1 Introduction
The Comblanchien limestone is a famous building stone used in France in many architectural buildings, both historic (e.g. the Opéra de Paris) and nowadays (e.g. Lyon railway station). At present, this commercial rock is extracted from Bourgogne (to the south-east of the Paris Basin, Fig. 1A) in only four quarries: SETP (Société d’Entreprise de Travaux Publics), Pierres Bourguignonnes, Rocamat and CMB (Carrières et Marbreries de Bourgogne) (Fig. 1B). The Comblanchien limestone exhibits physical and ornamental characteristics that make it an elegant building stone. As a consequence, temptation is great for some professionals to replace it by other building limestones that, if not necessarily of lesser ornamental or petro-physical (resistance to weathering, robustness) quality, remain cheaper. There is thus a need for the local economic sector to make sure that a given building limestone comes from the Comblanchien region or not. In this article, we propose an approach capable of verifying, and potentially recovering, the provenance of some rocks used as building stones. Studies addressing such a problematic usually concern archeological or historical monument applications (e.g. Fronteau et al., 2010; Kastenmeier et al., 2010).

A. Geological map of the Comblanchien area. B. Location of the four quarries working the Comblanchien limestone (modified from Rémond et al., 1985 and Gélard, 1978). SETP: Société d’Entreprise de Travaux Publics. C. Stratigraphic and geochronological position of the Comblanchien limestone. “calcaire de Comblanchien” corresponds to the Comblanchien building limestone.
A. Carte géologique de la région de Comblanchien. B. Localisation des quatre carrières exploitant le calcaire de Comblanchien (modifié d’après Rémond et al., 1985 et Gélard, 1978). SETP : Société d’Entreprise de Travaux Publics. C. Position stratigraphique et géochronologique du calcaire de Comblanchien exploité.
The aim of this article is: (1) to provide a quantitative characterization of the Comblanchien limestone using petrological and geochemical tools, in order to identify potential markers of a guarantee of its origin; and (2) to test the validity of the approach by comparing the Comblanchien limestone to one of its competitors from Portugal. Finally, as an example of application, we have studied three samples collected at a building site near Paris where building stones were sold as Comblanchien limestone, but the actual provenance of which was suspicious.
2 Regional setting
South-eastward from the Paris Basin, Comblanchien is a commune from Bourgogne located in between Beaune and Nuits-St-Georges, to the west of the Bresse Graben (Fig. 1A). The Comblanchien Formation is located between the “Oolithe Blanche” Formation (Bathonian) and the “Dalle Nacrée” Formation (Lower Callovian) (Delmas et al., 2010; Purser, 1975; Fig. 1C). The presence of the foraminifera Meyendorffina (Kilianina) bathonica and Orbitammina elliptica (Delance, 1964) in the Comblanchien Formation indicates an Upper Bathonian age (Middle Jurassic). The formation is diachronous and extends beneath the Paris Basin and towards the Jura (Gaumet, 1997; Purser, 1975). Its thickness varies from 25 m in West of Bourgogne to 50–60 m in East Bourgogne (Perrier, 1993). According to some authors (Delmas et al., 2010; Floquet et al., 1989; Garcia, 1993; Javaux, 1992), the Comblanchien Formation is somewhat heterogeneous with various coated grains (i.e. oncoids, ooids), and fossils within a micritic to a sparitic groundmass (sensu Flügel, 2004). The deposit environment corresponded to a large Bahamian-type lagoon (Delmas et al., 2010; Medus and Mojon, 1996; Purser, 1975). During the Cenozoic, successive deformation events have induced displacements along faults in the western margin of the Bresse Graben (Rocher et al., 2003). One event consists in north-south shortening possibly of Pyrenean age (Rocher et al., 2003). In the four quarries working the so-called Comblanchien limestone (Fig. 1B), quarrymen distinguish four commercial types of limestones within the Comblanchien Formation. From the bottom to the top, they are named: Bancs d’Adots, Comblanchien limestone (also including the Rocheron from the Rocamat quarry), Granité, and Bancs de découverte (Fig. 1C). This study focuses on the Comblanchien building limestone, which has been described by Rat (1991), but also provides data for the Granité.
3 Strategy
The Comblanchien limestone exhibits an overall visual homogeneity that justifies its commercial name. In details, it shows some variability (e.g. changes in colour and concentration of stylolitic joints marked by iron oxides) that can be observed either within a single quarry or from a quarry to the other. The strategy was therefore to perform an exhaustive sampling of the four present-day worked quarries. Within each quarry, most strata have been sampled directly on cliffs and, when necessary, the sample set has been completed by blocks provided by the quarrymen. The set of samples is presented in the stratigraphic section of Fig. 2. The analytical strategy was to use routine methods in their state-of-the-art, so that the procedure may be repeated quite easily. A total of 121 thin sections and 41 whole rock powders from 41 samples have been produced. Samples were photographed individually, petrographically described at both the macroscopic and the microscopic scales, under the naked eye, the binocular and the microscope (Fig. 3). For all samples, the O and C isotope compositions were measured. Eight samples were selected for both chemical analysis (major and trace elements) and Nd isotope geochemistry. They were those showing the largest variations in O and C isotope compositions, and we can reasonably assume that the other samples display compositions in the range defined by these eight ones.
