1 Introduction
Phosphorites form following a widely-accepted genetic scheme involving a massive flux of biomass to the sediments, the decay of which releases organic matter-linked phosphorus, and triggers carbonate-fluor-apatite (more specifically, francolite) precipitation (e.g., (Cha et al., 2005; Föllmi, 1996; Kim et al., 1999; Soudry, 2000)). The burial of phosphorus and the subsequent formation of phosphorites are typically observed in zones of the ocean with high surface productivity, such as modern suboxic to anoxic marine sediments of upwelling areas: off Namibia, Chile, Peru, in the Gulf of California and in the Arabian Sea ((Arning et al., 2009a; Arning et al., 2009b) and references therein). As authigenic phosphate enrichments originate from organic decay by bacterial activity, the role of microbes in the phosphogenesis has been much studied (Arning et al., 2008; Arning et al., 2009a; Arning et al., 2009b; Krajewski et al., 1994; Schulz and Schulz, 2005).
Francolite precipitation is generally considered as occurring under suboxic conditions (Cha et al., 2005; Föllmi, 1996; Froelich et al., 1988; Jarvis et al., 1994; Schuffert et al., 1998), that is, at the stage of denitrification and Fe-Mn oxihydroxide reduction, prior to the development of sulfate-reduction and fermentation reactions (anoxic conditions). However, the present study of Late Jurassic cryptalgal carbonate (the Laminites Bitumineuses Formation, Orbagnoux hamlet, Jura Mountains, France) yields several lines of evidence that francolite may have paradoxically precipitated under anoxic and strongly reducing conditions (concentrations in redox-sensitive trace metals, occurrence of sulfurized lipidic fractions of organic matter). Phosphatic stromatolites have already been observed (Martin-Algarra and Sanchez-Navas, 2000; Soudry, 2000) but the scenario of their genesis usually involves a multistep formation under contrasting redox conditions and with carbonate-fluor apatite precipitating during the non-anoxic stages. Here, to account for the paradox of francolite forming under anoxic conditions, we expose an interpretation grounded on early bacterial calcification induced by sulfate-reducing conditions, which lowered pore water alkalinity and triggered francolite formation.
2 An overview of phosphorus geochemistry in sediments
Phosphorus (P) is essential to all forms of life on Earth, as it plays a fundamental role in many metabolic processes and is a major constituent of skeletal material. Phosphorus is a structural element in DNA and RNA, as well as in many enzymes, phospholipids, and other biomolecules. Phosphorus is present with an average crustal abundance of 0.01%, but it shows a higher content in most marine sediments and sedimentary rocks (Föllmi, 1995; Föllmi et al., 2004; Mackenzie et al., 1993; Trappe, 1998). The main source of P to the sediments is the phytoplankton necromass that reaches the sediment–water interface, plus fish scales and bones. A further source of P is dissolved inorganic P that is transferred across the sediment–water interface (possibly with the help of microbial mats (Föllmi, 1996; Föllmi et al., 2005). Usually, P is released as PO43− from decaying organic matter during oxic, suboxic and anoxic bacterial degradation at/or below the sediment–water interface. Largely, the P remineralized to pore waters can either escape from the sediment back to the water column or be precipitated and trapped within the sediment (Louchouarn et al., 1997; Sannigrahi and Ingall, 2005; Span et al., 1992). Under anoxic conditions, phosphorus then generally diffuses upward from the sediment and returns to the water column. This P cycling is very efficient: it has been estimated that only 1% of organic phosphorus escapes cycling and is trapped in sediments and sedimentary rocks (Benitez-Nelson, 2000).
However, if their escape from sediments is not possible, phosphate ions released by organic decay can reach pore-water concentrations high enough for authigenic phases, namely, francolite, to precipitate. Francolite may be represented by the formula:
Francolite precipitation is conditioned by alkalinity, pH, Eh and bacterial activity (Benitez-Nelson, 2000; Reimers et al., 1996). Francolite can precipitate either rapidly (most probably replacing a short-lived and poorly crystallized precursor; (Föllmi, 1996)) or slowly (usually replacing calcite but possibly directly also (Föllmi, 1996; Jarvis et al., 1994; Piper and Perkins, 2004; Trappe, 1998). The P concentration must be high enough to support francolite supersaturation. This enrichment may result from a high organic matter supply in highly productive marine environments such as upwelling areas (Arning et al., 2009a; Arning et al., 2009b), but high productivity is not always a prerequisite. In low-productivity portions of the seas, it is often considered that P enrichment can be effectuated by redox cycling of iron, with P sorption onto iron-oxihydroxide coatings and Fe-P coprecipitation (e.g., (Jarvis et al., 1994; Piper and Perkins, 2004); n.b., manganese also can be involved in P enrichment, (Wang and van Cappellen, 1996)). An alternative mechanism for P retention may be the uptake by bacteria storing polyphosphates as energy source (Sannigrahi and Ingall, 2005).
