1 Introduction
Lake Nyos is a crater lake located in NW Cameroon, along the well-known “Cameroon Volcanic Line” (Déruelle et al., 2007), at about 300 km north-west of Yaounde. In the evening of August 21, 1986, a great quantity of lethal gases (mainly CO2) and water droplets bursted out of the lake, killing 1746 people and all cattle in the valley below. Two years before, also in August, a similar event, with luckily much less casualties, occurred in the neighboring Lake Monoun, distant from Lake Nyos by about 30 km. Understandably, these dramatic events have led to many studies (Evans et al., 1994; Freeth, 1990; Kusakabe et al., 1989, 2000; Pintér et al., 2009), concluding that around 1.24 Mt of CO2 were released to the exosphere during Lake Nyos 1986 event (Kusakabe et al., 1989) and that deep lake waters were progressively loaded with magmatic CO2. This has resulted in a lower density at depth, compared to the higher water density at the surface. Contrary to most crater lakes, in which this disequilibrium situation is constantly restored by a progressive migration of gas-loaded waters towards the surface, this type of density inversion (light deep against heavy surface waters) was very sudden and catastrophic, resulting in a gigantic wave of CO2 loaded foam which moved along the valley at great speed, a « nuée humide » which can almost be compared to the « nuées ardentes » of andesitic volcanoes.
If the magmatic origin of the gases is well established, notably from their isotopic signature (Coltorti and Grégoire, 2008), many questions remain open, concerning notably the gas source at depth and the way by which it could accumulate at the bottom of the lake. There was no sign of eruption prior to the outburst; Lake Nyos had been formed by a hydromagmatic eruption more than 400 years ago (Lookwood and Rubin, 1989), and since that time no sign of volcanic activity had been noticed. Alkaline basaltic lavas were erupted at this occasion, carrying a number of mantle xenoliths which bring much information on the nature and physico-chemical evolution of the Upper Mantle below the Cameroon Volcanic Line (Teitchou, 2008). It is well known that, especially in continental settings, these xenoliths carry CO2 – bearing inclusions (Roedder, 1984), which evidence the possible existence of a CO2-rich free fluid phase at depth, especially at the time of the volcanic eruption. The present study has been undertaken in order to see if these inclusions, until now never reported despite the number of investigations done in the region, did exist in Lake Nyos ejecta and, as they were rapidly discovered, which information they could provide on the sequence of events leading to the dramatic issue. Incidentally, it was found during the course of investigation that these inclusions show some interesting features, which give significant information on the CO2 origin, as well as the way by which it can occur as a free gaseous phase at mantle depth.
2 Mantle xenoliths in Lake Nyos lavas
Mantle peridotite xenoliths in basaltic lavas of the Lake Nyos volcanic unit are predominantly spinel-bearing lherzolites and harzburgites (Teitchou, 2008). Most of them (more than 60% of collected samples) are spinel lherzolites, composed of olivine (55–70%), orthopyroxene (7–30%), clinopyroxene (6–20%) and spinel (1–6%). Less abundant (30%) harzburgites are composed of olivine (71–83%), orthopyroxene (10–20%) and clinopyroxene (2–4%). The mineralogy and geochemistry of these xenoliths have been recently thoroughly investigated (Teitchou, 2008; Teitchou et al., accepted). A number of these samples was then investigated for fluid inclusions, focusing on lherzolites as these contain by far the most abundant inclusions (see below). As all samples present more or less the same characteristics, it was felt that five samples would give a good representation of the xenolith population as a whole: four lherzolites (NK05, NK09, NK11 and NK14) and one harzburgite (NK10). Texture of the latter is protogranular, against porphyroclastic for the lherzolites (for NK14, transitional porphyroclastic to equigranular). In fact, sample NK14 turned out to be the most typical and interesting, and it has served as a basis for this presentation. Other lherzolite samples are however rather comparable, showing basically the same features than those described in the present work.
