1 Introduction
1.1 Geological background
Chromitite, a rock chiefly containing the chromite mineral, is the major source of chromium and a common rock-type in many ophiolite massifs. The mechanism of chromian spinel concentration in podiform chromitites has been discussed in many papers (e.g. [4,5,19,24,27,34]), but it has not yet been completely solved. Chromitites are able to record petrogenetic processes in the upper mantle. However, the study of many ophiolites reveals that they are not equally distributed among a mantle section of ophiolite complexes. In some cases, chromitites are never observed, contrasting with the majority of ophiolites in which chromitite is present in the transition zone [8,22,23]. This important observation has been elaborated in 1985 by F. Boudier and A. Nicolas, with the LOT-and HOT- ophiolite types [8], see also [19,23,24]. Chromitite is generally absent in ophiolites that are characterized by a lherzolite-dominant mantle section (Lherzolite Ophiolite Type [LOT]). On the contrary, it is present in ophiolites where this section is harzburgitic (Harzburgite Ophiolite Type [HOT]).
As the initial source of chromium is contained in clinopyroxene (diopside) [8], the degree of partial melting plays an essential role for the eventual formation of chromitites. In LOT-peridotites, which have suffered a low-degree of partial melting, chromium hosted by clinopyroxene is still largely retained in this mineral. In HOT-systems, on the other hand, the clinopyroxene of fertile mantle peridotite has been molten. As a result, chromium has been incorporated into the basaltic melt. This increases the possibility for the later segregation of chromium into chromitites during basalt crystallization [24].
Petrography of mantle peridotites of the Jandaq ophiolite shows that this ophiolite is of the LOT type, and based on the general trend, it should not contain the chromitite. Despite the general rule, some LOT ophiolites have chromitite (e.g. [8,15,24]), and paucity of chromite mineralization in some HOT is presented (e.g. [33]). These exceptions show the necessity of chromitite potential study on the Jandaq ophiolite as one of the important Iranian ophiolites.
Chromitites are present in most of Iranian ophiolites (e.g. Neyriz, Naein, Esfandagheh, Sabzevar, Faryab and Ashin), all belonging to the HOT-type [30,32]. However, they have not been found in other occurrences (e.g. Anarak, Posht-e-Badam and Jandaq, Bayazeh), which until now have not been studied in great detail.
The main aim of this paper is to check the chromitite potential of mantle peridotites in Jandaq ophiolite. This ophiolite presents good exposures of mantle peridotites in northwestern part of the Central-East Iranian Microplate (CEIM). This occurrence will then be compared with the Ashin ophiolite (Central Iran) which is a HOT with considerable amount of chromite deposits (Fig. 1). It is hoped that the comparison of Jandaq chromitite absent LOT with Ashin chromitite bearing HOT, will be useful to evaluate the chromitite potential in Jandaq ophiolite.
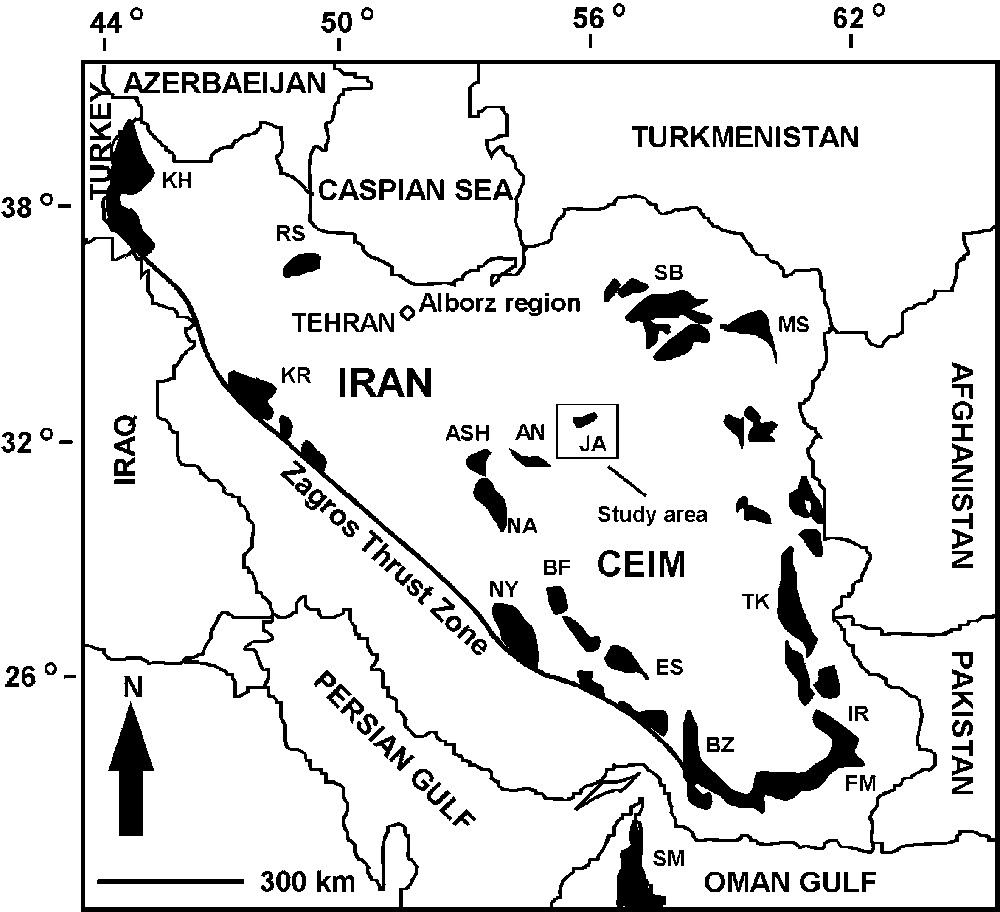
Main ophiolites of Iran and location of the Jandaq ophiolite. KH: Khoy; KR: Kermanshah; NY: Neyriz; BZ: Band Ziarat; NA: Naein; BF: Baft; ES: Esfandagheh; FM: Fanuj-Maskutan; IR: ranshahr; TK: Tchehel Kureh; MS: Mashhad; SB: Sabzevar; RS: Rasht; SM: Samail; ASH: Ashin; AN: Anarak; JA: Jandaq.
Principales ophiolites d’Iran, emplacement de l’ophiolite de Jandaq. KH : Khoy ; KR : Kermanshah ; NY : Neyriz ; BZ : Band Ziarat ; NA : Naein ; BF : Baft ; ES : Esfandagheh ; FM : Fanuj-Maskutan ; IR : ranshahr ; TK : Tchehel Kureh ; MS : Mashhad ; SB : Sabzevar ; RS : Rasht ; SM : Samail ; ASH : Ashin ; AN : Anarak ; JA : Jandaq.