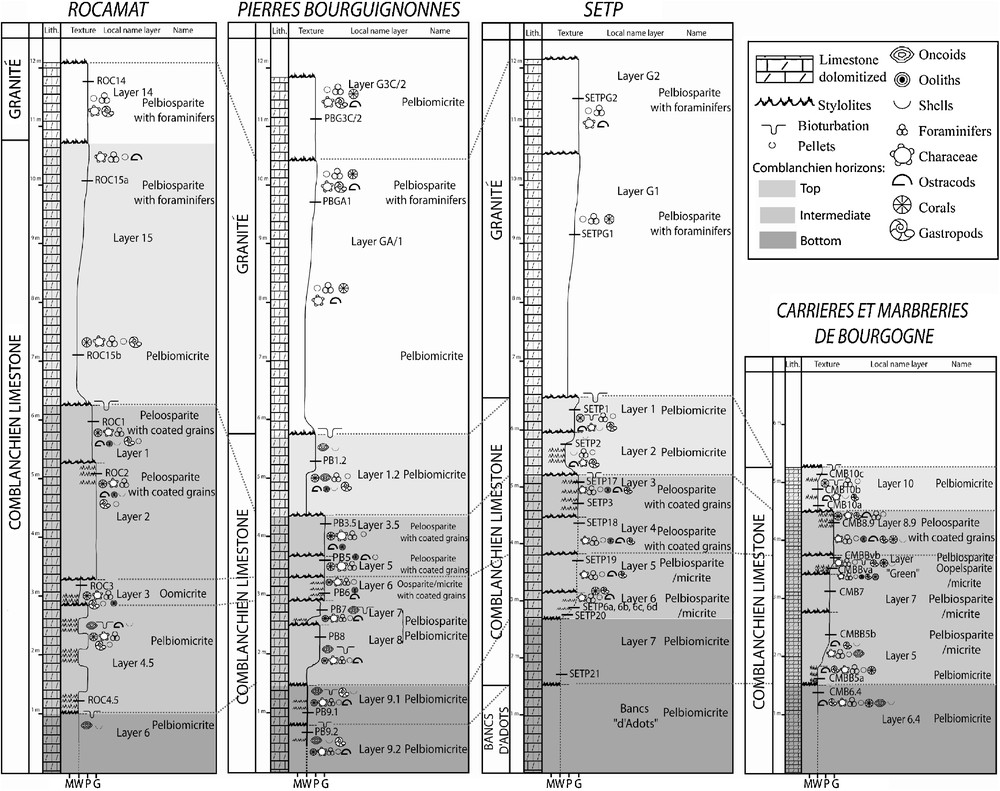
Stratigraphic logs of the four quarries of the Comblanchien limestone and stratigraphic locations of the samples are indicated. Dashed lines indicate proposed layer correlations from a quarry to the other. The classification used is that of Folk (1959).
Log stratigraphiques des quatre carrières du calcaire de Comblanchien. La localisation stratigraphique des échantillons est indiquée. Les tirets gris correspondent aux corrélations proposées entre les bancs d’une carrière à l’autre. La classification utilisée est celle de Folk (1959).

Photographs of the various Comblanchien limestone facies (bottom and top: wackestone; intermediate: grainstone) and of one of its Portuguese competitor (VATC grainstone). P: pellets, Ood: ooids, Bct: bioclasts and Gpd: gastropods.
Photographies des faciès variables du calcaire de Comblanchien (base et sommet : wackestone ; intermédiaire : grainstone) et de son concurrent portugais VATC (grainstone). P : pellets, Ood : ooïdes, Bct : bioclastes et Gpd : gastéropodes.
Searching for markers of the provenance of building limestones, the Sr isotope composition was not retained as it is not expected to vary significantly as a function of the geographic location within limestones of about the same age. Indeed, due to the long residence time of Sr in seawater, homogenization over large scales is achievable and the Sr isotope composition is nearly constant throughout the world's ocean at a given time. In contrast, Rare Earth Elements (REE), among which is Nd, display much shorter residence times. As a consequence, one expects that limestones of the same age but from distinct geographic location may exhibit different Nd isotope compositions. In this study, a limestone sample originating from Moleanos (Portugal) and named VATC (Vidraço ATaija Creme) was analyzed in the same manner. This limestone, of Callovian age (Perrier, 1993), corresponds to a commercial competitor of the Comblanchien (Bathonian) limestone. Three other samples of limestones of uncertain origin were picked-up at a building site near Paris (samples A, B and F) and have also been studied.
4 Analytical methods
Large hand specimens, in the range 5 to 10 kg, were sampled in order to get one to several thin sections and a whole rock powder per sample. Blocks were sawed and crushed, and final powders were obtained using an agate mortar.
4.1 Modal analysis
The relative proportions of the cement and clasts were measured by classical point counting on thin sections (30 × 45 mm2; 4000 points/section; Table 1). The uncertainty on modes can reach up to 2 vol.%, as estimated from standard deviations from repeated modal analyses on granitic rocks at Géosciences Rennes.
Textures, modes et compositions en isotopes stables (C et O) des calcaires de Comblanchien, de l’échantillon VATC et des échantillons A, B et F. (1) Pellets, (2) Grains enrobés (oncoïdes et ooïdes), (3) Foraminifères, (4) Characées, (5) Ostracodes, (6) Gastéropodes et (7) Coraux. (*) Échantillons sur lesquels les analyses chimiques ont été effectuées. Les valeurs en gras représentent les valeurs intéressantes pour la distinction Comblanchien, VATC, et A, B et F.