The degree to which remineralized organic P is retained as a reactive fraction in sediments depends on the redox conditions of the depositional system. In environments with at least intermittently oxic bottom waters, redox cycling of Fe within the sediment limits the diffusive flux of remineralized P to the overlying water column: Fe oxihydroxides that scavenge phosphate from sediment pore waters are precipitated above the oxic/anoxic interface and dissolved below it, leading to retention of P for a period sufficient to permit slow growth of authigenic phosphate phases. In permanently anoxic environments with sulfidic bottom waters, Fe-oxihydroxides do not precipitate within the sediment, reducing the potential for adsorption and complexation of remineralized organic P. Lastly, sulfate-reducing conditions are generally considered to prevent francolite formation because the correlative rise in alkalinity increases francolite solubility and prevents supersaturation to be reached and thus precipitation to take place ((Cha et al., 2005; Soudry, 2000; Trappe, 1998) and references herein). Consequently, francolite is generally presented as precipitating under suboxic conditions and not anoxic-sulfidic conditions (Föllmi, 1996; Froelich et al., 1988; Jarvis et al., 1994). However, in sediments, suboxic and anoxic conditions may be closely linked and sulfate-reducing bacteria may play an indirect role in francolite precipitation. This role is evidenced by the association of pyrite with carbonate-fluor-apatite at some growth stages of phosphate-rich grain coatings or sediment crusts (Arning et al., 2008; Arning et al., 2009a; Arning et al., 2009b; Krajewski et al., 1994; Schulz and Schulz, 2005). Lastly, recent papers ((Arning et al., 2009a) and references therein) illustrated the role of the interactions between sulfate-reducing bacteria and sulfide-oxidizing ones in phosphogenesis processes.
3 Previous work, material and methods
During Late Jurassic times (Late Kimmeridgian), the present-day French southern Jura was a vast carbonate platform (Bernier, 1984). On this platform, abundant organic matter (OM) accumulated within laminated limestones — called the Laminites bitumineuses Formation — in a few individual lagoons. The present study deals with one of these lagoons located close to the present-day hamlet of Orbagnoux, close to the Rhône River in the Ain administrative department; the outcrop is located along the Dorche River. Previous papers (Gorin et al., 2009; Mongenot et al., 1997; Mongenot et al., 1999; Pacton et al., 2006; Pacton et al., 2007; Tribovillard, 1998; Tribovillard et al., 1991; Tribovillard et al., 1992) extensively presented the sedimentological and geochemical framework of the depositional processes at Orbagnoux and discussed the abundance of OM in these sediments in terms of paleoenvironments and conditions of OM preservation. Other papers studied extensively the role of microbes in the genesis of the Laminites bitumineuses, indicating in particular that the early-diagenetic conditions were very favorable to the calcification of bacterial bodies or by-products, either in vivo or shortly post-mortem (Pacton et al., 2006; Tribovillard, 1998; Tribovillard et al., 2000). The main points relevant to the question at stake here will be summarized below in the following sections 3.1 and 3.2.
3.1 Facies description
During deposition of the Laminites bitumineuses Formation, the conditions largely alternated between periods of particle settling under shallow water conditions and periods of very shallow water conditions favoring plane stromatolite growth, which led to the formation of parallel laminae and undulated laminae, respectively ((Tribovillard et al., 1991), their plate 1; (Tribovillard et al., 1992), their plate 2).
- 1. Parallel laminae are made of alternating, submillimeter scale, more or less dark-colored laminae. These parallel, continuous, individual layers are clustered into millimeter or centimeter scale laminae, which appear more or less dark depending on the dominant color in individual layers. Various macro-and microfossils are present in the parallel laminae: rare ammonites, common benthic foraminifers and ostracods. In scanning electronic microscope (SEM) observation, the light-colored laminae appear as exclusively made of coccospheres and coccoliths ((Tribovillard et al., 1991), their plate 2). The dark-colored laminae contain less frequent coccoliths and coccospheres, embedded within a matrix of structureless, gel-like, OM ((Mongenot et al., 1997), their plate 1).