1.2 Geological setting
Ophiolite complexes of Iran are part of Middle East Tethyan ophiolite belts. They link to other Asian ophiolites, such as Pakistan in the east, or ophiolites in the Mediterranean region, such as Turkish, Troodos, and East Europe in the west [32]. These ophiolites rest as giant thrust sheets upon a continental substrate [23].
Iranian ophiolites (Fig. 1) have geographically been divided into four groups:
- (1) ophiolites of northern Iran along the Alborz mountain range including Rasht ophiolites;
- (2) ophiolites of Zagros suture zone including the Neyriz and Kermanshah ophiolites which are apparently the extension of the Oman ophiolites;
- (3) ophiolites and color mélanges of the Makran region, located in the South-East of Iran and;
- (4) ophiolites and color mélanges that mark the boundaries of the CEIM.
The ages of emplacement derived from field relationships suggest another three-fold classification [1]:
- (1) Proterozoic ophiolites, that crop out on the western edge of the Lut block in central Iran;
- (2) Pre-Jurassic ophiolites that are located within the Alborz range in the northern Iran and;
- (3) Post-Jurassic ophiolites that are the most abundant ophiolite terrains in Iran.
Most recent time of emplacement is not known, but suggested by Lippard et al. [22] to be pre-Paleocene.
The study area (Jandaq ophiolite) is located on the West of central Iran, and the southern margin of Great Kavir with highly deserted geographical conditions (Fig. 2), considered to be Upper Proterozoic [1,3,28] or Paleozoic [10,11]. The Jandaq ophiolite is a remnant of Paleo-Tethys, transferred to central Iran by anti-clockwise rotation of Central-East Iranian microcontinent (CEIM) [6,10,11]. The ophiolite massif is composed of mantle peridotite and serpentinized mantle peridotite, metagabbro, basic and ultrabasic metamorphosed dikes, metapyroxenite, amphibolite, rodingite, and listwaenite [31]. It has been covered by Paleozoic metamorphic rocks (schist and marble) (Fig. 2). Chromitite has not been found yet. Mesozoic granite and mylonitic granite intrusions crosscut the Jandaq ophiolite and the covering metamorphic rocks. Chahpalang formation with Upper Jurassic age, and lithology of sandstone, siltstone and conglomerate, together with the Cretaceous limestone, have been covered by the Jandaq ophiolite, metamorphic rocks and granitic intrusions (Fig. 3A).
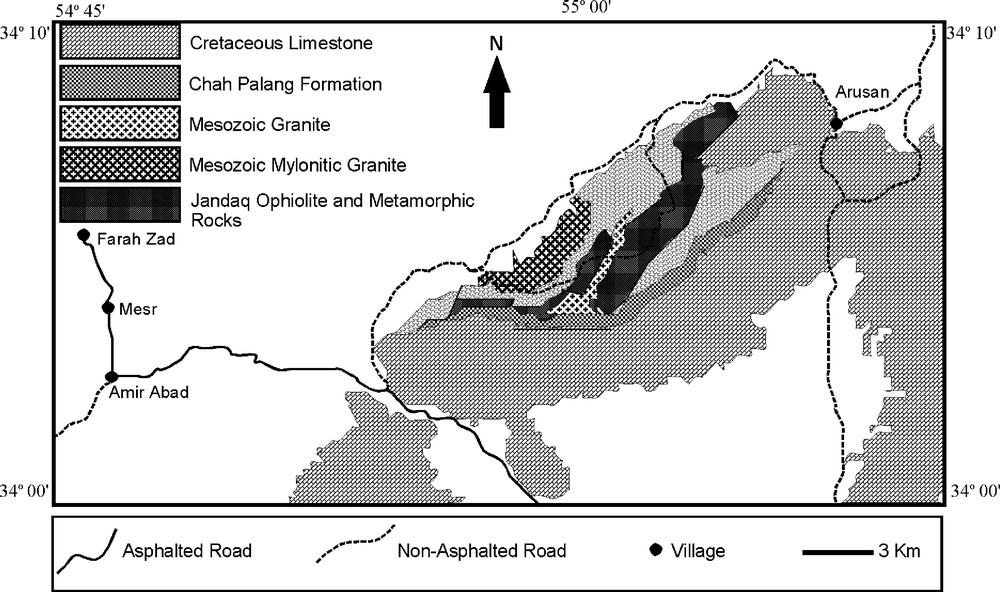
Simplified geological map of the study area.
Carte géologique simplifiée de la région étudiée.
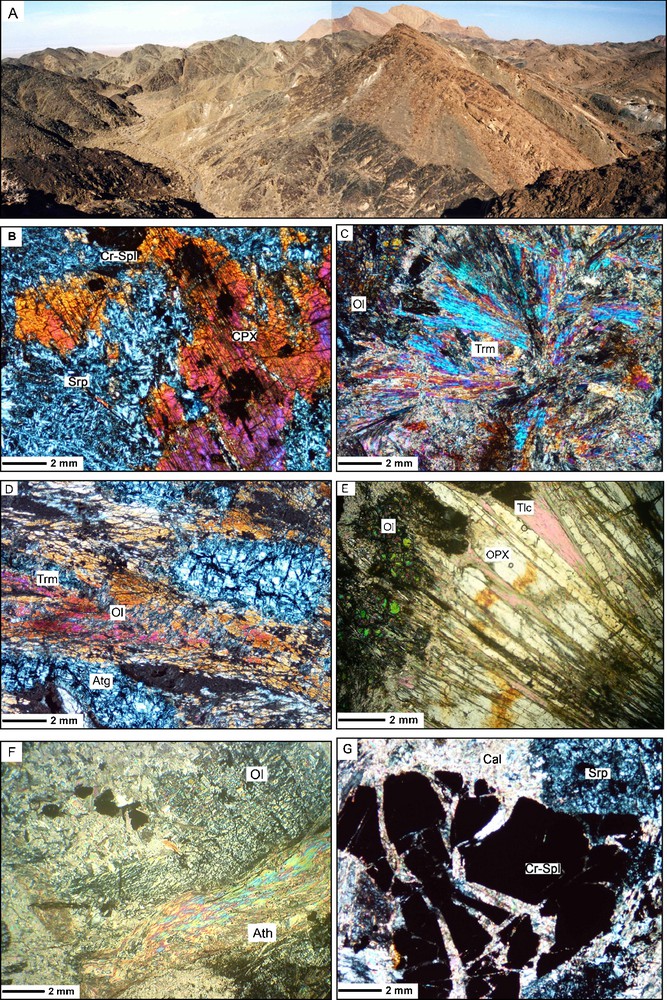
Field photograph and microscopic photomicrographs of the Jandaq ophiolite mantle peridotites. (A) General view of the Jandaq ophiolite. Mantle peridotites are in the foreground, and Chahpalang formation and Cretaceous limestones are in the background. (B) Relict of igneous clinopyroxene in a lherzolite. (C and D) Photographs of tremolite-bearing metalherzolites. (E and F) Metaharzburgites with metamorphic olivine, orthopyroxene, talc and anthophyllite. (G) Relict of an igneous spinel.