| Sample | Texture | Petrography | Stable Isotopes | |||||||||
| Groundmass C (vol.%) | Clasts B (vol.%) |
(1) | (2) | (3) | (4) | (5) | (6) | (7) | δ13C (‰ PDB) |
δ18O (‰ SMOW) |
||
| ROC14 | 34 | Sparite | 66 | 59 | 0.0 | 6.6 | 0.4 | 0.0 | 0.0 | 0.0 | 2.04 | 26.4 |
| ROC15a | 53 | Sparite | 47 | 38 | 0.0 | 7.8 | 0.7 | 0.3 | 0.0 | 0.0 | 2.06 | 26.3 |
| ROC15b | 54 | Micrite | 46 | 37 | 0.0 | 6.1 | 0.2 | 0.0 | 0.9 | 1.6 | 2.11 | 26.6 |
| ROCB1 (*) | 54 | Sparite | 46 | 27 | 13 | 1.6 | 1.5 | 1.2 | 0.9 | 0.3 | 2.17 | 27.2 |
| ROC2 | 29 | Sparite | 71 | 36 | 32 | 0.9 | 1.3 | 0.5 | 0.2 | 0.5 | 2.14 | 26.9 |
| ROC3 | 52 | Micrite | 48 | 17 | 26 | 0.4 | 2.6 | 1.2 | 0.0 | 0.5 | 2.16 | 27.1 |
| ROC4.5 | 69 | Micrite | 31 | 16 | 0.0 | 1.5 | 1.6 | 1.2 | 0.7 | 9.7 | 2.15 | 27.1 |
| PBG3 C/2 | 73 | Micrite | 27 | 17 | 0.0 | 6.7 | 1.4 | 1.4 | 0.3 | 0.1 | 1.88 | 27.1 |
| PBGA1 | 58 | Sparite/micrite | 42 | 34 | 0.0 | 5.3 | 0.7 | 0.8 | 0.6 | 0.4 | 1.84 | 26.9 |
| PB1.2 | 77 | Micrite | 23 | 19 | 0.0 | 2.0 | 0.6 | 1.1 | 0.1 | 0.9 | 1.97 | 27.6 |
| PB3.5 (*) | 21 | Sparite | 79 | 40 | 25 | 5.0 | 2.0 | 3.6 | 0.0 | 3.3 | 2.14 | 27.2 |
| PB5 | 53 | Sparite | 47 | 19 | 20 | 3.9 | 1.1 | 0.2 | 0.0 | 2.7 | 2.07 | 26.8 |
| PB6 | 29 | Sparite/micrite | 71 | 24 | 43 | 1.2 | 1.4 | 0.5 | 0.0 | 0.2 | 2.08 | 26.7 |
| PB7 | 56 | Sparite/micrite | 44 | 35 | 0.0 | 5.8 | 1.5 | 0.5 | 0.0 | 1.1 | 1.49 | 26.8 |
| PB8 (*) | 56 | Sparite/micrite | 44 | 35 | 0.0 | 5.8 | 1.5 | 0.5 | 0.0 | 1.1 | 1.75 | 26.5 |
| PB9.1 | 59 | Micrite | 42 | 33 | 0.0 | 4.4 | 1.6 | 1.4 | 0.2 | 1.2 | 1.97 | 26.8 |
| PB9.2 | 68 | Micrite | 32 | 25 | 0.0 | 5.6 | 0.3 | 0.3 | 0.3 | 0.3 | 2.18 | 26.9 |
| SETPG2 | 42 | Sparite | 58 | 47 | 0.0 | 10.3 | 0.2 | 0.0 | 0.0 | 0.1 | 1.36 | 27.0 |
| SETPG1 | 35 | Sparite | 65 | 59 | 0.0 | 4.3 | 0.0 | 0.0 | 0.0 | 1.6 | 0.72 | 27.1 |
| SETP1 | 87 | Micrite | 13 | 7.5 | 0.0 | 3.7 | 0.7 | 0.5 | 0.8 | 0.1 | 1.30 | 27.2 |
| SETP2 | 85 | Micrite | 15 | 11 | 0.0 | 2.4 | 0.9 | 0.3 | 0.1 | 0.0 | 2.00 | 27.0 |
| SETP17 | 43 | Sparite | 57 | 34 | 17 | 2.2 | 1.7 | 1.3 | 0.5 | 0.0 | 1.80 | 26.8 |
| SETP3 (*) | 46 | Sparite | 54 | 29 | 20 | 2.2 | 2.4 | 0.1 | 0.1 | 0.0 | 2.11 | 26.9 |
| SETP18 | 30 | Sparite | 70 | 37 | 30 | 1.4 | 0.7 | 0.3 | 0.3 | 0.4 | 2.25 | 26.6 |
| SETP19 (*) | 48 | Sparite/micrite | 52 | 43 | 0.0 | 6.7 | 0.9 | 0.8 | 0.7 | 0.0 | 1.14 | 26.1 |
| SETP6a | 45 | Sparite/micrite | 55 | 38 | 0.0 | 10.0 | 1.4 | 1.4 | 1.8 | 1.7 | 1.51 | 24.7 |
| SETP6b | 51 | Sparite/micrite | 49 | 34 | 0.0 | 8.2 | 1.7 | 1.5 | 1.4 | 1.6 | 1.33 | 24.9 |
| SETP6c | 66 | Sparite/micrite | 35 | 21 | 0.0 | 4.9 | 2.7 | 3.2 | 1.1 | 1.8 | 1.52 | 26.4 |
| SETP6d (*) | 56 | Sparite/micrite | 44 | 36 | 0.0 | 3.7 | 1.5 | 0.3 | 1.1 | 2.1 | −1.24 | 24.6 |
| SETP20 | 54 | Sparite/micrite | 46 | 35 | 0.0 | 6.3 | 1.7 | 1.3 | 0.2 | 0.8 | 1.57 | 26.4 |
| SETP21 | 80 | Micrite | 20 | 14 | 0.0 | 4.6 | 0.9 | 0.1 | 0.1 | 0.3 | 1.24 | 25.4 |
| CMB10c | 73 | Micrite | 28 | 20 | 0.0 | 4.0 | 0.9 | 1.5 | 0.9 | 0.6 | 1.84 | 26.6 |
| CMB10b | 81 | Micrite | 19 | 11 | 0.0 | 3.7 | 0.6 | 0.7 | 2.2 | 1.5 | 2.04 | 26.8 |
| CMB10a | 81 | Micrite | 19 | 12 | 0.0 | 2.6 | 1.3 | 0.1 | 1.4 | 1.6 | 1.72 | 27.2 |
| CMB8.9 | 24 | Sparite | 76 | 34 | 33 | 3.9 | 1.5 | 2.1 | 0.0 | 1.7 | 1.87 | 27.7 |
| CMBBvb (*) | 39 | Sparite | 61 | 48 | 0.0 | 10.0 | 1.4 | 0.4 | 0.4 | 0.4 | 2.10 | 27.1 |
| CMBBva | 42 | Sparite/micrite | 58 | 23 | 27 | 3.2 | 1.9 | 1.3 | 0.6 | 0.2 | 2.00 | 27.2 |
| CMB7 | 59 | Sparite/micrite | 41 | 33 | 0.0 | 3.2 | 1.1 | 0.8 | 0.1 | 3.0 | 1.42 | 26.0 |
| CMBB5b | 60 | Sparite/micrite | 41 | 33 | 0.0 | 3.4 | 1.7 | 0.4 | 0.1 | 1.7 | 2.21 | 26.6 |
