1 Introduction
Acid atmospheric pollutants deposited on sensitive ecosystems like forested granitic catchments cause increasing acidification of surface waters and soils (Chapman et al., 2008; Nakano and Tanaka, 1997; Reuss and Johnson, 1986; Vogt et al., 2007). The sensitivity of silicate soils in the forested Strengbach catchment observatory (Vosges mountains; http://ohge.u-strasbg.fr) to acid deposition has been confirmed by several isotope and trace element studies (Aubert et al., 2001, 2002a,b; Dambrine et al., 1998; Probst et al., 2000; Viville et al., 1993).
The neutralization of acid atmospheric depositions in silicate-rich soils is, at a short timescale, mainly controlled by ion exchange processes because they are more rapidly compared to chemical weathering (Norton and Vesely, 2003). These processes release basic cations such as Mg2+, Ca2+ and K+ into the draining surface waters and cause a depletion in these cations in the exchangeable pool of the upper (50 cm) soil compartments (Dambrine et al., 1998; Poszwa et al., 2003).
This might be harmful for such ecosystems, since the former cations are necessary for the growth of vegetation. Recent studies on soils from the Strengbach catchment have shown that with the exception of the leaf and needle litter layers, the upper 50 cm of the soils are strongly depleted in Ca, P and middle rare earth elements (REE) but strongly enriched in Al and Pb (Poszwa et al., 2003; Stille et al., 2009, 2011). The export of these elements from the watershed is partly interrupted due to their uptake by the plants’ root system and storage in the vegetation cells. Indeed, isotope data of Ca, Sr and Nd (one of the REE metals) from the Strengbach catchment indicate that these elements are partly taken up by the vegetation from the soil solution and, therefore, become recycled after leaf excretion, leaf fall and leaf decomposition (Cenki Tok et al., 2009; Schmitt et al., 2003; Schmitt and Stille, 2005; Stille et al., 2006). According to the studies of Miller et al. (1993) and Holmden and Bélanger (2010) the proportion of recycled Ca from litterfall by vegetation might increase with decreasing weathering flux from 80% to 90% of the annual Ca input to the Ca cycle. Leaching experiments indicate that the uppermost soil samples contain an important quantity of adsorbed and leachable atmosphere and/or soil solution derived components with low 87Sr/86Sr, 143Nd/144Nd and 206Pb/204Pb ratios, and that this mobile component, which is easily accessible for vegetation uptake, successively disappears with increasing depth, due to increasing pH of the soil solutions, the decrease of the soil cation exchange capacity and the increasing presence of primary, granite-derived minerals with depth; the mobile mainly atmosphere-derived component is replaced by another mobile reservoir with more geogenic, radiogenic Sr, Nd and Pb isotopic compositions (Stille et al., 2009, 2011). If a change of element sources occurred in the uppermost part of the soil system in the lifetime of a tree, then the change might have been recorded in its growth rings (Aberg et al., 1990; Drouet et al., 2005; Poszwa et al., 2003). Aberg (1995) and Aberg et al. (1990) observed that the Ca concentrations and 87Sr/86Sr ratios decrease from the center to the external part of the trunk and explained this phenomenon by the decrease of the contribution of Sr and Ca from the soil pool due to ongoing acidification and the constant contribution of rainwater Sr with a low 87Sr/86Sr. The 87Sr/86Sr isotopic variation in tree-rings with time has been successfully modeled by Poszwa et al. (2003) using a mechanistic model based on long term variations of 87Sr/86Sr in trees and soils. The approach included soil acidification and root uptake. The results confirmed that the 87Sr/86Sr ratios of soils and trees decrease with soil acidification (Aberg, 1995) and suggested that spruce growth rings are archives of past atmospheric pollution and acidification. Presently, only two preliminary studies observe small Ca isotopic variations within tree-rings which could be the result of changing Ca sources and/or hydrological stress (Farkaš et al., 2011; Nielsen et al., 2011). However, Cobert et al. (2011) showed that Ca isotopes may also be fractionated by physiological processes. The laboratory experiments of Cobert et al. (2011) on bean plants indicate that low pH and low Ca concentrations in the nutritive solution cause smaller Ca isotopic fractionations between bean organs and solution than higher pH and Ca concentrations. However, at this point it is not clear how far an extrapolation of these experimental bean plant data to natural, soil-grown trees is appropriate.