For all xenoliths, details of the geochemistry (with the same samples numbers) are given in Teitchou (2008) and Teitchou et al. (accepted). It will be sufficient here to say that REE-contents in clinopyroxenes, normalized to primitive mantle, point to the existence of two groups of xenoliths:
- (i) group 1, with “spoon” shaped patterns (HREE enrichment compared to MREE and LREE; [La /Sm] N: 0.08–5.50; [Sm /Yb] N: 0.47–1.17 and [La/Yb] N: 0.03–6.45). In the present study, this group includes lherzolites NK05, NK09 and NK11, as well as harzburgite NK10;
- (ii) group 2 (in the present study: lherzolite NK14) shows “LREE-enriched” clinopyroxenes; [La /Sm] N: 0.73–1.35; [Sm /Yb] N: 3–6.25 et [La /Yb] N: 3.25–4.5. HREE are also significantly fractionated, as evidenced by [Dy/Lu] N ratios ranging from 1.6 to 3 (group 1: 1–1.1).
As discussed in detail elsewhere (Teitchou, 2008; Teitchou et al., accepted), these geochemical features can be explained by a low degree of mantle melting (around 5%) followed by extensive metasomatic processes, which for group 2 can even erase the partial melting signature. In all cases, candidate for metasomatic agent has been estimated to be more or less alkaline- and carbonated – mafic silicate melts.
3 Fluid (and melt) inclusions
As usual, inclusions were observed in double polished plates, in the present case roughly 60 μm thick. Compared to most inclusion plates, this relatively small thickness (approximately the double of an ordinary thin section) has the disadvantage that dense fluids may have escaped from the cavity, the internal pressure in the inclusion, which as indicated below can reach up to 1 GPa at the time of the volcanic eruption, being still of the order of 100's bars at room temperature. But most inclusions would have leaked anyway, even in much thicker plate. A relatively thin one, much closer to an ordinary thin section, offers a better possibility to observe detailed relationships between the inclusions and the host mineral, in the present case the most interesting aspect of our study.
Compared to many other cases, e.g. in the French Massif Central (Bournac: Bilal and Touret, 1976) or southern Italy (Heblei: Sapienza and Scribano, 2000), Lake Nyos xenoliths contain relatively few inclusions. None were found in a number of investigated samples and, in those retained for the present investigation, inclusions occur only in few spots of any thin section (Fig. 1). Many mineral sections are inclusion-free, but in some of them inclusions, not especially small (size typically in the 5–30 μm range), can be found by careful observation, either along grain boundaries or, more interestingly, within mineral grains. Like in most lower crustal- or mantle xenoliths, these inclusions belong broadly to two categories:

Lherzolite NK14, general view. Center, the CO2 inclusion-rich clinopyroxene (Cpx) crystal shown in Figs.2C and 3.
Lherzolite NK14, vue d’ensemble. Au centre de la photo, le cristal (Cpx) riche en inclusions de CO2 des Fig. 2C et 3.
3.1 Late melt (mainly glass) inclusions
A number of xenoliths show signs of discrete, incipient melting, which has taken place in the enclosing lavas on the way up to the surface. A good sign is a reddish color of many minerals, especially olivine. More direct evidence is marked by intricate aggregates of mineral remnants and more or less devitrified glass, occurring typically at junctions of mineral boundaries (notably between olivine and clinopyroxene). A number of « trails » of very small inclusions, elongated and curved in shape, are frequently issued from these domains. These inclusions are filled with a clear glass, a small, relatively constant “shrinkage” bubble, corresponding to few % of the total volume. It is not uncommon to see several bubbles, all of the same size, in a single inclusion, indicating that the glass was slowly degassing at the time of the inclusion formation. No sign of detectable gas was found in the shrinkage bubble, showing that it has occurred at high temperature and low pressure, presumably during the volcanic eruption, at or very near of the surface. Such inclusions occur in virtually all volcanic ejecta and, if they could bring some information on the nature of the volcanic glass, they have little bearing with our major concern, namely the nature and source of gases at the mantle source.