| CMBB5a (*) | 66 | Micrite | 34 | 19 | 0.0 | 4.9 | 1.5 | 1.6 | 0.8 | 5.9 | 1.64 | 26.8 |
| CMB6.4 | 83 | Micrite | 17 | 9.1 | 0.0 | 4.1 | 1.7 | 1.2 | 0.6 | 0.4 | 2.00 | 26.2 |
| VATC (*) | 43 | Sparite | 57 | 44 | 10 | 1.9 | 0.7 | 0.3 | 0.0 | 0.2 | −0.40 | 26.5 |
| A (*) | 12 | Sparite | 88 | 67 | 15 | 1.8 | 0.5 | 3.2 | 0.0 | 0.0 | 0.41 | 26.4 |
| B (*) | 22 | Sparite | 78 | 40 | 31 | 1.7 | 3.4 | 1.6 | 0.0 | 0.9 | −1.43 | 26.5 |
| F (*) | 31 | Sparite | 69 | 45 | 9.9 | 0.8 | 9.4 | 0.4 | 0.0 | 3.3 | −1.55 | 26.2 |
4.2 Chemical analysis
Chemical compositions were measured by the Service d’Analyse des Roches et des Minéraux (SARM, CRPG-CNRS, Nancy, France). Analyses were performed by ICP-AES for major elements and ICP-MS for trace elements. Uncertainties and detection limits are provided in Table 2.
Compositions chimiques (en éléments majeurs et traces) et rapports isotopiques en 143Nd/144Nd en roche totale des différents calcaires (Comblanchien, VATC, A, B, et F). b.d.d. : en dessous de la limite de détection.
| Detection limit | Analytical uncertainty | ROC B1 |
PB 3.5 |
PB 8 |
SETP 3 |
SETP 19 |
SETP 6d |
CMB BvB |
CMB B5a |
VATC | A | B | F | |
| SiO2 (wt.%) | 0.5 | 10% | 0.60 | b.d.d. | 0.54 | b.d.d. | b.d.d. | b.d.d. | 0.51 | b.d.d. | 0.53 | 0.65 | b.d.d. | b.d.d. |
| Al2O3 | 0.02 | 25% | 0.31 | 0.25 | 0.29 | 0.17 | 0.18 | 0.21 | 0.27 | 0.16 | 0.28 | 0.44 | 0.08 | 0.06 |
| Fe2O3 | 0.01 | 5% | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 |
| MnO | 0.0005 | 10% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | 0.01 | 0.01 | b.d.d. | b.d.d. | b.d.d. | 0.01 | b.d.d. | b.d.d. |
| MgO | 0.02 | 10% | 0.61 | 0.62 | 0.55 | 0.61 | 0.53 | 0.37 | 0.62 | 0.62 | 0.28 | 0.33 | 0.27 | 0.24 |
| CaO | 0.035 | 2% | 54.4 | 54.9 | 54.5 | 55.0 | 55.1 | 55.1 | 54.5 | 55.1 | 55.0 | 54.8 | 55.7 | 55.6 |
| Na2O | 0.03 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| K2O | 0.01 | 25% | 0.08 | 0.07 | 0.07 | 0.05 | 0.05 | 0.06 | 0.07 | 0.05 | 0.03 | 0.03 | 0.01 | 0.01 |
| TiO2 | 0.001 | 25% | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.02 | 0.01 |
| P2O5 | 0.05 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| LOI | 43.43 | 43.46 | 43.45 | 43.76 | 43.48 | 43.57 | 43.53 | 43.62 | 43.43 | 43.36 | 43.30 | 43.59 | ||
| Total | 99.22 | 98.69 | 99.15 | 99.38 | 98.92 | 98.92 | 99.39 | 99.18 | 99.27 | 99.77 | 98.87 | 99.22 | ||
| As (ppm) | 1.10 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Ba | 1.50 | 25% | 4.66 | 4.04 | 4.68 | 3.45 | 16.63 | 5.92 | 4.12 | 2.95 | 1.87 | 3.10 | 2.38 | 1.68 |
| Be | 0.40 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Bi | 0.10 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Cd | 0.12 | 25% | b.d.d. | b.d.d. | 0.14 | b.d.d. | b.d.d. | 0.13 | b.d.d. | b.d.d. | b.d.d. | b.d.d. | 0.14 | 0.14 |
| Ce | 0.10 | 10% | 2.04 | 1.91 | 1.41 | 1.23 | 1.23 | 2.44 | 1.83 | 1.07 | 3.41 | 3.38 | 2.07 | 2.73 |
| Co | 0.35 | 25% | 0.72 | 0.69 | 0.76 | 0.74 | 0.98 | 1.03 | 0.77 | 0.71 | 0.76 | 0.88 | 0.68 | 0.71 |
| Cr | 4.00 | 25% | 4.50 | 5.03 | 4.34 | b.d.d. | b.d.d. | b.d.d. | 4.68 | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Cs | 0.15 | 25% | 0.35 | 0.29 | 0.33 | 0.18 | 0.18 | 0.21 | 0.33 | 0.16 | b.d.d. | 0.20 | b.d.d. | b.d.d. |
| Cu | 4.50 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Dy | 0.007 | 10% | 0.33 | 0.35 | 0.15 | 0.23 | 0.37 | 0.43 | 0.30 | 0.15 | 0.60 | 0.52 | 0.48 | 0.60 |
| Er | 0.003 | 10% | 0.23 | 0.24 | 0.11 | 0.16 | 0.22 | 0.25 | 0.22 | 0.10 | 0.35 | 0.30 | 0.31 | 0.35 |
| Eu | 0.004 | 10% | 0.07 | 0.07 | 0.03 | 0.05 | 0.09 | 0.12 | 0.06 | 0.03 | 0.15 | 0.13 | 0.11 | 0.14 |
| Ga | 0.20 | 25% | 0.35 | 0.29 | 0.32 | b.d.d. | b.d.d. | 0.27 | 0.32 | b.d.d. | 0.36 | 0.64 | b.d.d. | b.d.d. |