Annual growth rings of Picea abies have also been used as archives of Pb pollution trends in Central Europe (Novak et al., 2010, and citations therein). However, how far dendrochemical patterns are indeed reliable archives of past atmospheric Pb pollution is still a matter of discussion and it appears that there are important disagreements between dendrochemical and other Pb archives (Bindler et al., 2004; Hagemeyer and Schäfer, 1995; Nabais et al., 1999).
The aim of the present study was to test and discuss the suitability of the annual growth rings as environmental archives of acid rain and past atmospheric pollution. To do this, major, and trace element and Sr, Nd, Pb and Ca isotope determinations on growth rings of spruces from the forested Strengbach catchment have been performed. It is the first study on the Nd isotopic composition of growth rings. Earlier studies have shown that Nd isotopes are powerful tracers of atmospheric depositions on soils (Guéguen et al., 2012; Lahd Geagea et al., 2008a; Stille et al., 2006). It is also one of the first studies that reports and discusses the tree-ring Ca isotopic signatures. The sampled tree growth rings cover the period between 1916 and 1983 and, therefore, might have stored signs of the change in the relative contribution of Sr, Ca, Nd and Pb from the soil minerals and the atmosphere to the trees growth rings due to atmospheric pollution and increasing soil acidification.
2 Field settings
The forested Strengbach catchment is located in the Vosges mountains (north-east of France) (Fig. 1). It covers an 80 ha area at altitudes ranging from 880 m a.s.l. at the outlet to 1146 m a.s.l. at the top. The average annual pluviometry is around 1500 mm and the average temperature scatters around 6 °C; the climate is temperate mountainous and the west wind dominates. It has become a completely equipped environmental observatory with permanent sampling and measuring stations since 1986 (http://ohge.u-strasbg.fr).
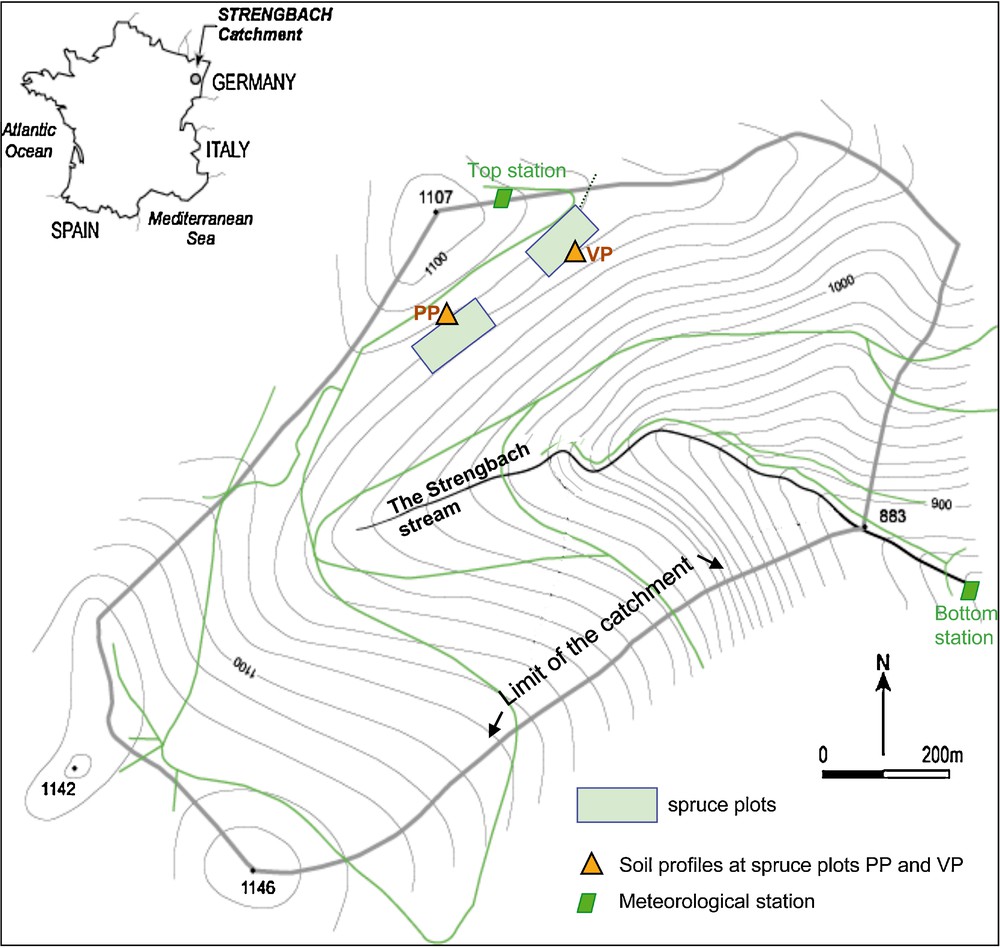
Strengbach catchment with sampling sites at spruce plots PP and VP.
Le bassin versant du Strengbach avec les sites d’échantillonnage PP et VP dans les parcelles recouvertes d’épicéas.