Affleurement et microphotographies des péridotites mantelliques de l’ophiolite de Jandaq. (A) Vue générale du massif ophiolitique. Les péridotites sont au premier-plan, la formation de Chahpalang et les calcaires crétacés en arrière–plan. (B) Relique de clinopyroxène igné dans une lherzolite. (C et D) Microphotographies de métalherzolites à trémolite. (E et F) Méta-harzburgite contenant olivine et orthopyroxène métamorphiques, talc et antophyllite. (G) Relique de spinelle magmatique.
All rock units of the Jandaq ophiolite have been highly metamorphosed, and they have suffered a high degree of serpentinization. Based on amphibolite suites [31], prograde metamorphism has reached upper amphibolite facies conditions (7.98–9.01 kbar and 714–737 °C). Several phases of metamorphism have certainly occurred, not deciphered in detail at present. Middle-Jurassic metamorphism is however indicated by new 40Ar-39Ar isotopic analyses [6] on samples from the northern slope of Rashid Kuh, one on muscovite in micaschist (163.86 ± 1.76 Ma plateau age), the second one on hornblende in amphibolite (156.56 ± 33.15 Ma plateau age).
For detailed study of the Jandaq ophiolite, 250 rock samples were collected from all rock units of the area, during 23 days of the field study.
2 Analytical data
Analysis of minerals was carried out with a Cameca SX-100 electron-probe micro-analyzer (WDS) at the Institute of Mineralogy, Leibniz University, Hanover, Germany. The analyses were performed under an accelerating voltage of 15 kV and a beam current of 15 nA with 3-μm probe beam diameter. Natural and synthetic minerals of known composition are used as standards. The Fe+3 and Fe+2 amounts of spinel were calculated assuming spinel (AB2O4) stoichiometry [13]. The Cr#, Mg# and Fe3+# are Cr/(Cr + Al), Mg/(Mg + Fe+2) and Fe3+/(Cr + Al + Fe3+) atomic ratio of minerals, respectively. Representative analyses of the minerals and calculated structural formulas are shown in Table 1. Mineral abbreviations in photomicrographs are from Kretz [18].
Analyses chimiques représentatives et formules structurales calculées des minéraux des péridotites mantelliques de l’ophiolite de Jandaq (Iran central).
| Sample | CPX | CPX | Spinel | Spinel | Olivine | Olivine | OPX | OPX | Tremolite | Tremolite | Anthoph. | Anthoph. | Talc | Talc | Chlorite | Chlorite | Serpentine | Serpentine |
| SiO2 | 52.42 | 52.92 | 0.00 | 0.25 | 40.72 | 41.29 | 57.43 | 57.31 | 58.34 | 57.80 | 55.62 | 56.99 | 59.91 | 61.74 | 34.48 | 33.74 | 42.28 | 43.38 |
| TiO2 | 0.35 | 0.27 | 0.00 | 0.41 | 0.00 | 0.01 | 0.00 | 0.04 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.02 | 0.03 | 0.01 | 0.02 |
| Al2O3 | 2.34 | 5.06 | 19.75 | 28.70 | 0.00 | 0.03 | 1.08 | 0.94 | 0.31 | 0.08 | 1.60 | 1.59 | 1.14 | 0.74 | 13.11 | 13.84 | 1.69 | 1.39 |
| Cr2O3 | 0.71 | 0.84 | 45.35 | 35.68 | — | — | 0.40 | 0.05 | 0.08 | 0.06 | 0.32 | 0.43 | 0.17 | 0.09 | 0.30 | 0.12 | 0.59 | 0.30 |
| FeO* | 3.46 | 2.16 | 23.16 | 19.78 | 10.88 | 7.60 | 7.05 | 5.95 | 2.31 | 3.66 | 2.90 | 2.82 | 1.40 | 1.31 | 6.50 | 6.62 | 1.92 | 1.86 |
| MnO | 0.17 | 0.08 | 0.21 | 0.77 | 0.10 | 0.07 | 0.12 | 0.15 | 0.03 | 0.05 | 0.00 | 0.00 | 0.08 | 0.03 | 0.09 | 0.10 | 0.06 | 0.05 |
| MgO | 17.07 | 15.83 | 9.17 | 12.94 | 48.64 | 51.38 | 34.25 | 35.91 | 23.83 | 22.64 | 32.99 | 34.16 | 30.53 | 30.53 | 32.47 | 32.56 | 38.74 | 39.25 |
| CaO | 23.22 | 21.68 | 0.01 | 0.04 | 0.00 | 0.00 | 0.08 | 0.09 | 13.26 | 13.60 | 0.00 | 0.04 | 0.02 | 0.03 | 0.06 | 0.02 | 0.02 | 0.00 |
| Na2O | 0.33 | 0.82 | 0.02 | 0.07 | 0.01 | 0.01 | 0.02 | 0.00 | 0.07 | 0.05 | 0.01 | 0.03 | 0.46 | 0.31 | 0.02 | 0.02 | 0.00 | 0.00 |
| K2O | 0.01 | 0.03 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.03 | 0.03 | 0.01 | 0.01 | 0.03 | 0.01 | 0.00 | 0.00 |
| NiO | 0.00 | 0.05 | 0.00 | 0.21 | 0.00 | 0.00 | 0.00 | 0.04 | — | — | — | — | — | — | — | — | 0.07 | 0.08 |
| Total | 100.08 | 99.75 | 97.68 | 98.57 | 100.36 | 100.39 | 100.43 | 100.48 | 98.17 | 97.90 | 93.16 | 95.67 | 93.56 | 94.70 | 87.08 | 87.06 | 85.38 | 86.32 |
| Oxygens # | 6 | 6 | 32 | 32 | 4 | 4 | 6 | 6 | 23 | 23 | 23 | 23 | 21 | 21 | 28 | 28 | 7 | 7 |
| Si | 1.906 | 1.922 | 0.000 | 0.061 | 0.998 | 0.997 | 1.977 | 1.955 | 7.880 | 7.927 | 6.611 | 6.586 | 7.699 | 7.866 | 6.577 | 6.452 | 2.013 | 2.033 |