| Gd | 0.02 | 10% | 0.31 | 0.33 | 0.16 | 0.22 | 0.38 | 0.47 | 0.29 | 0.15 | 0.67 | 0.59 | 0.51 | 0.70 |
| Ge | 0.11 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Hf | 0.030 | 25% | 0.08 | 0.07 | 0.08 | 0.05 | 0.06 | 0.06 | 0.08 | 0.05 | 0.08 | 0.09 | 0.04 | b.d.d. |
| Ho | 0.001 | 25% | 0.07 | 0.08 | 0.03 | 0.05 | 0.08 | 0.09 | 0.07 | 0.04 | 0.13 | 0.11 | 0.10 | 0.13 |
| In | 0.10 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| La | 0.06 | 5% | 2.3 | 2.4 | 1.2 | 1.6 | 2.4 | 2.9 | 2.1 | 1.1 | 4.2 | 3.5 | 3.4 | 4.3 |
| Lu | 0.001 | 10% | 0.03 | 0.04 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.02 | 0.04 | 0.04 | 0.04 | 0.04 |
| Mo | 0.30 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Nb | 0.06 | 15% | 0.34 | 0.28 | 0.37 | 0.19 | 0.24 | 0.25 | 0.32 | 0.19 | 0.25 | 0.32 | 0.06 | b.d.d. |
| Nd | 0.03 | 10% | 1.39 | 1.39 | 0.80 | 0.94 | 1.79 | 2.39 | 1.23 | 0.65 | 2.84 | 2.43 | 2.02 | 2.71 |
| Ni | 4.50 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Pb | 0.90 | 10% | b.d.d. | 1.01 | b.d.d. | b.d.d. | 1.09 | 1.84 | 1.12 | b.d.d. | 1.00 | 1.91 | b.d.d. | 0.91 |
| Pr | 0.008 | 10% | 0.35 | 0.34 | 0.20 | 0.23 | 0.44 | 0.60 | 0.30 | 0.17 | 0.67 | 0.59 | 0.47 | 0.64 |
| Rb | 0.30 | 25% | 2.84 | 2.35 | 2.63 | 1.71 | 1.58 | 2.12 | 2.50 | 1.38 | 0.81 | 1.32 | b.d.d. | b.d.d. |
| Sb | 0.10 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Sm | 0.007 | 10% | 0.29 | 0.27 | 0.16 | 0.19 | 0.34 | 0.49 | 0.25 | 0.13 | 0.57 | 0.53 | 0.42 | 0.57 |
| Sn | 0.40 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Sr | 1.40 | 5% | 193.3 | 218.1 | 169.0 | 211.3 | 172.3 | 129.9 | 199.9 | 198.7 | 144.9 | 142.1 | 121.0 | 90.1 |
| Ta | 0.015 | 15% | 0.02 | 0.02 | 0.03 | b.d.d. | 0.02 | 0.02 | 0.02 | b.d.d. | 0.02 | 0.03 | b.d.d. | b.d.d. |
| Tb | 0.004 | 5% | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 |
| Th | 0.02 | 15% | 0.25 | 0.20 | 0.19 | 0.12 | 0.13 | 0.14 | 0.21 | 0.10 | 0.35 | 0.41 | 0.14 | 0.17 |
| Tm | 0.005 | 10% | 0.03 | 0.04 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.01 | 0.05 | 0.04 | 0.04 | 0.05 |
| U | 0.03 | 15% | 1.12 | 1.06 | 1.22 | 0.75 | 1.23 | 0.62 | 1.07 | 1.26 | 0.48 | 0.68 | 0.23 | 0.35 |
| V | 0.45 | 25% | 3.38 | 3.24 | 3.42 | 3.64 | 2.65 | 3.68 | 3.81 | 2.70 | 3.17 | 3.88 | 1.59 | 1.85 |
| W | 0.20 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Y | 0.40 | 25% | 4.56 | 5.34 | 2.08 | 3.44 | 4.88 | 4.34 | 4.46 | 2.11 | 6.79 | 5.47 | 6.54 | 7.67 |
| Yb | 0.003 | 10% | 0.21 | 0.23 | 0.11 | 0.14 | 0.19 | 0.21 | 0.21 | 0.09 | 0.28 | 0.25 | 0.24 | 0.29 |
| Zn | 14.00 | 25% | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. | b.d.d. |
| Zr | 0.80 | 25% | 2.91 | 2.43 | 3.09 | 1.84 | 2.08 | 2.21 | 3.43 | 1.78 | 2.67 | 3.63 | 1.43 | b.d.d. |
| 143Nd/144Nd | 0.512192 | 0.512195 | 0.512161 | 0.512210 | 0.512172 | 0.512152 | 0.512215 | 0.512191 | 0.512165 | 0.512176 | 0.512197 | 0.512187 |
4.3 Oxygen and carbon isotopes
Stable isotope analyses were performed at the stable isotope laboratory at Géosciences Rennes. About 10 mg of powder was reacted with anhydrous H3PO4 at 50.0 °C overnight. Isotopic compositions were measured on a VG-SIRA 10 triple collector mass spectrometer. The results are quoted in Table 1 using the δ notation with respect to SMOW for δ18O and PDB for δ13C. Isotopic measurements were normalized using repeated analyses of an “in-house” standard (Prolabo Rennes) and of the NBS 19 international limestone standard. Taking into account (1) the analysis of five duplicates and (2) the uncertainty on measurements of the standards, we estimate the total uncertainty on isotopic compositions at ±0.1‰ and ±0.15‰, for δ13C and δ18O, respectively.