The forest covers 90% of the area and corresponds to about 80% of spruces (mainly Picea abies L.) and 20% of beech (Fagus sylvatica). The bedrock is a hydrothermally altered Hercynian granite (Boutin et al., 1995). The soils are on average 80 cm deep, sandy and stony, and belong to a brown acidic to ochreous podzolic soil series (El Gh’Mari, 1995; Fichter et al., 1998a). Two neighbouring experimental soil profiles (PP and VP) at 1070 m a.s.l. altitude from the northern part of the watershed covered with spruces are regularly analysed (Fig. 1). Shallow soils (upper 50 cm) are acidic (pH = 3.7–5; Aubert et al., 2001; Poszwa et al., 2003; Solovitch-Vella et al., 2007). Their acidity is caused by bedrock lithology (Brezouard base-poor leucogranite), deposition of acid atmospheric pollutants (acid rain; Février et al., 1999; Party, 1999) and the formation of important quantities of organic acids derived from the slow decomposition of organic matter (leaf and needle litter; Hansson et al., 2010; Lorenz et al., 2000). The studied spruces and soil solutions (Cenki Tok et al., 2009) are from the VP plot. Their ages range between 110 and 120 years and the tree density was 557 stems/ha (Biron, 1994). In 2008 we estimated a similar density of 575 stems/ha.
3 Sampling and analytical methods
Spruce trunk slices of 58–73 cm diameter were recovered in September 2005 from stumps (0 m height) of 3 spruce specimens freshly harvested for lumber from the VP plot (Fig. 1). Sampling at the standard height of 1.5 m was therefore not possible. The growth rings were counted at the Chrono-environment laboratory in Besançon. In order to recover at least 20 g of wood for the different chemical and isotope analyses (in particular for 143Nd/144Nd), groups of growth rings rather than individual rings were sampled. Typically, groups covering 8 rings were extracted by splitting with an inox blade from the center (label ‘C’ in Table 1), the intermediate (‘M’), and the external portion (‘E’) of each individual slice of trunk. The ages given in Table 1 are average values for each growth ring package. Growth rings younger than 1983 were not analyzed in order to avoid the active sapwood, which is an open isotopic system because of continuous re-equilibration by sap flow (Houle et al., 2008). For chemical and Sr, Nd and Pb isotope analyses, about 20 g of sample were split into match-scale splints and calcined using a step-wise heating procedure (350, 450, and 500 °C during 90, 90, and 120 min., respectively). The large sample size was necessary in order to have enough Nd for the determination of its isotopic composition. Nevertheless, two Nd isotope measurements failed due to the low Nd concentrations (< 5 ppb). Of course, this sampling strongly reduced the temporal resolution of the dendro-isotopical signal.