3.2 Early CO2 inclusions
Far more interesting is the second type of inclusions, dark and monophase (gaseous), which as first sight look like empty cavities (Figs. 1 and 2). Most of them are indeed now empty, fluids inside the cavity having escaped during the eruption or sample preparation. However, the shape (elongated tubes or, more typically, negative crystal), general appearance, and location within the mineral host are not ambiguous: as first shown in 1965 by E. Roedder (1984), these inclusions do or did contain a supercritical fluid, which in most cases investigated so far turned out to be pure (or almost pure) CO2. A complete investigation would require microthermometry measurements, in order to determine the fluid density, as well as Raman analyses to check its purity. These measurements were not done in the present study, but an independent investigation (Pintér et al., 2009), that was brought to our attention after the submission of this manuscript, provided us with additional information, which could be used in our discussion. In the literature, average maximum CO2 densities recorded so far in mantle xenoliths are close to 1 g/cm3, corresponding to an homogenization temperature (to liquid) of about −10 °C (Bilal and Touret, 1976, Evans et al., 1994, Honda et al., 1995). This gives an internal overpressure of roughly 1 GPa at the surface at the time of the eruption, illustrating the remarkable strength of the mineral host in the rapidly evolving volcanic system. These values would correspond to about 30 km depth for the formation of these inclusions. These estimates are however very minimal. We discuss below the reasons why some inclusions at least were made at the time of mineral equilibration in the xenoliths, with P = 2 GPa and T = 1300 °C as reasonable estimates (Teitchou, 2008, Teitchou et al., accepted). The depth of inclusion formation would then be about 60 km, with a CO2 density, estimated from the PVT equation of state (Touret and Bottinga, 1979), above 1.2 g/cm3.
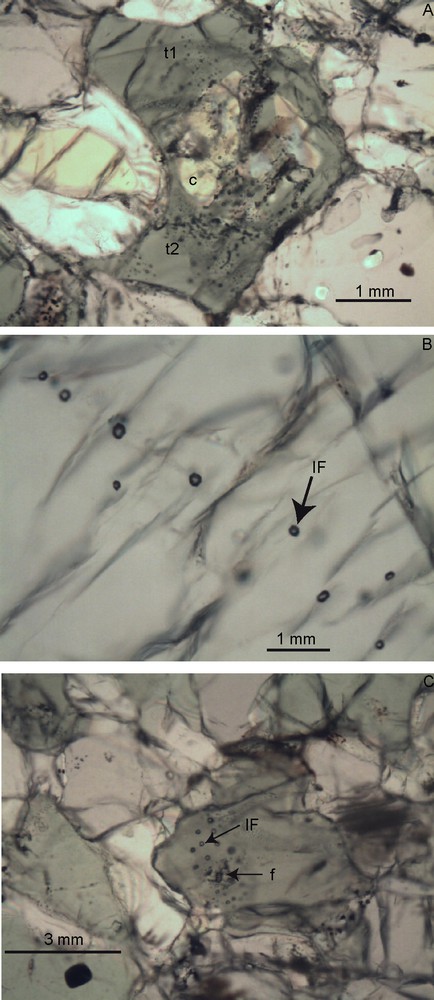
Fluid inclusions (CO2) in Lake Nyos lherzolites. A. Sample NK14. Another Cpx crystal with many inclusions, clustered in the core of the crystal (c), surrounded by relatively early trails, partly transposed (t1) and late trails made of mostly exploded inclusions (t2). B. Sample NK05. Well-defined trail of CO2 inclusions (IF), oriented at right angle on the Cpx major cleavage. C. Sample NK14, detail of the inclusion cluster shown in Figs. 1 and 3. IF: Fluid (CO2) inclusions, f = “melted zone” (see text)
Inclusions fluides (CO2) dans les lherzolites de Nyos. A. Un autre cristal de clinopyroxène (échantillon NK14) contenant de très nombreuses inclusions de différentes générations. B. Échantillon NK05, traînée bien définie d’inclusions (IF) grossièrement orthogonale sur la direction du clivage du Cpx. C. Échantillon NK14, détail du groupe (cluster) d’inclusions décrit dans les Fig. 1 et 3. IF: Inclusions fluides (CO2), f = « zone fondue » (cf texte).