| Ti | 0.010 | 0.007 | 0.000 | 0.075 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.003 | 0.004 | 0.000 | 0.001 |
| Al | 0.10 | 0.217 | 6.061 | 8.190 | 0.000 | 0.000 | 0.044 | 0.038 | 0.049 | 0.013 | 0.224 | 0.216 | 0.173 | 0.111 | 2.952 | 3.121 | 0.095 | 0.077 |
| Cr | 0.020 | 0.024 | 9.333 | 6.830 | — | — | 0.011 | 0.001 | 0.009 | 0.006 | 0.030 | 0.039 | 0.003 | 0.001 | 0.045 | 0.018 | 0.020 | 0.011 |
| Fe2+ | 0.033 | 0.066 | 4.448 | 3.296 | 0.223 | 0.153 | 0.203 | 0.121 | 0.000 | 0.306 | 0.000 | 0.000 | 0.150 | 0.140 | 0.954 | 1.034 | 0.076 | 0.073 |
| Fe3+ | 0.072 | 0.000 | 0.600 | 0.709 | 0.00 | 0.00 | 0.000 | 0.048 | 0.261 | 0.115 | 0.288 | 0.273 | 0.000 | 0.000 | 0.082 | 0.025 | 0.000 | 0.000 |
| Mn | 0.005 | 0.003 | 0.006 | 0.158 | 0.002 | 0.001 | 0.003 | 0.004 | 0.003 | 0.006 | 0.000 | 0.000 | 0.009 | 0.003 | 0.015 | 0.016 | 0.002 | 0.002 |
| Mg | 0.925 | 0.857 | 3.558 | 4.671 | 1.778 | 1.849 | 1.757 | 1.826 | 4.798 | 4.629 | 4.970 | 5.885 | 5.849 | 5.798 | 9.232 | 9.281 | 2.750 | 2.742 |
| Ca | 0.905 | 0.844 | 0.003 | 0.010 | 0.000 | 0.000 | 0.003 | 0.003 | 1.919 | 1.998 | 0.000 | 0.005 | 0.003 | 0.004 | 0.012 | 0.004 | 0.001 | 0.000 |
| Na | 0.023 | 0.058 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.018 | 0.014 | 0.002 | 0.007 | 0.115 | 0.077 | 0.015 | 0.015 | 0.000 | 0.000 |
| K | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.003 | 0.005 | 0.004 | 0.002 | 0.002 | 0.015 | 0.005 | 0.000 | 0.000 |
| Ni | 0.000 | 0.002 | 0.000 | 0.041 | 0.000 | 0.000 | 0.000 | 0.001 | — | — | — | — | — | — | — | — | 0.000 | 0.000 |
| Sum | 4.000 | 3.999 | 24.008 | 24.000 | 3.001 | 3.001 | 4.000 | 4.000 | 14.941 | 15.015 | 13.007 | 13.016 | 14.001 | 14.001 | 19.903 | 19.975 | 4.937 | 4.928 |
The major and trace element analyses were carried out on whole rocks at the Central laboratory of University of Isfahan by Bruker S4 Pioneer XRF, and Activation Laboratory of Isfahan (MNSR Department), by NAA method. Forty-four samples of mantle peridotites from the Jandaq and Ashin ophiolite were selected for analyses. Whole rock geochemical data are presented in Table 2.
Compositions chimiques des péridotites mantelliques des ophiolites de Jandaq (en haut, échantillons J) et Ashin (en bas, échantillons A). (Éléments majeurs en pds%, trace en ppm, * : ppb). Lz : lherzolite, Hz : harzburgite, Du : dunite.
| Sample | SiO2 | TiO2 | Al2O3 | Cr2O3 | Fe2O3* | MnO | MgO | CaO | Na2O | K2O | NiO | LOI | Co | Sc | V | La | Sm | Dy | Yb |
| J685 (Lz) | 44.18 | 0.08 | 1.19 | 0.10 | 6.25 | 0.12 | 33.68 | 5.41 | 0.03 | 0.01 | 0.12 | 9.05 | 47 | 4 | 14 | 0.31 | 0.33 | 2.33 | 0.33 |
| J688 (Lz) | 39.70 | 0.23 | 3.91 | 0.31 | 13.5 | 0.17 | 33.32 | 2.01 | 0.04 | 0.01 | 0.29 | 7.10 | 77 | 5 | 86 | 4.80 | 2.29 | 2.67 | 2.47 |
| J715 (Lz) | 39.88 | 0.13 | 3.55 | 0.50 | 12.07 | 0.15 | 35.79 | 1.26 | 0.22 | 0.01 | 0.18 | 6.94 | 97 | 17 | 59 | 0.22 | 0.15 | 1.24 | 0.31 |
| J716 (Lz) | 42.99 | 0.12 | 4.14 | 0.66 | 8.14 | 0.09 | 26.59 | 11.32 | 0.07 | 0.01 | 0.25 | 6.53 | 56 | 36 | 123 | 0.28 | 0.30 | 1.85 | 0.52 |
| J718 (Lz) | 41.36 | 0.13 | 5.65 | 0.42 | 11.25 | 0.13 | 29.34 | 5.57 | 0.03 | 0.01 | 0.14 | 6.52 | 73 | 18 | 60 | 0.95 | 0.22 | 1.53 | 0.40 |
| J719 (Lz) | 39.81 | 0.23 | 5.95 | 0.42 | 9.06 | 0.10 | 27.54 | 10.47 | 0.11 | 0.02 | 0.17 | 6.71 | 72 | 29 | 96 | 0.28 | 0.33 | 2.07 | 0.46 |
| J720 (Lz) | 41.44 | 0.12 | 3.61 | 0.34 | 10.71 | 0.11 | 33.11 | 2.95 | 0.03 | 0.01 | 0.20 | 7.92 | 96 | 14 | 38 | 0.19 | 0.11 | 2.11 | 0.37 |
| J721 (Lz) | 39.58 | 0.15 | 8.07 | 0.03 | 7.82 | 0.09 | 29.97 | 7.39 | 0.05 | 0.01 | 0.14 | 6.86 | 71 | 16 | 52 | 0.23 | 0.14 | 2.00 | 0.37 |
| J722 (Lz) | 41.42 | 0.13 | 5.48 | 0.35 | 8.94 | 0.11 | 32.38 | 4.32 | 0.06 | 0.01 | 0.15 | 7.14 | 85 | 20 | 62 | 0.35 | 0.17 | 2.01 | 0.38 |
| J723 (Lz) | 41.67 | 0.18 | 4.08 | 0.72 | 7.35 | 0.11 | 25.47 | 12.33 | 0.13 | 0.02 | 0.27 | 8.66 | 91 | 47 | 149 | 0.43 | 0.45 | 2.00 | 0.86 |
| J724 (Lz) | 44.77 | 0.23 | 4.57 | 0.75 | 7.25 | 0.11 | 23.48 | 12.45 | 0.15 | 0.02 | 0.01 | 6.97 | 78 | 48 | 150 | 0.34 | 0.46 | 2.37 | 0.63 |
| J725 (Lz) | 43.32 | 0.13 | 5.82 | 0.22 | 9.58 | 0.11 | 30.49 | 3.74 | 0.04 | 0.01 | 0.22 | 6.77 | 86 | 13 | 44 | 0.21 | 0.13 | 2.25 | 0.42 |