Concentrations en Ca, Sr, Pb et Nd dans des cernes d’épicéas.
| Sample | Age interval | Median age | Ca | Sr | Pb | Nd |
| Years | ppm | ppm | ppm | ppb | ||
| VPE-1–E | 1961–1969 | 1965 | 426.8 | 1.99 | 2.59 | 4.23 |
| VPE-1-M | 1934–1942 | 1938 | 429.0 | 1.79 | 1.88 | 4.65 |
| VPE-1–C | 1917–1925 | 1921 | 605.6 | 4.01 | 2.76 | 5.86 |
| VPE-2–E | 1968–1976 | 1972 | 329.0 | 1.08 | 3.91 | 5.32 |
| VPE-2–M | 1933–1941 | 1937 | 397.6 | 1.45 | 2.74 | 5.96 |
| VPE-2–C | 1918–1926 | 1922 | 709.8 | 3.24 | 3.68 | 9.51 |
| VPE-5–E | 1979–1987 | 1983 | 376.8 | 1.40 | 2.01 | 5.06 |
| VPE-5–M | 1951–1959 | 1955 | 382.1 | 1.26 | 6.18 | 4.93 |
| VPE-5–C | 1912–1920 | 1916 | 475.4 | 1.59 | 0.74 | 6.27 |
The ashed samples were digested under clean-lab conditions with 6 mL of 15 molar, distilled HNO3 in closed teflon vessels. After 30 minutes at room temperature, the samples were progressively heated in a microwave stove (135, 160, and 100 °C during 10, 10, and 20 min., respectively), evaporated, taken up in 15 mL of 1 molar HNO3 and divided into different aliquots for Nd, Sr, and Pb isotope analysis. The same digestion procedure was used for concentration analysis by ICP-MS, but with only 1 g of sample material.
For Ca isotope measurements about 100 mg of dried (not ashed) grinded trunk samples were digested in savillex™ vials using a hot oxidative acid method (double distilled HNO3, double distilled HCl and H2O2 suprapur).
The atmospheric particulate matter (PM) were collected with passive « Sigma-2 » samplers (Grobéty et al., 2010) close to the VP plot at the top station (Fig. 1; Guéguen et al., 2010, 2012). The sampling technique is based on the sedimentation principle (Stokes's law) and collects particles in the size range < 100 μm. Compared with the active samplers it allows a more precise trace element analysis since the PM can quantitatively be transferred, but it necessitates a much longer sampling time of two to four weeks.
The major and trace element concentrations were measured by ICP-AES and ICP-MS at the Laboratoire d’Hydrologie et de Géochimie de Strasbourg (LHyGeS, Strasbourg). The analytical error is < 5% and the detection limit is 0.01 μg/L (Chabaux et al., 2011; Steinmann and Stille, 1997; Stille et al., 2009). For all elements analyzed, our results were in good agreement with those of the certified reference materials (e.g. AGV-1, GSN, BEN and AIEA lichen standard).
The recovered solutions were evaporated and then prepared for Nd, Sr, Pb and Ca isotope analysis using standard techniques (Lahd Geagea et al., 2008a; Schmitt et al., 2009; Steinmann and Stille, 1997). Sr and Nd were separated from other elements using Eichrom's Sr Resin in series with Eichrom's TRU Resin and Eichroms Ln Resin according to Pin and Zalduegui, 1997. The Pb was separated from other elements using Bio-Rad AG1-X8 anion exchange resin and 0.6 N HBr and 6 N HCl as eluents. Ca was separated from matrix elements using a high selectivity automated ionic chromatography (HPIC, ICS-3000, DIONEX) (Schmitt et al., 2009). The procedural blanks were 0.35 ng, 0.13 ng and 0.005 ng for Pb, Sr and Nd, respectively. The procedural Ca blank was < 150 ng which corresponds to max. 2.5% of the total amount of Ca analyzed.