The lines above were written at the time of the submission of the present manuscript, on early September 2009. By a lucky coincidence, an independent investigation by Pintér et al., 2009, presented during the XX ECROFI meeting in Granado (Spain) on 29 September 2009, provided a number of supplementary data on these interesting samples. These authors have also studied fluid inclusions in mantle xenoliths, not only from lake Nyos, but also from neighboring Lake Barombi. With some differences (see below), their observations broadly confirm ours. Most important, they were able to perform microthermometry measurements (homogenization and melting temperatures) which, if they indicate that the range of composition might be more complicated than we had anticipated, confirm the existence of high-density CO2 fluids, according to the prediction already done in this work.
4 The unique case of Lake Nyos: CO2-rich inclusions occur dominantly in clinopyroxenes
It is known by experience that, even for volcanoes occurring in apparently similar settings, the number of inclusions occurring in any xenolith varies grossly from one volcano to the other. In some cases (e.g. Bournac, French Massif Central and Heblei, Sicily), peridotite xenoliths contain quite abundant inclusions, occurring in most mineral phases virtually, whereas others are virtually inclusion-free. This is notably the case for peridotites with equigranular texture. The case of Lake Nyos is intermediate, in this respect most interesting. CO2-inclusions may be locally abundant, but only within some mineral grains, which in all cases observed so far in the samples we have investigated, turned out to be clinopyroxenes. Pintér et al., 2009 report “… an older inclusion generation occurring randomly in olivine and orthopyroxene, partly or completely decrepitated…” (p. 189, slightly summarized). We have not observed such inclusions in the samples we have studied.
In the example shown on Fig. 1 (sample NK14), only two pyroxene grains in the whole thin section did contain inclusions. These are shown in Figs. 1 and 2 A and C, respectively. Inclusions are isolated or, more typically, they form tridimensionnal “clusters” (Touret, 2001) dispersed within the mass of the mineral host. Some clusters, containing few 10’ inclusions, are limited to few spots in the mineral host, whereas others contain many more inclusions, giving to the host a vacuolar, spongy appearance (Fig. 2A). In this case, neighboring inclusions are often disposed along a roughly planar surface (“trails”), which may eventually cross the grain boundary and grade into the next crystal. Such a trail is shown in Fig. 2B.
The most typical example of well-defined cluster that we have found, in lherzolite sample NK 14, is shown in Figs. 1 and 2C, respectively. About 30 inclusions, all almost identical in size (diameter about 30 μm) and shape (roughly spherical, close to negative crystal) are regularly disposed in the volume of the mineral host, not along any planar surface. The center of the cluster does not contain any inclusion. It is occupied by a low-polarizing zone with indistinct boundaries, which includes an intricate mixture of microcrystals and low-birefringence mineral phases, possibly devitrified glass. A total of 33 microprobe analyses have been done on three clinopyroxene crystals from samples NK14: 25 within the cluster shown in Fig. 2C, the other eight on two crystals containing spinel, either in inclusions (analyses 26 to 29), or in exsolutions (analyses 30 to 33). All analyses (available upon request to the corresponding author) give roughly comparable results, with the following composition, expressed in % of clinopyroxene solid solution end members (wollastonite [Wo(Ca)], enstatite [En (Mg)] and ferrosilite [Fs(Fe2+)]: Wo [Ca]) between 44.6 and 46.2, En (Mg) between 47.7 and 49.7, and Fs(Fe2+) between 4.3 and 6.8. The Mg number (X Mg = Mg /Mg+Fe2+) is also relatively constant at 0.8 to 0.9. For this last parameter, the most sensible indicator of xenolith mineral equilibration, this small variation range is not at random. The 25 analyses mentioned above have been done along two orthogonal profiles, indicated as lines 1 and 2 in Fig. 3. Analyzed spots, with corresponding numbers, are reported on the profiles. As shown on the graph of Fig. 3, the X Mg number along these profiles shows a distinct decrease towards the center of the “fusion” zone, indicating that the composition of the mineral host has not fully homogenized after the formation of the melting zone and related inclusion cluster.