4.4 Nd isotopes
Nd isotope analyses were performed on 100 mg of rock powders using a 7-collectors Finningan MAT-262 mass spectrometer at Géosciences Rennes (Table 2). Powders were dissolved twice with a mixture of 6 N HCl acid in a sealed Savilex beaker on a hot plate during 3 to 4 days. They were then dried and taken up with concentrated 2.5 N HCl acid. During the analytical session, measurements of the AMES Nd standard gave a mean 143Nd/144Nd ratio of 0.511945. Blanks values for Nd were lower than 300 pg and no correction was made to the measured isotopic ratios.
5 Results
5.1 Petrography
5.1.1 Description
The Comblanchien and the Granité limestones are fine-grained and beige coloured. They are stratified and the front quarries provide exceptional conditions to observe the sub-horizontal metric layers. Dolomitized zones are locally visible, either as horizontal brownish-purplish bands (e.g. sample CMBB5a, Fig. 3) or in burrows resulting from bioturbation. The limestone is made up of various clasts embedded in a microcrystalline matrix (micrite; e.g. sample PB1.2) or in a sparry calcite cement (sparite; e.g. sample PB3.5; see also Fig. 2). Sparite and micrite are present in all the quarries, and locally coexist within a single sample. The clasts consist in pellets, coated grains (oncoids and ooids; Flügel, 2004) and various fossils (foraminifera, ostracods, gastropods, characea and corals). Except for the pellets (20–50 μm), the size of elements varies between 50 and 150 μm. Iron oxides are disseminated in the rocks or concentrated within stylolitic joints subparallel to bedding. By combining field and microscopic observations, we have established facies correlations between quarries (see grey dashed lines on Fig. 2). These correlations define three horizons within the Comblanchien limestone. From the bottom to the top, they are (Fig. 2): (1) a pelbiomicrite bottom horizon, (2) an intermediate horizon with pelbiosparite/micrite, oomicrite and peloosparite and (3) a pelbiomicrite top horizon. Each of these horizons is made of several layers which are named differently from a quarry to the other but the limits of the horizons can be used to laterally correlate the layers. For example, layer 9.1 of the Pierres Bourguignonnes quarry correlates with layer 7 of the SETP quarry (see column “Local name layer”, Fig. 2). In addition, there is no significant petrographic distinction between the Granité and the bottom and the top horizons of the Comblanchien limestone.
The VATC sample is a fine-grained and compact limestone, beige colored with a sparry calcite cement. It contains pellets, coated grains (oncoids and ooids) and bioclasts (e.g. foraminifera, characea, ostracods). Clasts are thus about the same as those in the intermediate horizon of the Comblanchien limestone. In details, foraminifera Meyendorffina (Kilianina) bathonica and Orbitammina elliptica (Delance, 1964) known in the Comblanchien limestone are not present in the VATC sample. Samples of unknown provenance A, B and F resemble both the VATC sample and the intermediate layers of the Comblanchien limestone.
5.1.2 Modal composition
The relative abundance and the nature of the groundmass (C: sparite/micrite) and of the clasts (B) are reported in Table 1. Among clasts, pellets and coated grains are the most abundant, the former being present in all the samples but with variable proportions (7.5–59.1 vol.%). Coated grains are only present in the intermediate sparitic layers. The fossils are also found everywhere; the most abundant are the foraminifera.
Sample VATC is clast-rich (57 vol.%) and contains coated grains; this sample thus resembles qualitatively and quantitatively to the intermediate sparitic layers of the Comblanchien limestone. Samples A, B and F are much more clast-rich (up to 88 vol.%) and also contain coated grains.
5.2 Stable isotope geochemistry
The Comblanchien limestone and the Granité exhibit rather homogeneous δ18O (25.4–27.7‰) and δ13C (1.24–2.25‰) values, which compare well with unaltered marine Jurassic limestones (Veizer et al., 1999) (Table 1, Fig. 4). Some samples from the SETP quarry have lower δ18O values down to 24.6‰ and δ13C values down to –1.24‰, which are likely signs of some secondary alteration.

δ13C (PDB) versus δ18O (SMOW) for Comblanchien limestone, VATC, and A, B and F samples.
δ13C (PDB) versus δ18O (SMOW) pour les échantillons de calcaires de Comblanchien, VATC, et A, B et F.
The VATC sample has a δ18O value comparable to most of the Comblanchien samples (26.5‰) but shows a negative δ13C value (–0.40‰) that thus compares well with some SETP samples. Samples A, B and F have a comparable δ18O value (near 26.5‰) and a somewhat variable δ13C value between –1.55 and 0.41‰.
5.3 Complementary geochemical characterization
5.3.1 Major and trace elements
The chemical composition of the Comblanchien limestone reflects the nearly total absence of terrigenous mineral phases: excluding the major oxides classically entering in carbonate structural formulas (CaO, MgO and LOI assumed as mainly CO2), the sum of oxides is less than 1 wt.% (Table 2). Consistently, characteristics of trace elements are typical of marine limestones: Sr content is about 200 ppm, REE patterns are not fractionated relative to the upper continental crust and exhibit a large Ce negative anomaly (Ce/Ce* about 0.49) (Fig. 5).