- 2. The undulating laminae facies comprises irregularly undulated, alternating light- or dark-colored, laminae, grossly parallel to bedding. Thin section observations show an alternation of dark laminae, consisting of bundles of very thin seams, and of light-colored, thicker, carbonate laminae, commonly containing abundant peloids ((Tribovillard, 1998), plates 1 & 2; (Tribovillard et al., 2000), plate 1). These peloids are interpreted as being induced by cyanobacterial activity (Tribovillard, 1998; Tribovillard et al., 2000). The peloids originate from the in vivo or shortly post-mortem calcification of sheaths of coccoid cyanobacteria. Such a calcification must have operated in an environment with strong alkalinity (Tribovillard, 1998). Acid-etched samples also evidence the filamentous texture of the dark, irregular, seams of the dark laminae from the plane stromatolites ((Tribovillard, 1998), plates 1 & 2; (Tribovillard et al., 2000), plate 1). In the undulating laminae, no coccoliths have been observed. The biolaminations must have resulted from self-burial processes, i.e. mat-by-mat overgrowth, in the absence of particle settling.
3.2 Summary of published geochemical data
The organic content is high and expectedly higher in the dark laminae than in the light ones (4.47–8.55 wt.% vs. 0.65–8.03 wt.%; (Tribovillard et al., 1991; Tribovillard et al., 1992)). The values of the Hydrogen Index of the kerogens are always very high (755–966 mg hydrocarbons per g TOC). Elemental analyses performed on the isolated kerogens indicate that organic sulfur is very abundant (12–17.6 wt.% of the kerogens; (Mongenot et al., 1997)). Consequently, the OM studied belongs to the so-called Type I S (Sinninghe Damsté et al., 1993). The simultaneous occurrence of abundant organic matter and reducing conditions favored intense sulfate reduction. Hence, large amounts of sulfide were released and trapped within the sediment below the cyanobacterial barrier. As iron was very limited in the depositional environment in relation to the absence of terrestrial supply (mean Fe content: 0.11%, n = 19; (Tribovillard et al., 1999)), sulfide reacted with organic molecules; this early sulfurization of organic matter acted as an efficient agent of organic matter preservation (Mongenot et al., 1997; Mongenot et al., 1999).
The major- and trace-element composition was determined for a few samples of the Laminites bitumineuses (Tribovillard et al., 1999). Phosphorus appears to be present in proportions fluctuating between 76 and 264 μg g−1 (n = 19, mean 165,9 μg g−1) for a Ca content bracketed between 34 and 38% (average carbonates show 400 μg g−1 and 30% for P and Ca, respectively; (Wedepohl, 1991)). The Laminites bitumineuses are enriched in Mo, V, Ni, Cu, Zn and Ba (Tribovillard et al., 1999). The enrichment in Mo was studied by Tribovillard et al., 2004 to evidence the efficient capture of Mo by sulfurized organic matter. Within the Laminites bitumineuses, the parallel laminae as well as the undulated laminae subfacies are markedly enriched in redox-sensitive Mo, thus indicating that anoxic conditions prone to sulfate reduction developed just below the sediment–water interface (Tribovillard et al., 1999). However, the concentration of Mo and transition metals is governed by the abundance of organic matter. The marked enrichment in redox-sensitive metals testifies to the prevalence of reducing pore-water conditions. Thus, the Laminites bitumineuses are depleted in P relative to average carbonate standard but the P concentrations are not negligible and may even be considered as relatively important if one considers that the very reducing and alkaline pore-water conditions should have opposed to P retention in the sediment.
3.3 Analysis
We observed and analyzed 10 rock thin sections representing the various facies of the Laminites bitumineuses using a FEI Quanta 200 scanning electronic microscope equipped with a Rontec energy-dispersive-spectroscopy (EDS) microprobe, looking for discrete P concentrations. We also observed thin sections that were etched with diluted HCl, because this technique proved successful to reveal calcified bacterial bodies and structures in the Laminites bitumineuses Fm. (Tribovillard, 1998). We used a Raman microspectrometer to analyze some P-rich concretions (see below) but the laser beam destabilized the concretions and no spectrum could be acquired.