| J728 (Lz) | 43.16 | 0.08 | 0.79 | 0.48 | 8.22 | 0.11 | 37.95 | 0.70 | 0.04 | 0.01 | 0.27 | 8.93 | 71 | 7 | 31 | 0.15 | 0.10 | 2.34 | 0.29 |
| J729 (Lz) | 44.22 | 0.09 | 0.89 | 0.39 | 7.75 | 0.13 | 35.83 | 0.78 | 0.04 | 0.02 | 0.34 | 10.26 | 118 | 7 | 33 | 0.19 | 0.12 | 2.10 | 0.34 |
| J731 (Lz) | 42.83 | 0.07 | 0.85 | 0.50 | 8.38 | 0.07 | 36.97 | 0.91 | 0.04 | 0.01 | 0.29 | 9.87 | 74 | 12 | 34 | 0.18 | 0.13 | 1.81 | 0.37 |
| J732 (Lz) | 44.93 | 0.06 | 1.15 | 0.51 | 7.79 | 0.08 | 36.14 | 0.70 | 0.04 | 0.01 | 0.42 | 9.10 | 99 | 10 | 42 | 0.16 | 0.11 | 1.91 | 0.32 |
| J740 (Lz) | 43.93 | 0.08 | 0.98 | 0.31 | 6.73 | 0.05 | 37.88 | 0.98 | 0.04 | 0.01 | 0.31 | 9.32 | 77 | 7 | 18 | 0.19 | 0.12 | 1.21 | 0.51 |
| J741 (Lz) | 44.46 | 0.08 | 1.36 | 0.34 | 7.25 | 0.09 | 32.40 | 5.04 | 0.20 | 0.16 | 0.23 | 8.96 | 66 | 7 | 35 | 1.14 | 0.11 | 2.24 | 0.58 |
| J748 (Lz) | 40.52 | 0.15 | 2.95 | 0.75 | 13.01 | 0.13 | 34.40 | 0.70 | 0.02 | 0.01 | 0.24 | 8.12 | 94 | 11 | 67 | 0.16 | 0.11 | 2.31 | 0.37 |
| J772 (Lz) | 43.76 | 0.08 | 0.66 | 0.39 | 7.06 | 0.07 | 37.47 | 0.98 | 0.04 | 0.01 | 0.32 | 9.87 | 83 | 7 | 33 | 0.22 | 0.14 | 1.79 | 0.33 |
| J773 (Lz) | 40.79 | 0.22 | 2.72 | 0.38 | 9.44 | 0.10 | 36.44 | 0.98 | 0.03 | 0.01 | 0.34 | 9.28 | 77 | 9 | 62 | 1.84 | 0.75 | 2.09 | 1.15 |
| J622 (Hz) | 43.62 | 0.10 | 1.29 | 0.35 | 5.45 | 0.08 | 38.96 | 0.84 | 0.02 | 0.01 | 0.31 | 9.64 | 77 | 7 | 37 | 0.12 | 0.10 | 1.67 | 0.36 |
| J762 (Hz) | 43.75 | 0.20 | 2.10 | 0.04 | 7.55 | 0.08 | 35.35 | 0.77 | 0.68 | 0.01 | 0.20 | 9.52 | 67 | 10 | 43 | 0.41 | 0.18 | 1.70 | 0.42 |
| J769 (Hz) | 37.20 | 0.08 | 3.70 | 0.10 | 10.14 | 0.11 | 40.47 | 0.84 | 0.02 | 0.01 | 0.19 | 7.43 | 89 | 8 | 26 | 0.25 | 0.15 | 1.99 | 0.34 |
| J770 (Hz) | 38.64 | 0.12 | 3.91 | 0.03 | 11.18 | 0.13 | 36.94 | 0.91 | 0.02 | 0.01 | 0.17 | 8.15 | 92 | 12 | 35 | 0.20 | 0.12 | 2.02 | 0.32 |
| Mean | 42.08 | 0.13 | 3.17 | 0.38 | 8.87 | 0.11 | 33.53 | 3.77 | 0.09 | 0.02 | 0.23 | 8.22 | 80 | 15 | 57 | 0.55 | 0.29 | 1.98 | 0.53 |
| A50 (Lz) | 42.61 | 0.10 | 2.06 | 0.42 | 8.19 | 0.13 | 36.03 | 1.58 | 0.03 | 0.01 | 0.25 | 8.58 | 140 | 14 | 68 | <0.08 | <0.10 | <0.45 | <0.14 |
| A201 (Lz) | 34.46 | 0.08 | 2.72 | 0.38 | 8.28 | 0.13 | 39.09 | 2.57 | 0.06 | 0.01 | 0.25 | 11.96 | 92 | 14 | 80 | <0.05 | <0.10 | <0.30 | 0.25 |
| A222 (Lz) | 36.10 | 0.08 | 2.97 | 0.35 | 8.72 | 0.12 | 40.02 | 2.92 | 0.13 | 0.01 | 0.24 | 8.33 | 98 | 14 | 83 | <0.07 | <0.12 | <0.30 | <0.10 |
| A249 (Lz) | 37.68 | 0.06 | 1.49 | 0.45 | 7.72 | 0.12 | 40.19 | 1.87 | 0.03 | 0.01 | 0.22 | 10.15 | 90 | 12 | 64 | 48* | <0.10 | <0.30 | <0.08 |
| A287 (Lz) | 38.82 | 0.06 | 1.23 | 0.34 | 7.71 | 0.11 | 37.63 | 0.95 | 0.03 | 0.02 | 0.24 | 12.86 | 100 | 11 | 47 | <0.08 | <0.11 | <0.30 | <0.12 |
| A322 (Lz) | 41.89 | 0.09 | 1.34 | 0.44 | 9.49 | 0.13 | 30.87 | 6.46 | 0.06 | 0.01 | 0.11 | 9.10 | 95 | 32 | 98 | <0.07 | 0.12 | <0.40 | <0.35 |
| A424 (Lz) | 37.58 | 0.06 | 1.17 | 0.41 | 7.72 | 0.11 | 37.53 | 1.34 | 0.03 | 0.01 | 0.29 | 13.74 | 105 | 10 | 46 | <0.06 | <0.10 | <0.36 | <0.10 |
| A425 (Lz) | 40.37 | 0.07 | 3.06 | 0.42 | 8.21 | 0.12 | 35.98 | 2.52 | 0.09 | 0.02 | 0.28 | 8.86 | 100 | 13 | 72 | <0.08 | 0.23 | <0.40 | 0.33 |
| A468 (Lz) | 37.21 | 0.06 | 1.23 | 0.42 | 7.43 | 0.11 | 38.93 | 1.43 | 0.02 | 0.01 | 0.29 | 12.86 | 101 | 10 | 49 | <0.06 | <0.09 | <0.30 | <0.10 |
| A473 (Lz) | 41.29 | 0.07 | 2.82 | 0.38 | 7.72 | 0.11 | 34.87 | 2.84 | 0.13 | 0.02 | 0.31 | 9.45 | 97 | 13 | 75 | <0.08 | 0.14 | 0.27 | 0.44 |
| A399 (Hz) | 39.04 | 0.05 | 0.81 | 0.37 | 7.56 | 0.08 | 38.41 | 0.36 | 0.03 | 0.01 | 0.31 | 12.97 | 104 | 6 | 28 | 62* | <0.08 | <0.26 | <0.50 |
| A400 (Hz) | 38.15 | 0.06 | 1.06 | 0.58 | 7.53 | 0.07 | 38.66 | 0.53 | 0.02 | 0.00 | 0.32 | 13.01 | 108 | 8 | 43 | <0.10 | <0.09 | <0.27 | <0.10 |
| A419 (Hz) | 37.67 | 0.07 | 1.97 | 0.51 | 8.62 | 0.11 | 36.61 | 0.36 | 0.02 | 0.01 | 0.32 | 13.73 | 120 | 14 | 83 | <0.08 | <0.09 | <0.36 | <0.12 |
| A476 (Hz) | 38.36 | 0.06 | 0.76 | 0.37 | 6.95 | 0.11 | 39.31 | 0.31 | 0.02 | 0.01 | 0.33 | 13.42 | 96 | 8 | 37 | <0.07 | <0.10 | <0.30 | <0.10 |
| A504 (Hz) | 38.61 | 0.06 | 1.36 | 0.47 | 6.98 | 0.11 | 36.66 | 1.75 | 0.03 | 0.02 | 0.28 | 13.68 | 93 | 11 | 53 | <0.06 | <0.11 | 0.29 | <0.10 |