The Pb and Sr isotopic compositions were determined using a fully automatic VG Sector thermal ionization mass spectrometer at the LHyGeS with a 5-cup multicollector after enrichment and separation from the bulk sample. During the measurement period the NBS 987 standard yielded 87Sr/86Sr = 0.710259 ± 0.000021 (2σ, n = 12). The Pb isotopic data presented here were adjusted for mass fractionation by repeated analyses (n = 30) of the NBS 981 standard. The 2 σ-errors of the reported isotopic ratios are for 206Pb/204Pb ± 0.021, 207Pb/204Pb ± 0.024, 206Pb/207Pb ± 0.00062 and 208Pb/206Pb ± 0.0021. The Nd isotopic compositions were determined using a Nu instruments MC-ICP-MS at the branch of Isotope Geology at the University of Berne. The in-house standard yielded 143Nd/144Nd = 0.511061 ± 0.000020 (2σ, n = 23) corresponding to a value for the La Jolla standard of 0.511843. The Ca isotopic compositions were measured on a Triton thermal ionization mass spectrometer at the LHyGeS using a 42Ca-43Ca double spike and following a procedure adapted from Holmden, 2005 and presented in Schmitt et al., 2009. The results were expressed as δ44/40Ca (‰) using the NIST SRM 915a standard (Eisenhauer et al., 2004). The 2SD long-term external reproducibility was found equal to ± 0.12‰ based on repeated standard and sample measurements.
4 Results
The Ca, Sr, Nd and Pb concentration and isotope data are given in Tables 1 and 2, respectively. The growth rings of the 3 spruce trunks show a similar record with decreasing Ca, Sr, and Nd concentrations from the core to the external portions of the trunk slides (Figs. 2a–c). The strongest relative concentration change is between the innermost growth rings, which formed in the same time period between 1916 and 1922, and the intermediate segments, which formed just before 1940. The Pb concentrations manifest no time-dependent progression (Fig. 2d).