4 Results
Scarce phosphate concretions are observed, scattered through the samples rich in organic matter. They appear as laminae parallel to the bedding plane, generally with a length of several hundreds micrometers and a maximum thickness of a few tens of micrometers (Fig. 1A and B). They occasionally look like flat bodies, being pinched at both ends when observed in thin sections (Fig. 1E). The phosphate concretions appear as dense, homogeneous bodies looking like solidified gels, but Fig. 1F shows that the bodies may show an internal lamination. In addition, the gel-like phosphate concretions have a brittle aspect (Fig. 1C and D) that suggests that solidification occurred prior to compaction during early diagenesis.
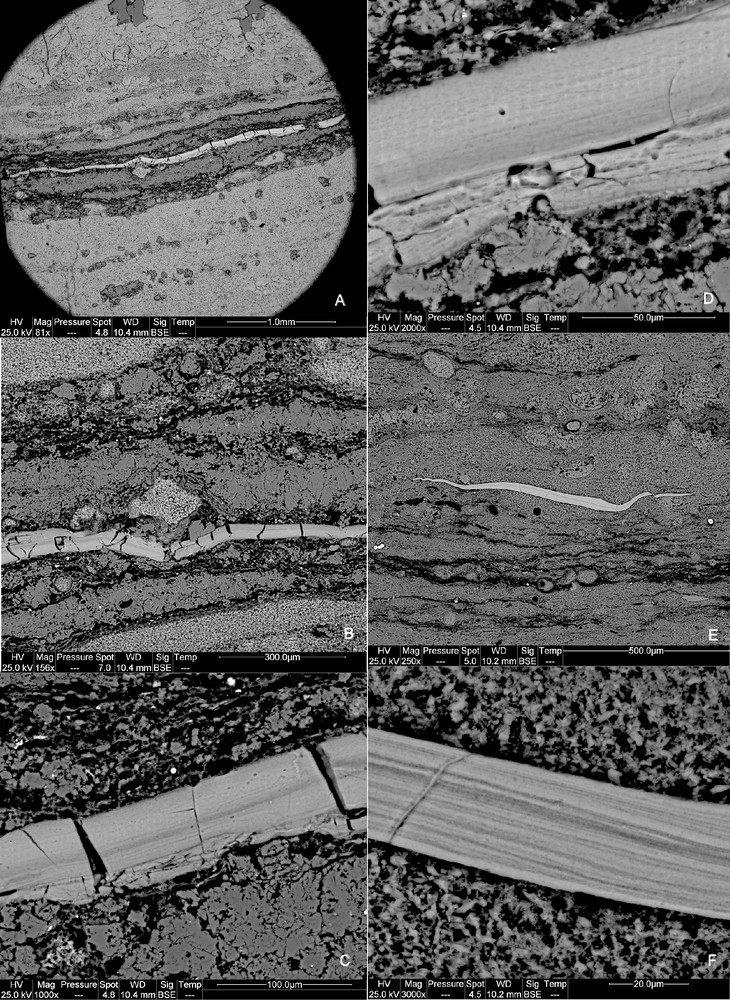
SEM imaging of rock thin sections using back scattered electron mode. A–D: views at different magnifications of a francolite lamina. In A, the lamina is the light-colour level in the centre of the picture. E–F: two views at different magnifications of an elongated francolite body.
Images prises au microscope électronique à balayage en mode électrons rétrodiffusés des lames minces de roches des faciès stromatolithiques. A–D : vues à différents grossissements d’une lamine de francolite. En A, la lamine est le niveau clair au centre de l’image. E–F : vues à deux grossissements différents d’un corps allongé en francolite.
EDS analyses and elemental mapping using SEM show that the phosphatic laminae and bodies are made of oxygen, phosphorus, calcium, fluorine, sulfur and sodium (Fig. 2). The composition of the various samples analyzed is rather constant and the mean concentrations are O: 34.48 wt.%, F: 10.59 wt.%, P: 12.14 wt.%, Ca: 41.16 wt.%, Na: 0.54 wt.% and S: 1.10 wt.% (n = 7). This chemical composition suggests that the P concentrations are made of francolite. We tried to confirm it with a Raman spectrometer but the material is destabilized under the laser beam and we could not get any spectrum.