| A514 (Hz) | 37.21 | 0.06 | 1.21 | 0.29 | 6.58 | 0.08 | 40.34 | 0.34 | 0.02 | 0.01 | 0.29 | 13.58 | 94 | 10 | 45 | <0.06 | <0.11 | <0.32 | <0.10 |
| A503 (Du) | 38.43 | 0.07 | 1.98 | 0.70 | 7.35 | 0.13 | 36.95 | 0.32 | 0.11 | 0.01 | 0.32 | 13.62 | 107 | 21 | 90 | <0.09 | <0.10 | <0.32 | <0.14 |
| A505 (Du) | 33.51 | 0.07 | 1.42 | 0.47 | 10.08 | 0.18 | 39.49 | 1.12 | 0.03 | 0.01 | 0.22 | 13.41 | 119 | 9 | 40 | 50* | <0.09 | <0.35 | <0.10 |
| A506 (Du) | 33.85 | 0.06 | 0.70 | 0.92 | 8.66 | 0.14 | 37.83 | 0.48 | 0.02 | 0.01 | 0.28 | 17.05 | 117 | 7 | 36 | <0.06 | <0.10 | <0.35 | <0.10 |
| Mean | 38.04 | 0.07 | 1.65 | 0.46 | 7.97 | 0.12 | 37.65 | 1.58 | 0.05 | 0.01 | 0.27 | 12.12 | 104 | 12 | 60 | — | — | — | — |
The serpentine mineral was identified at the Central laboratory of the University of Isfahan as antigorite on the basis of its X-ray diffraction pattern by Bruker D8 Advance XRD machine.
3 Petrography and mineral chemistry
In outcrop, these metamorphosed ultramafic bodies are grayish-green to dark-green. Generally, they are extensively serpentinized, metamorphosed and sheared, retaining very little primary relict texture. Main textures of the investigated rocks are porphyroblastic, granoblastic, nematoblastic, poikiloblastic and mesh texture. The textural relationships of mantle peridotites in Jandaq ophiolite demonstrate a prograde metamorphism from partially serpentinized peridotite to metaperidotite in response to a regional metamorphism in upper amphibolite facies conditions. A retrograde metamorphism has been developed after the progression. Therefore, the studied rocks have undergone three main stages of recrystallization. An initial retrograde hydration was followed by a progressive dehydration and a final retrograde hydration. The evidence of this complex hydration and dehydration reactions is presented by minerals description:
- - Clinopyroxenes (diopside with Mg# 0.91–0.97) and chromian spinels (Cr# 0.46–0.61) are relicts of the primary igneous mineralogy and are the most resistant minerals against the metamorphism and alteration. Inner part of spinels and most of clinopyroxenes are intact (Fig. 3B and 3G). The modal abundance of clinopyroxene in some rock samples reaches to 30% (very high) which partly are changed to tremolite during the metamorphism. Spinels altered to chromian magnetite in margin and their modal abundance in all samples is less than 3%. Cr2O3 contents of clinopyroxenes ranges from 0.71–1.31 wt% (Table 1).
- - Olivines (Fo88–Fo92) are metamorphic, produced during progressive metamorphism. This is suggested by very irregular grain boundary relations of olivines with other metamorphic minerals and fine magnetite inclusions [26]. Olivine grains show alteration to serpentine that is one of the evidences of last hydration. Concentrations of minor elements as TiO2, Al2O3 and CaO are very low. MnO content ranges up to 0.14 wt.%.
- - Orthopyroxenes (enstatite with Mg# 0.89–0.96) are elongate and much coarser-grained than coexisting olivines. The margins of all orthopyroxens are replaced by serpentine. This texture indicates that the orthopyroxene retrograde serpentinization has occurred. Al2O3 and Cr2O3 contents of analyzed orthopyroxenes are 0.49–1.94, and 0.03–0.40 wt.%, respectively. Concentrations of minor elements are low. Compositionally, the studied orthopyroxenes are similar to the reported metamorphic orthopyroxenes from other ultramafic metamorphosed rocks [14]. Orthopyroxenes occur as granoblasts to bladed grains with scalloped borders in contact with olivine and interpreted to record the prograde reaction (Fig. 3E):
- - Tremolite (Mg# 0.89–1) is common in these ultramafic bodies and present prismatic crystals and some times jack-straw texture (Fig. 3C and D). Some tremolites are poikiloblastic, having inclusions of opaque minerals and chlorite that are common around igneous clinopyroxene relicts; others present intergrowth with olivine and serpentine. Tremolites and clinopyroxenes are present as the most important Ca-bearing silicates in most of these rocks. Main chemical characteristics of analyzed tremolites are low concentration of Na2O (< 0.45% wt.%) and Al2O3 (< 1.62 wt.%), and high Mg# (> 0.89).