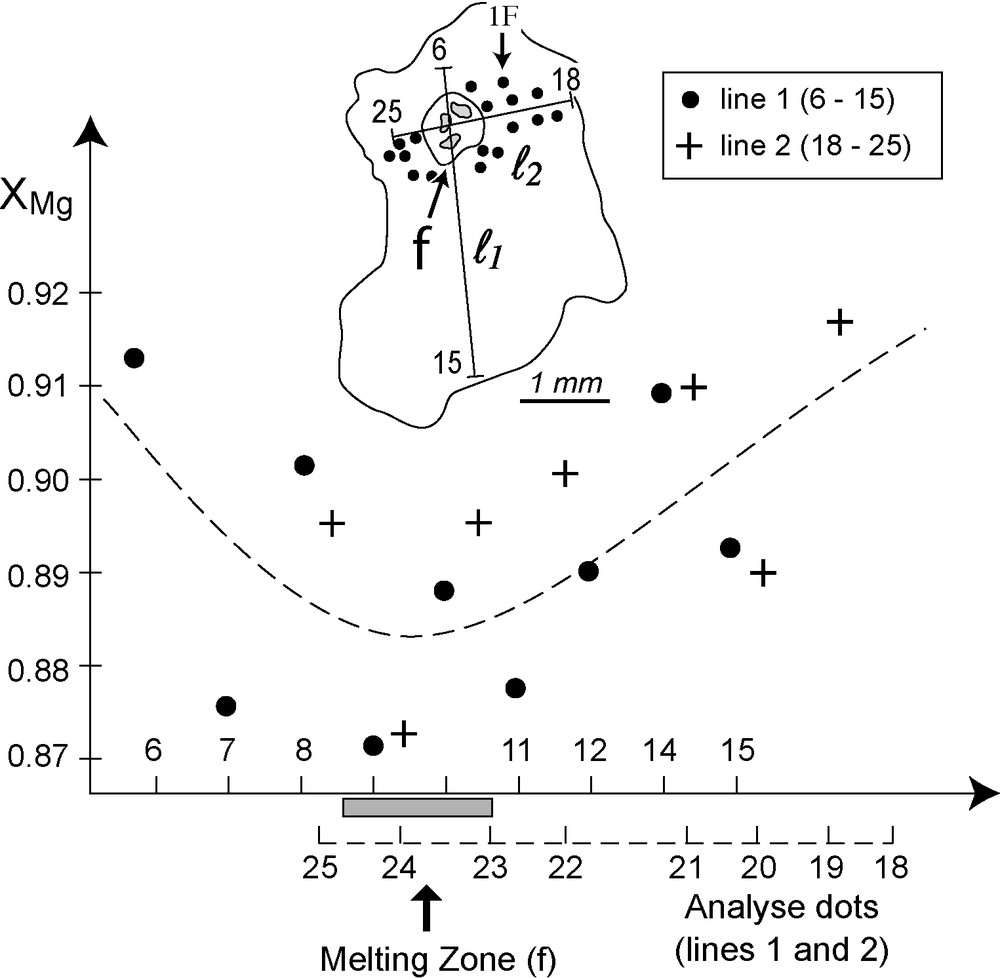
Electron microprobe analysis of the Cpx crystal shown in Figs. 1 and 2C. XMg: Mg/Mg+Fetotal.Lines 1 and 2, emplacement of the analytical spots, with indication of analyzed numbers, f = “melted zone” (see text).
Analyse par microsonde électronique du cristal de Cpx représenté dans les Fig. 1 et2C. XMg : Mg/Mg + Fetotal. Lignes1 et 2: emplacement des points d’analyse, f = “zone fondue”.