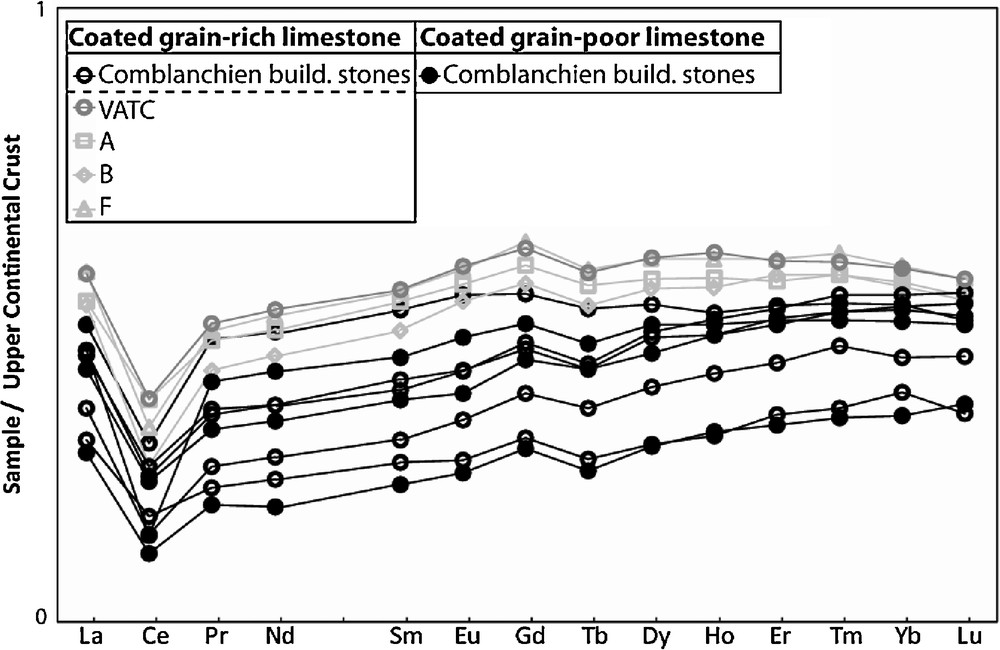
Upper Continental Crust-normalized Rare Earth Elements patterns for the Comblanchien limestone, VATC, and A, B and F samples. Normalisation values from Rudnick and Gao (2003).
Spectres de Terres Rares normalisés à la croûte supérieure continentale du calcaire de Comblanchien, des échantillons VATC et A, B et F. Valeurs de normalisation de Rudnick and Gao (2003).
The VATC sample is also a nearly pure and typical marine limestone. Despite it resembles to the Comblanchien limestone, sample VATC is slightly but significantly richer in REE, Y and Th, and poorer in MgO, Ba, Rb, Sr (only sample SETP6d is poorer in Sr than VATC) and U. Interestingly, these elements are also enriched (REE, Y, ±Th) or depleted (MgO, Ba, Rb, Sr) in samples A, B and F compared to the Comblanchien samples.
5.3.2 Nd isotopes
The 143Nd/144Nd ratios of the Comblanchien limestone are homogeneous in the range 0.51215–0.51221 (Table 2). The VATC sample exhibits an unspecific ratio of 0.512165 that lies within the Comblanchien range. Samples A, B and F also have 143Nd/144Nd ratios in the same range, between 0.51218 and 0.51219.
6 Discussion
6.1 Variability within the Comblanchien limestone
The Comblanchien limestone samples define a rather homogeneous population. Nevertheless, small heterogeneities occur in the data. Petrographically, the most prominent feature is the presence or the absence of coated grains in the samples. Actually, coated grains were only observed in the intermediate layers of the exploited strata and can thus be considered as a good marker of these layers. Geochemically, the stable isotope compositions of some samples from the SETP quarry are distinguished from the others by lower δ13C and δ18O values. In particular, the negative δ13C value, which corresponds to the lowest δ18O value (sample SETP6d), has to be related to a secondary effect because no negative value is to date documented in sedimentary elements from Bathonian sequences (see fig. 9 E in Brigaud et al., 2009). Interestingly, there is no correlation between the petrographic peculiarity (presence of coated grains) and the isotopic anomaly (low δ13C and δ18O values). These two features should thus relate to two independent processes that did not significantly alter the whole rock chemical compositions or the Nd isotope compositions. Both are indeed invariant throughout the Comblanchien population. The Sr content of sample SETP6d lower than that of the other SETP samples may reflect some alteration. In carbonate rocks, as the Comblanchien limestone, the main causes of heterogeneities are lateral and vertical variations of facies, variations in deposition environment, variations of the terrigenous input and of the diagenetic overprint. The latter may be related to heterogeneities in fluid circulations during compaction and re-crystallization controlled by variations in porosity and permeability. For the Comblanchien limestone, it is reasonable to exclude effects of recent weathering to explain the differences of some SETP samples because the rocks of lesser quality are excluded from the commercial channels. Likewise, local isotopic perturbations due to climatic effects during deposition are likely negligible because of the relatively short time span recorded by the studied sequence.
The specific isotopic signature of some SETP samples is not restricted to given layers in the sequence, but is rather characteristic of the SETP quarry as a whole. As a consequence, the alteration lowering both δ13C and δ18O values of some SETP samples should relate to the location of the SETP quarry on a map-scale fault (Fig. 1B). A possible explanation for the isotopic alteration of the SETP samples is therefore preferential fluid flow along the fault. Such fluid-rock interactions might have occurred either as early as the diagenetic history of the limestones or later during the Cenozoic tectonic activity of the area (Rocher et al., 2003), depending on the age of the fault. Both contexts are indeed capable of lowering the isotopic signatures through carbonate-water interactions.