Compositions isotopiques en Pb, Sr, Nd et Ca dans des cernes d’épicéas.
| Sample | Median age (years) | 206Pb/204Pb | 207Pb/204Pb | 208Pb/204Pb | 206Pb/207Pb | 87Sr/86Sr | ± 2smean | 143Nd/144Nd | ± 2smean | d44/40Ca | ±2smeana | n |
| VPE-1–E | 1965 | 18.070 | 15.565 | 37.86 | 1.1610 | 0.72466 | 0.00001 | 0.51196 | 0.00002 | 0.49 | 0.08 | 2 |
| VPE-1-M | 1938 | 18.505 | 15.606 | 38.42 | 1.1858 | 0.72649 | 0.00001 | 0.56 | 0.08 | 2 | ||
| VPE-1–C | 1921 | 18.153 | 15.609 | 38.11 | 1.1629 | 0.73056 | 0.00001 | 0.51206 | 0.00003 | 0.44 | 0.08 | 2 |
| VPE-2–E | 1972 | 18.218 | 15.591 | 38.12 | 1.1684 | 0.72255 | 0.00001 | 0.51205 | 0.00002 | 0.31 | 0.08 | 2 |
| VPE-2–M | 1937 | 18.021 | 15.587 | 37.96 | 1.1562 | 0.72341 | 0.00001 | 0.51202 | 0.00002 | 0.40 | 0.08 | 2 |
| VPE-2–C | 1922 | 18.197 | 15.563 | 38.02 | 1.1692 | 0.73146 | 0.00001 | 0.51217 | 0.00007 | 0.41 | 0.08 | 2 |
| VPE-5–E | 1983 | 18.246 | 15.597 | 38.15 | 1.1698 | 0.72537 | 0.00001 | 0.51206 | 0.00004 | 0.42 | 0.08 | 2 |
| VPE-5–M | 1955 | 18.593 | 15.619 | 38.50 | 1.1904 | 0.72826 | 0.00001 | 0.46 | 0.08 | 2 | ||
| VPE-5–C | 1916 | 18.533 | 15.619 | 38.45 | 1.1865 | 0.73047 | 0.00001 | 0.51206 | 0.00003 | 0.25 | 0.08 | 2 |
| PM catchment | 0.71354 | 0.00003 | 0.51195 | 0.00010 | ||||||||
| PM #1 urban | 0.70919 | 0.00001 | 0.51198 | 0.00008 | ||||||||
| PM #9 urban | 0.70867 | 0.00001 | 0.51190 | 0.00001 |
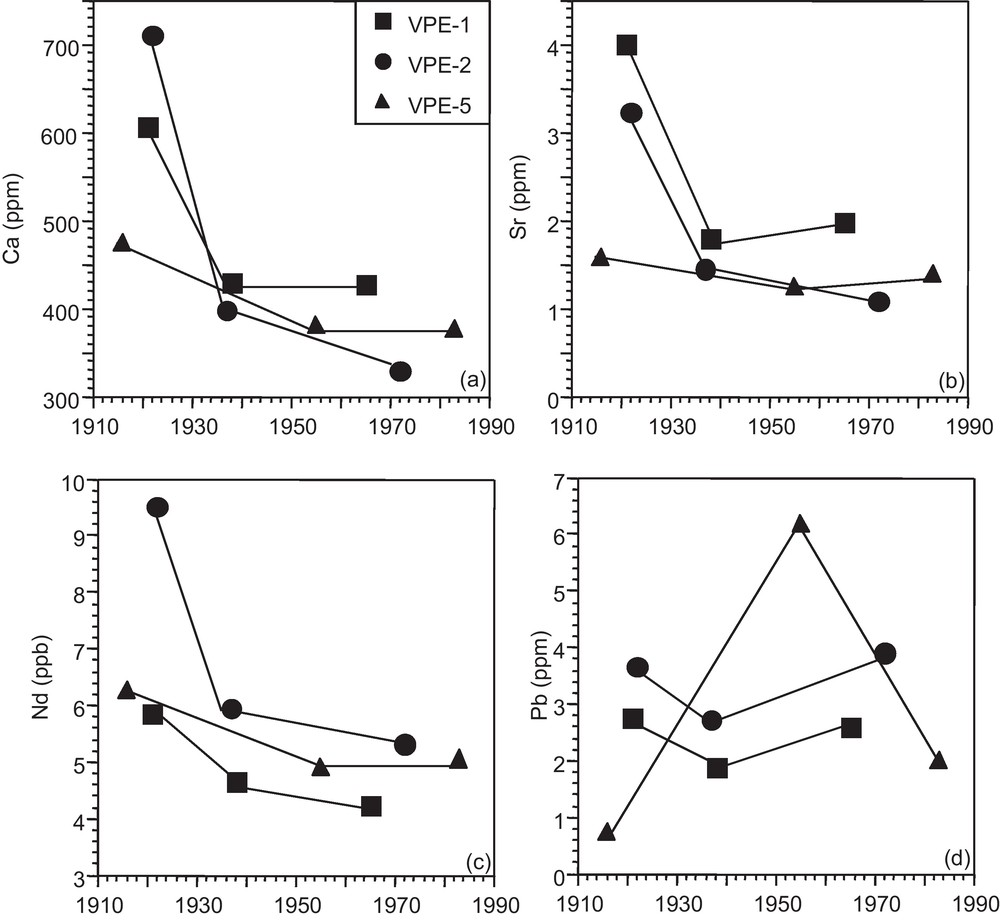
Ca, Sr, Nd and Pb concentrations in tree growth rings through time (1916–1983).
Concentrations en Ca, Sr, Nd et Pb dans les cernes d’épicéas au cours du temps (1916–1983).