5 CO2 formation: a consequence of mantle metasomatism
The fact that CO2-inclusions occur dominantly in clinopyroxenes, also that they are not related to local melting in basaltic lavas, precludes the possibility that fluids were issued from the xenolith host-basalts. If this was the case, the late melt inclusions would contain CO2, which would also occur in secondary trails, cutting all minerals. This situation has been observed in a number of volcanic xenoliths, notably in the French Massif Central (e.g. Bournac: Bilal and Touret, 1976). It is possible that some late trails reported by Pintér et al., 2009 for their “younger generation” have this origin. We believe however that it is more probable that they are “pseudo-secondary” features (Roedder, 1984), generated inside the mineral host. What we have observed in our samples is different: all inclusions that we have seen, either primary, made at the time of the mineral equilibration of the pyroxene host, or, if occurring in clusters, “pseudo-secondary”, resulting from the decrepitation of a former primary inclusions. The “melted” zone shown in Fig. 3 is a good example. It contains only inclusions which have been formed inside the crystal host, without evidence of fluids brought from outside. CO2 there must have been generated “in-situ”, from a mineral reaction which has led to the local melting of the pyroxene. A most plausible explanation is the destabilization of a calcium-rich carbonate (calcite) at the time of pyroxene melting. This would not only liberate CO2, but also Ca ions, which by diffusion in the pyroxene structure could depress XMg, as shown in Fig. 3. Such a mechanism is further supported by a number of arguments. We know that peridotites under the Cameroon Volcanic Line show a strong evidence of mantle metasomatism, caused by carbonate-rich percolating melts (or fluids) (Teitchou et al., accepted). We know also that clinopyroxenes are the “sensible” minerals in “fertile” peridotites, being the first mineral phases destroyed during partial melting and formation of basaltic melts. The cluster shown in Fig. 3, with its central melted zone, is then believed to be a direct illustration of this process.
In conclusion, we believe that CO2 was first produced at mantle depth by carbonate destabilization during interaction between percolating metasomatic melts (or fluids) and mantle peridotites. Phase stability in a carbonated mantle (i.e. in presence of CO2 fluids, carbonate melts, and /or carbonates) has been thoroughly studied experimentally (e.g. Frezzotti et al., 2009). At “normal” mantle temperatures (i.e. about 1250 °C), carbonates, either occurring as mineral phases or dissolved in melts, decompose at a pressure of about 2 GPa (depth about 60 km). As indicated above, cations (Ca, Mg, Fe) can be incorporated into silicate structures notably pyroxenes, whereas free CO2 considerably depresses the dry peridotite solidus (by about 200 °C, leading to partial melting and formation of alkaline basalts, see e.g. Fig. 6 in Frezzotti et al., 2009). Free CO2 appears thus when alkali basalt melts are first generated, eventually supplying a considerable amount of potential energy to boost the basalts towards the surface. Even if, in the present case, there is no reliable mineral P–T indicators in the xenoliths themselves, we can have an idea of possible P–T conditions at which this sequence of events has happened from commonly assumed conditions of mantle partial melting during the formation of basaltic melts: T about 1250–1300 °C, P = 2–2.5 GPa. CO2 equation of state would then give a CO2 density (or molar volume), of about 1.2 g/cm3 (35 cm3/mole) (CO2 PVT extrapolated from Touret and Bottinga, 1979, pers. com. M.L. Frezzotti). Microthermometry measurements made by Pintér et al., 2009, even if they raise new problems, are in line with these estimates. They have measured in their investigated samples, both in Nyos and Barombi, low homogenization temperatures to liquid, typical for dense CO2-rich fluids. For the pure CO2 system, the relation between homogenization temperature and fluid density is immediate, from CO2-PVT data. But the result in terms of density (or molar volume) can be somewhat modified, due to the possible occurrence of other gases. Some melting temperatures measured by Pintér et al. (2009) are very low, between −68 and −95 °C for some Nyos samples. These very low values are certainly due to the presence of a polar gas, such as CH4 or, more probably N2. However, in all cases of comparable melting temperatures so far encountered by the senior author, homogenization did occur to gas, indicating low-density fluids. On the other hand, fluids which homogenize to liquid in the vicinity of CO2 triple point (−56.6 °C), as measured by Pintér et al., 2009 in Barombi samples, have always proven to be almost pure CO2, which can reasonably well be expressed in term of “CO2-equivalent. The “quite low homogenization temperature of CO2-rich phase to liquid phase, ranging from −53 to −44 °C in Nyos and −52 to −38 °C in Barombi xenoliths” (p. 190, Pintér et al., 2009) corresponds presumably to this type of fluids. For Nyos, these data would correspond to a CO2-equivalent density of 1.15 and 1.13 g/cm3, respectively (Touret and Bottinga, 1979). Raman measurements would be very desirable, and hopefully they will be done in the future. We feel however confidant that they will not change the main conclusion of the present work for the scope of this paper, namely that the CO2 density at the source was well above that of liquid water.
