4.5–96) and from Roberts Victor eclogites (YbN = 2.2–22.6).
7 Discussion and origin of the protoliths
The different xenoliths brought up to the surface along the south-western margin of the Kaapvaal Craton are compared to their diamond-bearing cratonic counterparts from the Roberts Victor mine. As seen earlier, clinopyroxenes from off-craton xenoliths generally exhibit lower contents in MgO and higher contents in LREE and MREE than on-craton clinopyroxenes. Contents of HREE are similar between the on-craton and the off-craton clinopyroxenes. Similarly, garnets from off-craton xenoliths contain lower MgO abundances than garnets from the Roberts Victor xenoliths, and MREE and HREE abundances are higher in the former, compared to the on-craton garnets. LREE abundances in on-craton and off-craton garnets overlap. Temperatures of equilibration for the different circum-craton xenoliths (at P = 30 kb) cover a wide range of values, increasing from the garnet websterites (TEG = 742–781 °C) through the garnet clinopyroxenites (TEG = 715–830 °C) to eclogites (TEG = 707–1056 °C, mean value of 913 °C), with sample HGX23 (corundum-bearing eclogite) yielding the highest value (TEG = 1258 °C). In addition to yielding high temperatures of equilibration, sample HGX23 plots off the various geochemical trends, suggesting distinct conditions of formation and equilibration for this sample (in which omphacites are depleted in Na2O). Pressures of equilibration obtained for the garnet websterites vary between 16 and 33 kb (corresponding to depths of ∼50–100 km), 18 kb for sample RDK-1 (TEG = 743 °C) and 23 kb for sample 42103. Based on geophysical studies indicating the occurrence of 45 to 50 km of crust underneath the region [6,11,20,21,24,26], we can assume that the suite as a whole originate from the upper mantle rather than from the Lower Proterozoic crust. Extremely low temperatures of equilibration were obtained for the garnet websterite 20063 (TEG: 655 °C, TK: 575 °C, TBK: 642 °C), in which clinopyroxenes exhibit severe exsolutions of spinel. Although care was taken to only analyze “fresh” cores in the clinopyroxene, it appears that the calculated temperatures of equilibration for this sample correspond to the subsolidus event during which clinopyroxene exsolved spinel. Interestingly, other garnet websterites exhibiting spinel exsolutions in clinopyroxene (20123, 20770), but showing less exsolution than sample 20063, yield temperatures of equilibration similar to the temperatures obtained for the garnet websterite 20043, in which clinopyroxene do not exhibit such exsolution features. Samples exhibiting exsolution of orthopyroxene in clinopyroxene also yield equilibration temperatures that are within the range of values defined by the other samples.
Xenoliths from on-craton localities such as those from Roberts Victor (Kaapvaal craton, South Africa) are well known for widespread diamond and other high-pressure mineral occurrence, which indicate deeper conditions of equilibration for these rocks, in contrast to off-craton xenoliths that do not contain such minerals. The high temperatures of equilibration obtained for eclogites from Roberts Victor by Harte and Kirkley [13] at an assumed pressure of 50 kb (947 to 1285 °C) reflect the conditions of equilibration in the upper mantle underlying the Kaapvaal craton. The lower temperatures of equilibration obtained in this study for the off-craton xenoliths, even at an assumed pressure of 50 kb, indicate that the latter must have equilibrated at much lower pressures than 50 kb (outside the diamond field), given the fact that the continental geotherm under the Kaapvaal craton is much steeper than the one under the Namaqua–Natal belt.
As evidenced in the previous sections, the textural, petrographical and geochemical characteristics of the two groups of xenoliths (omphacite-bearing group and diopside-bearing group) are similar (although not identical) and show a clear evolution from one group to the other. This overall similarity suggests a probable cogenetic origin. Recently acquired oxygen isotope data on garnets yield δ18Ognt values of 5.25–6.78 ‰ for the nine eclogites analysed and 5.24–7.03 ‰ for the four garnet clinopyroxenites analysed. The garnet websterite 20770 yields a δ18Ognt value of 5.15 ‰. Garnets in six samples show values typical of mantle garnets (5.3 ± 0.2; 20), whereas garnets from the eight other samples display much higher values. δ18O values higher than mantle values are commonly interpreted as resulting from low-temperature alteration of sea-floor basalts and related rocks. We may therefore assume that the studied off-craton xenoliths represent such as already proposed for the South African on-craton eclogite xenoliths [26,28] originally oceanic crustal rocks, the eclogitic varieties deriving from originally plagioclase–rich protoliths and the pyroxenitic varieties from plagioclase-free protoliths. During subduction, the plagioclase-rich protoliths equilibrated at mantle depths to omphacite bearing eclogites, whereas those without plagioclase re-equilibrated to diopside and garnet-bearing pyroxenites.
Acknowledgments
This work was made possible by the financial support of the University of Cape Town, the National Research Foundation and the Centre national de la recherche scientifique. The authors warmly thank Andreas Späth, Philippe de Parseval and Frédéric Candaudap for their help during data acquisition.