There are some implications of the above observations in the context of building stones characterization. Because coated grains are restricted to intermediate layers, they represent good markers of these layers. By extension, as they locate within intermediate layers, it becomes possible to assign a precise stratigraphic origin for the coated grain-bearing Comblanchien limestone. In addition, because stable isotope anomalies are restricted to the SETP samples, it is possible to guarantee that a given sample with such anomaly (taken for example from a recent building) comes from that specific quarry. Finally, the so-called Granité, which forms the top of the sequences worked in Rocamat, Pierre Bourguignonnes and SETP quarries, appears as a strict equivalent of the Comblanchien limestone. So, even if the Granité was sold as a building stone, which is actually not the case as it is used to make aggregates, one would have had difficulties in discriminating between the Granité and the Comblanchien limestone with the tools used here. This illustrates the difficulty to precisely define commercial building stones.
Our study is restricted to the four quarries near the Comblanchien village. It is therefore not possible to ascertain that no other quarry, in the Paris Basin or the Jura, mining the Comblanchien Formation, could or not provide limestones with the same petrographical, geochemical and paleontological characteristics as the present Comblanchien limestone.
6.2 Comblanchien versus VATC limestones
The VATC sample displays similarities with some Comblanchien limestones, both petrographically and geochemically. Most of the qualitative and quantitative characteristics of the VATC sample can be recognized in some Comblanchien limestones. Actually, as foraminifera Meyendorffina (Kilianina) bathonica and Orbitammina elliptica (Delance, 1964) are absent from VATC, the faunal content could constitute an efficient tool of discrimination. However, the determination of foraminifer species can only be made by specialists and dating using biozones requires strong expertise. So the present strategy constitutes an alternative and quite easily accessible method of discrimination.
Only the few Comblanchien samples from the intermediate coated grain-bearing layers with a sparitic cement compare to VATC. From petrographic observations alone, it is thus impossible to discriminate between the coated grain-bearing Comblanchien and VATC limestones. Conversely, coated grain-free building stones from Comblanchien cannot be confused with VATC type ones, and petrography alone seems there discriminative.
VATC sample has O and C isotope compositions in the range defined by the Comblanchien limestone. In details, a single Comblanchien sample (SETP6d) has a δ13C lower than that of the VATC sample. Considering the large number of analyses of the Comblanchien limestone, one could say that the δ13 C tool is a rather good marker, but not absolute since a negative δ13C value does not guarantee the VATC origin. To circumvent this uncertainty, one could follow an isotopic reasoning in which the negative δ13C value of sample SETP6d is accompanied by a lower δ18O relative to the other Comblanchien samples, this peculiar δ18O being absent from the VATC sample. One could then argue that the low δ13C values of SETP6d and VATC samples reflect distinct processes, a fluid-related one (as discussed above) and a primary signature one, respectively.
The efficient discrimination between the Comblanchien limestone and the VATC sample comes from a combination of analytical methods. VATC is coated grain-bearing, as do some Comblanchien limestones (11 samples, which coated grain contents are highlighted in bold in Table 1). The latter have δ13C values between +1.75 and +2.25‰ (bold values in Table 1), well above the VATC value (−0.4‰). In addition, the chemical compositions of eight Comblanchien samples (Table 2) differ from that of the VATC sample, especially for MgO and REE (see also Fig. 5). Thus, we infer that the combination of the coated grains and δ13C tools is efficient and sufficient to discriminate between Comblanchien and VATC.
6.3 Origin of the erratic samples A, B and F
Samples A, B and F were collected on a building site near Paris in early 2010. On this site, building stones were assumed to come from the Comblanchien area. Our previous results provide some clues confirming that these samples did not origin from the Comblanchien area. Indeed, coated grain-bearing samples A, B and F have δ13C values between +0.41 and −1.55‰, which are different from the Comblanchien coated grain-bearing limestone isotopic characteristics.
Finally, from our results, the question to know if samples A, B and F come from Portugal as does sample VATC can also be addressed. At this stage, it is somewhat hazardous to conclude since the intrinsic petrographical and geochemical variability of VATC cannot be defined from a single sample. Nevertheless, sample VATC and samples A, B and F share many petrographical (sparitic cement, coated grains as clasts) and geochemical characteristics (low to negative δ13C value, identical δ18O value, low MgO, Ba and Sr content, high REE and Y content), so that all these samples likely share the same Portuguese provenance.
7 Conclusions
This work provides some useful tools to fingerprint the Comblanchien building limestone extracted from quarries near the Comblanchien village. Among the Comblanchien limestones, it was possible to recognize different building stones, some being coated grain-bearing (intermediate layers) or coated grain-free and others being specific of a quarry (SETP) cut by a map-scale fault. The study further shows that simple combined petrographical and geochemical analyses are useful to distinguish the Comblanchien limestone from some of its commercial analogues. Actually, the difference between the coated grain-bearing Comblanchien limestone and the VATC Portuguese limestone is based on the double observation of coated grain as clasts and of distinctive δ13C values. Chemical compositions also display characteristics that are consistent with this conclusion. On the other hand, the coated grain-free Comblanchien limestone cannot be confused with VATC. Based on these results, we show that samples of unknown provenance can be certified as non-Comblanchien limestone. As such an approach may provide a robust tool for discriminating between currently mined limestones of different provenances, the study opens the possibility to fingerprint current limestones and to attach them identity cards, and if required commercial labels.
Acknowledgements
Quarrymen are thanked for facilitating access to their quarries, for help during sampling and for constructive discussions. We thank X. Le Coz for thin sections and Y. Lepagnot for his help during field work and sample preparation. We also thank J. Marin (SARM, Nancy) and D. Vilbert (Géosciences Rennes) for performing geochemical analyses. F. Michel is thanked for fruitful discussions. Two anonymous reviewers helped to clarify some aspects of the present article. This work was supported by the Centre Technique de Matériaux Naturels de Construction (CTMNC).