6 Not one, but two successive density inversions!
All petrographical evidences outlined above indicate that CO2 first liberated at the mantle source had a density at least equal, most probably significantly higher that of liquid water (1 g/cm3). This result is by no mean specific to lake Nyos: from the experience of the first author, high-density CO2 inclusions have been found (notably in the French Massif Central) or can be suspected in most mantle xenoliths investigated so far. In most eruptions, the volcanic column continues without interruption up to the surface. Volume is not limited, and deep CO2 progressively expands, with a considerable expansion and rapid decrease of the density. This phenomenon is very general, explaining the considerable amount of mantle-derived CO2 released during or after the eruptions in some volcanic areas (e.g. southern Italy, Sapienza and Scribano, 2000). The case of a crater lake, like in Nyos, is different: water may infiltrate in the chimney at some kilometer depth, forming a fixed volume in which deep CO2 has no possibility to expand. Then, like for any inclusion (also a constant volume system), CO2 has to follow an isochore (equal density line). For a reference density of 1 g/cm3, the CO2 isochore starts in the vicinity of CO2 triple point, at about −50 °C. It crosses the almost vertical liquid H2O isochore at a temperature of about 30 °C and a pressure of about 0.1 GPa (Fig. 4). These P–T conditions (I in Fig. 4) define the density inversion for pure H2O (liquid) and 1 g/cm3 CO2 systems. At higher temperature, the relevant CO2 isochore crosses successively H2O isochores of lower density: CO2 fluids are heavier than H2O, they tend to sink. At lower temperature, they are lighter. CO2-fluids tend then to rise to ultimately reach the surface. Intersections of CO2 and H2O-isochore pairs of identical densities define the complete density inversion curve, shown as l in Fig. 4. The situation that we know at the Earth's surface, namely gaseous CO2 bubbles rising in liquid H2O, is then only valid for a limited domain of geological interest, not exceeding a few kilometer depth. Below this level, CO2 tends to sink in liquid water, as long as the temperature remains above few 10's °C. Such a situation has been observed on several occasions in deep-sea ocean waters. It had been reported in early (unfortunately secret) Soviet literature (A. Chupin, Novosibirsk, pers. com. to J.T.). But it has also been found more recently (1995) during submersible experiments in Japan (Shinkai 6500 expedition, Honda et al., 1995). H2O–CO2 density inversion was then observed to occur at a depth of 2569 m at the low temperature (about 4 °C) of deep ocean water. This low temperature may somewhat complicate the problem, notably through the possible formations of gas hydrates (clathrates). Nevertheless, it gives a reasonable order of magnitude, which confirms the data shown in Fig. 4.