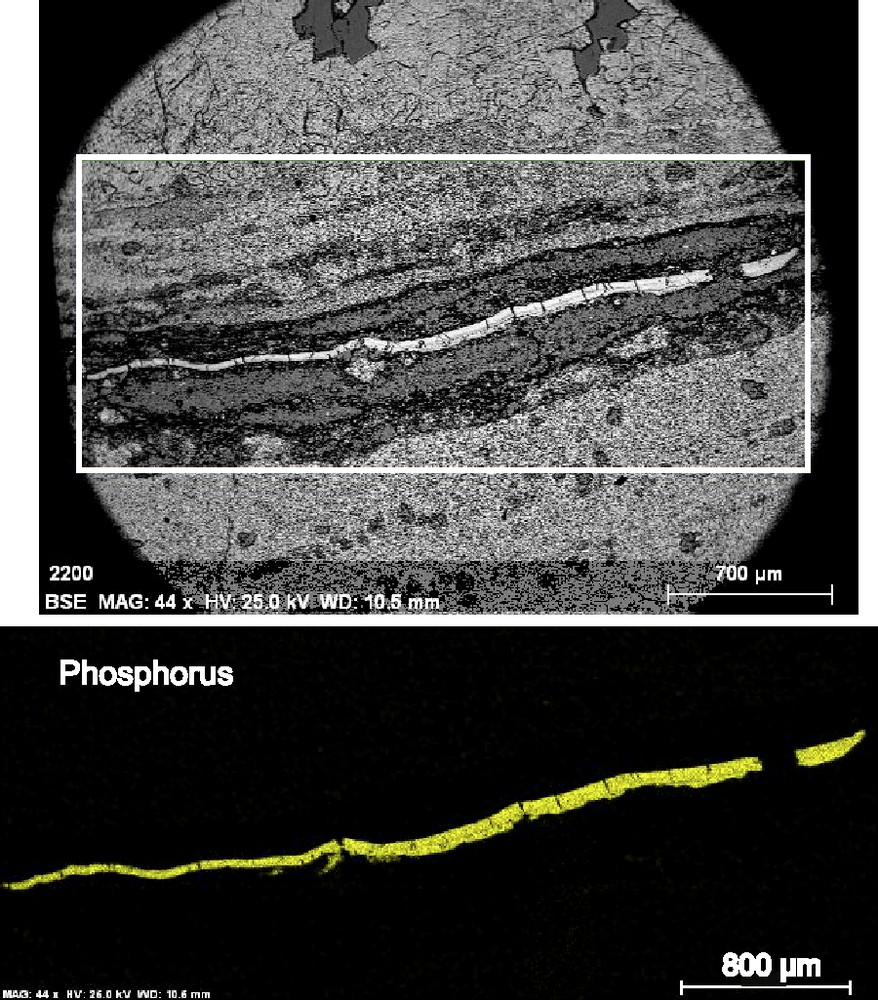
Example of the chemical mapping performed using EDS-equipped SEM imaging. Same lamina as in Fig. 1A. The bottom part of the figure shows phosphorus distribution in the studied part of the thin section: P is only present in the francolite lamina and never scattered through the rock.
Exemple de cartographie chimique réalisée au microscope électronique à balayage équipé d’une sonde EDS. Il s’agit de la lamine illustrée dans la Fig. 1A. La partie inférieure de la figure montre la distribution du phosphore dans la lame mince : le phosphore n’est présent que dans la lamine et jamais en imprégnation de la roche.
The phosphate concretions are observed in the stromatolitic facies (undulated laminae) and not in the parallel laminae resulting from settling processes. In other words, the P bodies are in close association with the facies resulting from bacterial activity. Pictures of acid-etched samples (Fig. 3) show that the P bodies are always included in seams with the typical alveolar networks interpreted as bacterially-induced fabrics (calcification of exopolymeric substances or eps (Gorin et al., 2009; Tribovillard, 1998)).