- - Anthophyllite (Fe3+# 0.52–0.53) is magnesian amphibole of these metamorphosed peridotites and is much less abundant than tremolite. Anthophyllite can be recognized by its parallel extinction and lack of twinning. This mineral makes sheaf-like aggregates (Fig. 3F). Concentrations of MgO ranges from 33 to 34.16 wt.%, and abundance of Na2O is very low (< 0.03 wt.%). This orthoamphibole has a restricted stability field between approximately 600 and 800 °C at pressures below 12 kbar [29].
- - Chlorite (peninite with Mg# 0.90–0.91) is present with faintly green pleochroic in matrix and sometimes in veinlets of studied rocks. Presence of this mineral is another evidence of the last retrograde metamorphism in greenschist facies. Cr2O3 content of analyzed chlorites is 0.08 to 0.30 wt.%.
- - Antigorite (Mg# 0.89–0.98), which is colorless or less commonly pale green, is dominant constituent of Jandaq ophiolite metamorphosed mantle peridotites. It occurs mostly as fine-grained crystals. Antigorite has partially replaced and surrounded the metamorphic olivines by the last metamorphic episode. This mineral identified by microprobe analyses and XRD pattern. Antigorites have a wide range of Mg# (0.89–0.98) and Al2O3 contents (1.35–4.12 wt.%). The upper stability limit of antigorite is between 500 and 600 °C [31].
- - Talc (Mg# 0.98) is common in orthopyroxene bearing rock samples. It is not aligned with respect to a foliation. The Na2O and Al2O3 contents of analyzed talcs range from 0.11–0.46 and 0.45–2.12 wt.%, respectively.
Based on the petrography and mineral assemblages, two rock types are distinguished in mantle peridotite section of Jandaq ophiolite:
- (1) Metalherzolites (clinopyroxene + spinel + olivine + tremolite + chlorite + serpentine + magnetite ± calcite);
- (2) Metaharzburgites (olivine + orthopyroxene + talc + anthophyllite + magnetite + spinel ± low amounts of chlorite and tremolite).
4 Whole rock chemistry
Table 2 lists the chemical compositions of 44 whole rock samples. Twenty-five samples from mantle peridotites of the Jandaq ophiolite and 19 samples from the Ashin ophiolite analysed to find the chemical nature of studied ultramafic system. The mean of analyzed samples from each ophiolite is presented in lower row.
All the analyzed samples from the Jandaq ophiolite are rich in MgO (23.48–40.47 wt.%), with wide range in the concentrations of Fe2O3* (5.45–13.5 wt.%), Al2O3 (0.66–8.07 wt.%) and CaO (0.70–12.45 wt.%). Cr2O3 and TiO2 contents are 0.04–0.75 wt.% and 0.06–0.23 wt.%, respectively. Considerable amounts of LOI confirm the extensively serpentinization of these rocks and presence of hydrous minerals. The lherzolite samples which contain considerable amount of relict clinopyroxene (J716, J719, J723 and J724), present the highest contents of CaO, Sc and V. Low-CaO lherzolites are essentially made by serpentine and fine-grained tremolite.
Study of whole rocks chemical composition shows similarity of all analyzed rock samples with the pattern of mantle peridotites from ocean basins. Comparison of whole rocks chemical composition from the Jandaq and Ashin ophiolites and using the mean of analyses reveals that the Jandaq ophiolite samples have lower contents of MgO, Cr2O3, MnO, NiO, Co, V and higher amounts of SiO2, TiO2, Al2O3, Fe2O3*, CaO, Na2O, K2O, Sc and REE. These chemical characteristics show the undepleted nature of mantle peridotites in Jandaq ophiolite.
5 Discussion
5.1 Metamorphic condition of P-T equilibration
The dominant metamorphic mineralogy of these rocks included olivine, tremolite, orthopyroxene, talc, anthophyllite, chlorite, serpentine and magnetite. Based on the mineralogical composition and using the scheme of Evans [14], these mineral assemblages correspond to tremolite peridotite, which is indicative of the amphibolite facies that followed by green schist facies. These results are confirmed by Table 13- 2 in Spear [28], mineralogical study of amphibolites, and using the amphibole barometry and amphibole-plagioclase thermometry [33]. Subsequent amphibolite to greenschist facies retrogression modified the mineralogy of these progressively metamorphosed mantle peridotites, as evidenced especially by serpentinization of metamorphic olivines and orthopyroxenes, late growth of chlorite and thin veins of serpentine developed in the ultramafic rocks.
5.2 Petrogenesis
The fertile lherzolite in the mantle section of the Jandaq ophiolite is a predominant rock unit based on the field and the petrographical studies. Abundance of lherzolite, low amount of harzburgite and dunite, and limited amount of amphibolite (formerly basic volcanic rocks) in this ophiolite reveals that the partial melting and continual melt production is not an extensive phenomenon in this mantle section. Therefore, the volume of ascending melt should be low and wall rock/melt interactions would not occur in large scale. According to the quantitative melting indicator for mantle residues [16] and using the composition of spinels from the Jandaq ophiolite, the degree of partial melting is lower than 18%. But the field investigations, small harzburgite layers which are uniformly dispersed within the lherzolite and considering the very high lherzolite/harzburgite ratio, show that these partial meltings are discrete and non-pervasive episodes in this ophiolite.
Highly variable contents of Al2O3 in analyzed samples (0.79–8.07 wt.%) is one of characteristics of orogenic lherzolites [9]. Low amounts of Cr2O3 associated with highly variable content in Al2O3 appear as characteristics of peridotite massifs [9], compared to cratonic and abyssal peridotites that show a large spread of Cr2O3 whole rock content correlated with Al2O3 content. The contents of other mildly incompatible elements as V, Sc and Yb, in whole rock chemical data of Jandaq mantle peridotites, and Cr2O3 content of clinopyroxenes, support this idea. It resembles that the Jandaq mantle peridotites provide us the Earth's mantle with unique exposure. Mineral chemistry of clinopyroxenes (diopsides) reveals the sub-oceanic crust mantle origin of the Jandaq peridotites (Fig. 4A and B).