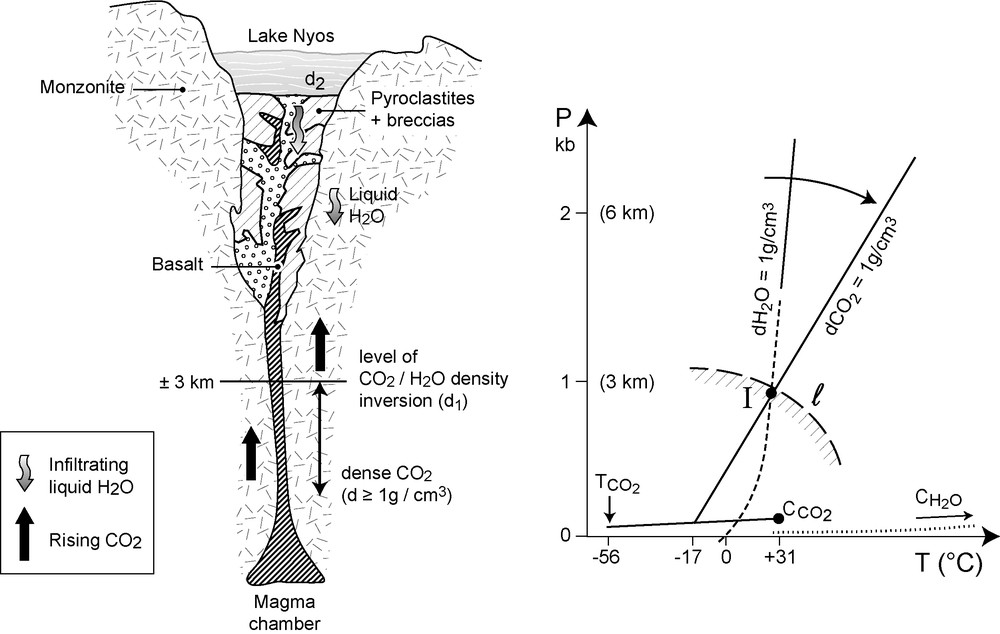
Density inversions in Cameroon lake volcanoes. Left: Cross section of the Lake Nyos volcanic system (after Lockwood and Rubin, 1989, modified). d2: shallower density inversion (lake waters), d1: deep density inversion between descending waters and dense volcanic CO2. Right: H2O and CO2 isochores for the reference density of 1 g/cm3. I: inversion density point, l = line for different densities. T = triple, C = critical points for CO2 and H2O, respectively.
Inversions de densité dans les lacs volcaniques du Cameroun. À gauche: section du système volcanique du Lac Nyos (d’après Lockwood et Rubin, modifié). d2 : inversion de densité superficielle, dans les eaux du lac. d1 : inversion de densité profonde entre eaux du lac infiltrées et CO2 volcanique dense. À droite, isochores de CO2 et H2O, pour la densité de référence de 1 g/cm3. I = point d’inversion des densités, l: ligne pour des densités différentes. T et C : points triples et critiques pour CO2 et H2O, respectivement.
In conclusion, we propose that the sudden gaseous bursts observed in the Cameroon volcanoes are caused, not by one, but by two successive density inversion phenomena. First of all, as advocated by all students of these remarkable eruptions, a shallow one, indicated as d2, in Fig. 4. Strictly confined to the lakes, it corresponds to a density inversion between deep, CO2-loaded waters near the bottom of the lake, and denser, CO2-free waters near the surface. But the results of the present study suggest that this d2 density inversion has been preceeded (and fed) by a deeper density inversion (d1, Fig. 4), at the level between infiltrating lake waters in the volcanic pipe, to a depth of about 3 km. Trapped dense CO2, issued from mantle depth during the last eruption, some 500 Ky ago (Aka et al., 2008), could quietly remain in a deep reservoir, between the magma chamber and the water level, as long as the regional temperature at depth remains above the density inversion temperature. The heat accumulated during the eruption, the high geothermal gradients in rifted volcanic areas, suggest that the major part of this deep CO2 is still probably under these conditions. Only in the contact zone with descending waters, temperature may be low enough to initiate the d1 inversion, leading to a regular flow of gaseous CO2 towards the surface. Understandably, the present-day gas recharges in both Nyos and Monoun lakes are carefully controlled, notably through the international Nyos and Monoun Degassing Project (NMDP) (Issa et al., 2006). Measurements between August 1987 and January 2004 have indicated a recharge in lake Monoun of 8 Mmol/year, constant during this entire time interval. This constancy indicates that a large part of the “deep” reservoir remains untouched at depth, with (in the absence of external hazards, such as earthquakes or new eruptions) no reason for the recharges to change significantly in a foreseeable future.
Acknowledgments
We thank M. Cuney (Nancy) for providing facilities for microphotography, as well as helpful discussion and suggestions. We also thank P. de Parseval for his help with the electron microprobe analysis as well as Christiane Cavare-Hester for her help with Figs. 3 and 4. Constructive review comments by M.L. Frezzotti and P. Barbey are gratefully acknowledged. Maria Luce is also thanked for having in due time (last minute!) called the attention of the senior author on the works presented at the XX ECROFI in Granada.