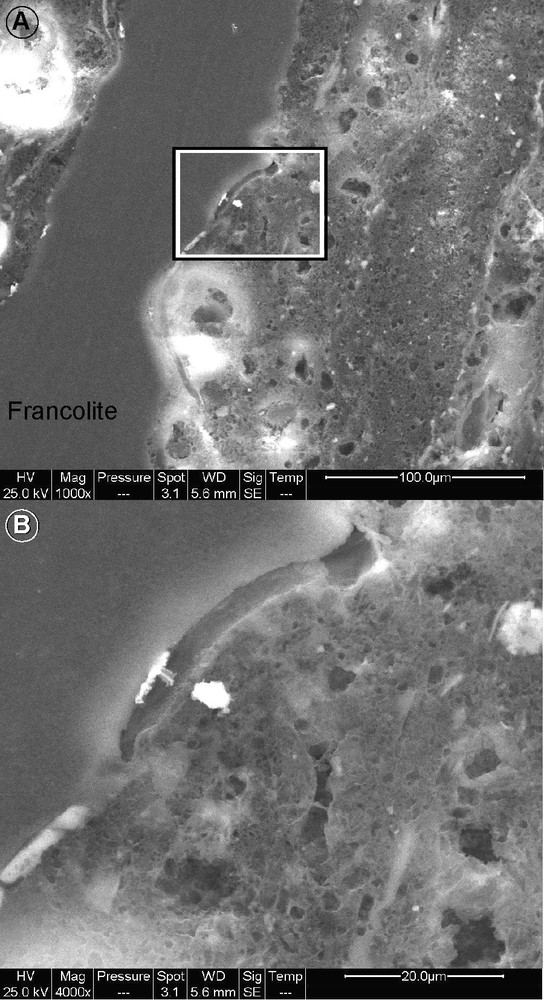
SEM imaging (secondary electron mode) of an HCl-etched sample of undulated laminae (stromatolite) showing the sharp contact between francolite and the spongy network interpreted as calcified extrapolymeric substances produced by bacteria. Picture B is an enlargement of the frame within picture A.
Images prises au microscope électronique à balayage en mode électrons secondaires d’un échantillon de roche qui a subi une légère attaque acide, afin d’accentuer le relief. Il s’agit d’un échantillon de stromatolithe plan (lamines ondulées). L’image montre le contraste sans transition entre la francolite et le réseau spongiforme calcaire, interprété comme la calcification des substances secrétées par les bactéries (eps). L’image B est un agrandissement de la partie indiquée par un cadre dans l’image A.
5 Interpretation
5.1 Redox conditions of deposition
Using the previous works about Orbagnoux evoked above, the following points may be specified. Throughout the various types of laminites, the common presence of oxygen-demanding planktic and benthic organisms supports the conclusion that the water column must have remained (almost) continuously oxic, whereas the underlying sediments were constantly bathed by anoxic pore waters. The much rarer occurrences of benthic organisms in the undulated (stromatolitic) facies indicate that oxygen was probably only episodically refuelled to the sediment surface. The sharp chemical interface was caused by the presence of cyanobacterial biofilms acting as a barrier between the two contrasting environments. The samples studied here are enriched in redox-sensitive trace elements (notably Mo and V) evidencing that strongly reducing conditions must have prevailed during early diagenesis, hence contemporaneously with francolite precipitation because strong authigenic enrichment in vanadium and molybdenum requires the presence of abundant H2S. See detailed explanations for the rapid development of sulfate-reducing conditions below the sediment–water interface in (Tribovillard et al., 1999). The presence of sulfurized OM is also evidencing sulfate-reducing conditions because sulfur incorporation to OM requires the presence of sulfide ions during early diagenesis (Aycard et al., 2003).
The above-mentioned previous interpretations about the Laminites bitumineuses allow stating that francolite must have formed under prevailing reducing, sulfidic conditions. Thus francolite precipitation occurred under sulfate-reducing conditions although the development of such conditions is generally considered to prevent francolite formation because the correlative rise in alkalinity increases francolite solubility (Cha et al., 2005; Soudry, 2000; Trappe, 1998). The paradox of coeval reducing condition and francolite formation was already addressed by Soudry (Soudry, 2000) about the Negev phosphorites. For this author, the maximization of francolite-precipitation conditions requires that most of the organic-matter degradation takes place at shallow burial depth, above the sulfate-reduction zone where carbonate precipitation occurs. However, in the case of the Laminites bitumineuses, the sulfate-reducing reactions developed very close to the sediment–water interface. The question is consequently how can be combined sulfidic conditions and francolite presence?