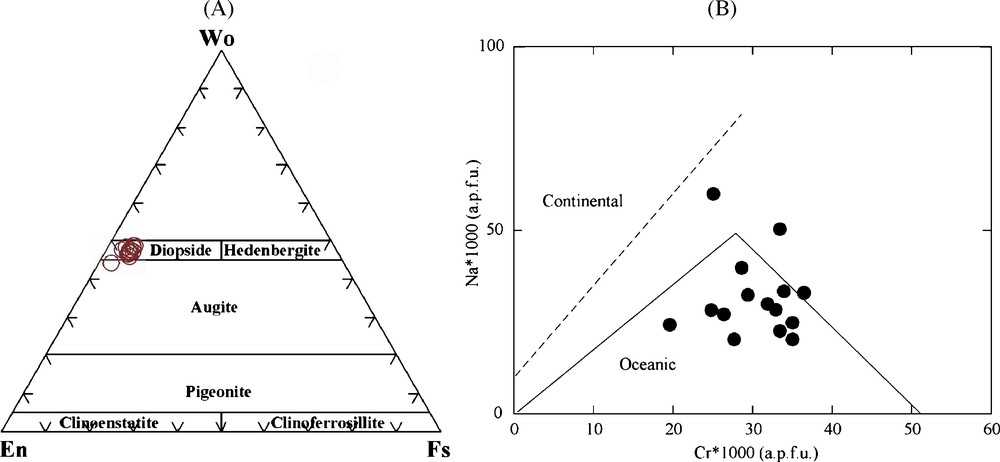
Chemical diagrams of clinopyroxenes. (A) Ternary of pyroxenes classification and diopside composition of Jandaq clinopyroxenes, (B) Atomic Cr and Na content of clinopyroxenes formula unit to confirm oceanic nature of the Jandaq peridotites. The diagram is from [17].
Diagrammes chimiques des clinopyroxènes. (A) Classification ternaire des pyroxènes, indiquant la composition diopsidique des clinopyroxènes de Jandaq, (B) Pourcentage atomique Cr et Na dans la formule-unité des clinopyroxènes, confirmant la nature océanique des péridotites de Jandaq. Le diagramme provient de [17].
Several recent contributions (e.g. [7,20,21]) suggest that a number of lherzolites could represent refertilized mantle, that is depleted (harzburgitic) mantle in which percolating magma crystallized secondary clinopyroxene. In this case, the Cr2O3 content of clinopyroxene is not marker of the initial fertility of the peridotite concerning this element. However, in the case of Jandaq ophiolite, field studies, transitional succession of lherzolite – harzburgite – dunite, absence of lherzolite – dunite contact, homogeneous rock samples in mineralogy, and low degree of partial melting, reject this hypothesis. Using Le Roux [21], whole rock versus mineral chemistry discriminant diagram, supports partial melting trend instead of refertillization one.
About of using the chemical composition of whole rocks and minerals, in Paleo-tectonic reconstruction of ophiolites, Nicolas and Boudier [25] discuss the validity of geochemical discrimination diagrams in their application to ophiolite environments. In particular, they question the validity of Cr# in spinel by reference to abyssal peridotite data [2,12]. They reveal that the common used discriminant diagrams do not discriminate anything. Therefore, the geotectonic discrimination diagrams are not used in this article.
5.3 Characterization of the ophiolite type
According to the petrography and considering the clinopyroxene relict and tremolite modal contents, it can be concluded that the protolith of poly-metamorphosed mantle peridotites of the Jandaq ophiolite have originally been lherzolite (most) and harzburgite (less), and dunites are rare in this ophiolite. This points to LOT characteristic of this ophiolite, but anyway, it should be noted that the distinction between LOT and HOT ophiolites is not straightforward by petrography of poly-metamorphosed mantle peridotites and more evidences are needed (e.g. whole rock chemical data).
Considerable amounts of TiO2, Al2O3, CaO, Na2O, K2O, Sc and REE in the Jandaq samples, points to LOT nature of the Jandaq mantle peridotites and supports the petrographical results. By wide spread partial melting and continual basaltic melt production, the contents of these elements will decrease. Presence of lherzolites with high content of CaO (10.47–12.45 wt.%), and considerable amount of modal clinopyroxene (ranges up to 30%), can be interpreted as intact peridotites of mantle. Alternately, the low TiO2, Al2O3, CaO, Na2O, K2O, Sc and REE contents in the Ashin ophiolite mantle peridotites argue for the HOT nature of this ophiolite, high degree of pervasive and continual partial melting and clinopyroxene removal for production of primary basaltic melt.
5.4 Implications for chromitite potential
The above two sections (5.2 and 5.3) reveal that the Jandaq ophiolite is characterized by a lherzolitic mantle section and belongs to the Lherzolite Ophiolite Type (LOT). Therefore, based on the general rule, the chromitite should be generally absent. Arai [4] shows that in Al-rich mantle peridotites (as Jandaq ophiolite that amount of Al2O3 reaches up to 8.07 wt.%), the podiform chromitite is almost absent or very small in amount.
Comparison of chemical whole rock data from the Jandaq (as a LOT) and Ashin ophiolites (as a HOT) reveals that the Cr2O3 content of mantle peridotites from the Ashin ophiolite (0.46 wt.%) is higher than that in the Jandaq ophiolite (0.38 wt.%), in spite of abundant chromitite formation in the Ashin ophiolite. This difference shows that the Cr2O3 content of the Jandaq ophiolite originally was low and this mantle is not suitable for considerable chromitite formation through high degree of partial melting and wide spread melt-wall rock reaction.
The study of chemical composition of clinopyroxenes shows that the chromium hosted by pyroxenes, is still largely retained by these mineral and may form limited chromitite after high degree of partial melting. The range of Cr2O3 content in clinopyroxenes is 0.71–1.31 wt %.
6 Conclusions
In Jandaq meta-ophiolite, the predominant mantle peridotite is lherzolite, which composed of clinopyroxene, spinel, olivine, tremolite, chlorite, serpentine and magnetite. Geochemically, the minerals and whole rock samples appear as orogenic peridotite massifs. The main geochemical characteristics of these fertile peridotites are low amount of Cr2O3 and highly variable content of Al2O3. Based on the field and petrographical studies, chemistry of minerals and whole rock samples, the Jandaq peridotites belong to the LOT (Lherzolite Ophiolite Type), and have no chromite concentration potentiality, following the thermal structure and chemical composition.
Acknowledgment
The author is indebted to Professor Adolphe Nicolas, Professor Jacques Léon Robert Touret and Professor Françoise Boudier for providing useful comments on the manuscript. Supports of the University of Isfahan, Dr. Jurgen Koepke and Leibniz University are gratefully acknowledged.