5.2 Calcification of bacterial structures
In the present case, the layers where francolite formed show abundant calcified bacterial structures ((Tribovillard, 1998), plates 1 & 2; (Tribovillard et al., 2000), their plate 1). Carbonate mineralization of the bacterial structures probably took place during anaerobic degradation of the matted sediments (Tribovillard, 1998). Early calcification of bacteria may be triggered by a marked rise in pore water alkalinity in vivo or shortly post-mortem (Awramik, 1984; Castanier et al., 2000; Kazmierczak et al., 1996; Kempe, 1990; Kempe and Kazmierczak, 1990; Knorre and Krumbein, 2000; Martin-Algarra and Sanchez-Navas, 2000; Riding, 1991) if the environment became supersaturated with respect to calcite (early in situ calcification can operate only in conjunction with carbonate supersaturation and alkalinity, but is prevented by high PCO2). However, carbonate precipitation is conditioned by pH. The two main points characterizing the Laminites Bitumineuses are the presence of abundant, sulfurized organic matter, and the prominent influence of cyanobacterial biofilms upon sedimentation. These points may be clues that help deciphering the chemical conditions that affected the pore space. The biofilms acted as barriers and thus hampered the replenishment of pore waters in dissolved oxygen. This situation must have favored the early onset of sulfate-reducing conditions, which raises pore water alkalinity:
The biofilms also (at least partly) retained the chemical products of sulfate-reduction reaction within sediment pore waters (presence of benthic epifauna). As stated above, the depositional environment was depleted in iron, which limited the reaction between reactive iron and sulfides usually leading to iron sulfide formation. Consequently, sulfide ions could react with organic matter, ending in organic matter sulfurization. It is not known (to our knowledge) whether organic matter sulfurization may be a phenomenon quantitatively sufficient to have a detectable influence on pore water pH, but from a theoretical point of view, organic matter sulfurization would tend to raise the pH. Two main types of reactions must be envisioned to account for natural sulfurization processes (Adam et al., 2000): H2S addition to C double bonds (i), and reductive sulfurization of carbonyls, transforming a ketone or an aldehyde into a thiol (ii).
- (i) In this first case, H2S molecules are transformed to sulfides or thiols, which raises the pH (thiols are potentially acidic but their pKa are much higher than that of H2S).
- (ii) In this second case, the first step of the process is the reaction between H2S and a carbonyl, which induces the formation of a thiocarbonyl and H2O (hence, a pH rise). The second step is the reduction of a thiocarbonyl into a thiol. H2S intervenes as a reductant. Thus, at each step, two HS bonds are substituted for by one SS bond (polysulfides or elemental sulfur), H being incorporated into the thiols. Consequently, in this second case too, organic matter sulfurization corresponds to H2S consumption and to the formation of thiols or sulfides less acidic than H2S or neutral, and inorganic S species such as S8 that will not influence the pH value of the pore waters. To sum up, organic matter sulfurization may participate to a relative pH rise in pore waters and thus facilitates carbonate precipitation, which is the most plausible explanation for the observed early-diagenetic bacterial calcification.
5.3 Explanatory model
These observations allow us to put forward an explanation for the paradoxical francolite precipitation under sulfidic conditions: the rapid bacterial calcification would capture carbonate ions and thus would decrease pore water alkalinity. The alkalinity fall would in turn allow francolite precipitation, since the presence of abundant dissolved CO32−/HCO3− conditions prevents francolite precipitation. The decay of abundant OM must have released phosphate ions in relatively large amounts and soluble-state phosphorus accumulated within the pore waters. Thus, we propose a relatively simple model to account for the P enrichment of the Laminites bitumineuses. The presence of an abundant biomass in the sediment would supply the “raw material” to the reactional system, i.e., the initial P budget. The development of cryptalgal, bacterial mats at the sediment–water interface would have limited exchanges between the water column and the pore space, hampering pore water renewal and favoring the early onset of sulfate-reducing reactions. The onset of sulfate reduction would increase pore water alkalinity, potentially preventing francolite precipitation. In addition, the presence of sulfide ions would induce active organic matter sulfurization inducing to a relative pH rise. The alkalinity and pH rises would trigger the development of conditions leading to carbonate supersaturation, inducing bacterial-structure calcification. The sudden fall in alkalinity induced by early calcification would allow francolite precipitation, despite the sulfidic conditions. Lastly, the phosphate concentrations are scarce throughout the Laminites bitumineuses, and only present in the most OM-rich levels. Thus, the francolite precipitation process presented here operated only sporadically, suggesting some possible threshold effects.
5.4 Undulated vs. parallel laminae
Both stromatolitic facies (undulated laminae) and particle-settling facies (parallel laminae) are enriched in redox-sensitive trace metals (notably Mo and V), thereby evidencing anoxic and sulfidic conditions below the sediment–water interface, and thus echoing the activity of sulfate-reducing bacteria in both cases. In addition, the settling facies largely contain more abundant OM — hence more abundant phosphate-carrier phases — than stromatolitic ones do. Under these conditions, it may be wondered why the phosphate concretion only occurs in stromatolites and not in parallel laminae. Both facies being anoxic and prone to OM-sulfurization, a prominent role of redox conditions may be ruled out to account for the difference between the two facies. What distinguishes above all the facies is the intensity of bacterial activity. In the case of the parallel laminae, the lagoon was relatively less confined and a rather diversified life existed, notably with benthic epifauna feeding on microbial mats (ceritid-type gastropods and foraminifers). In this case, sulfate-reducing bacteria developed at shallow depth below the sediment–water interface as is the case for most of OM-rich deposits. In the case of the stromatolitic facies, the lagoon was more confined in relation with shallower water depth, the salinity was higher inducing gypsum precipitation and strongly limiting the presence of mat grazers. Thus, microbial mats could develop into plane stromatolites. Consequently, bacterial activity was much more intense at and below the sediment–water interface in the case of the undulated laminae than in that of parallel laminae.
The more intense bacterial activity led to the modification of the pore-water chemistry and the bacterial proliferation induced early calcification of bacteria and/or eps, whereas such calcification could not occur in the parallel laminae. This situation is well illustrated by the deposition of the “kopara” facies on the present-day French Polynesia atolls or Kiritimati Island (Pacific Ocean), which is a good analog of the Orbagnoux depositional environment (Tribovillard et al., 1999). Thus, the alkalinity fall induced by bacteria/eps calcification, being the sine qua non condition to francolite precipitation was only met in the stromatolitic facies.
In addition, the microbial mats acted as a sort of physical barrier that must have limited rapid transfer of dissolved substances from pore waters to the water column. The dissolved phosphate released by OM remineralization could accumulate in the pore fluids. By contrast, the absence of such barrier at the sediment–water interface in the case of parallel laminae favored the release of dissolved phosphate from the sediment to the water column, so that francolite saturation could not be reached.
To conclude, the main factor accounting for the exclusive occurrence of phosphate concretions in the stromatolites is the early development and intensity of bacterial activity.
5.5 Original or early-diagenetic phosphate concretions?
The phosphatic laminae and concretions observed here are scarce and strictly discrete: the surrounding sediment shows no traces of phosphate impregnation. In addition, they show brittle deformation and internal laminations. So they differ somewhat from usual phosphate concretions. Lastly, some laminae show a rather large extension but most of the concretions are of millimeter-scale length. These observations suggest that the phosphate concretions, at least those showing internal lamination and compatible dimensions, may originate from arthropod remnants, more specifically crustacea, and among them ostracods (Karl Föllmi, personal communication). Crustacea are known to be able to live in lagoonal settings and to face low dissolved O2 conditions (e.g., (Hervant, 1997) and references therein). So their presence is plausible in the Orbagnoux lagoon. In addition, marine crustacea have carapaces made of chitin, calcite and apatite; consequently, it may be suggested that the phosphate concretions observed here resulted from the early-diagenetic phosphatization of phosphate-bearing precursors of biological origin. In the case of ostracods that primarily calcify their carapaces, the P-concretions would result from the early-diagenetic replacement of calcite by francolite. In this view, the genetic scheme described here to account for the formation of francolite concretion implies an early-diagenetic step, namely, the phosphatization of a biogenic precursor, probably originally P-bearing. However, none of the observed concretions did show identifiable structures allowing us to assign an unambiguously biological origin.
6 Conclusions
This study highlights the role played by the development of cryptalgal mats during early diagenesis. The presence of mats caused sulfate-reducing conditions to develop at (or at very shallow depth below) the sediment–water interface. The development of sulfate-reducing conditions initiated a chain of reactions ending with francolite precipitation despite the prevalence of anoxic conditions. The model proposed here is not restricted to the Laminites bitumineuses Formation of Orbagnoux but may also apply to other depositional milieus combining a high organic matter supply (the “raw material”) and the development of bacterial mats.
Acknowledgements
Thanks to Jean-Philippe Jenny for his help in the lab. Thanks to Pierre Adam and Armelle Riboulleau for their help with sulfurization. We are grateful to Karl Föllmi and an anonymous reviewer for their useful comments that helped us improve substantially the manuscript, and to Cécile Robin for her editorial work.