The 87Sr/86Sr isotope ratios of the tree growth rings show a time-dependent evolution with high values in the innermost growth rings (1916–1922) that scatter comparatively weakly (0.7305–0.7315) and are significantly higher than those of the outermost segments (Figs. 3 and 4a). Growth rings of spruce from the VP site (Fig. 1) have previously been studied (Poszwa, 2000; Poszwa et al., 2003). Unfortunately, they only analyzed tree-rings from 1950 to 1990. Their 87Sr/86Sr ratios show, with the exception of one sample, a similar range of variation (Fig. 4a). In VPE-1 and, to a lesser extent, in VPE-5 samples, high 87Sr/86Sr ratios are associated with higher Ca and Sr concentrations (Fig. 3a,b).
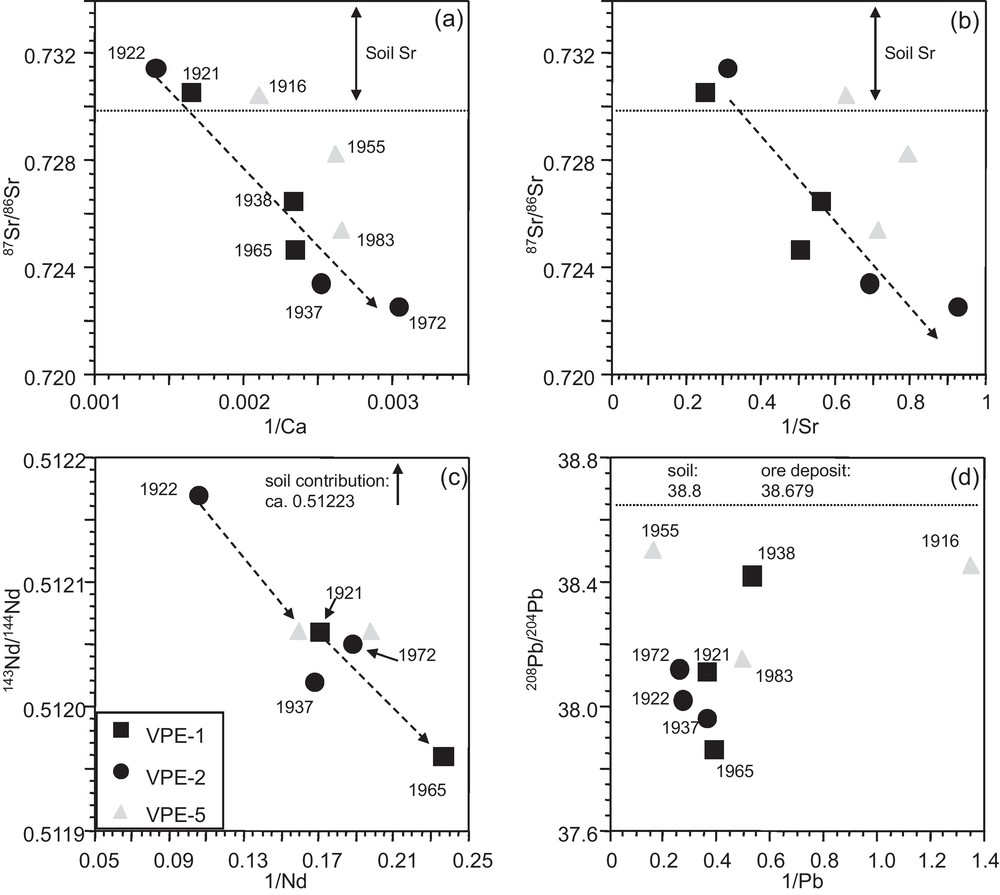
Relationships between (a), (b) Ca-Sr concentrations and 87Sr/86Sr isotopic compositions, (c) Nd concentrations and 143Nd/144Nd ratios and (d) Pb concentrations and 208Pb/204Pb isotope ratios in spruce growth rings. Time dependent evolutions are observable for Ca, Sr and Nd but not for Pb.
Relations entre (a), (b) les concentrations en Ca et en Sr et les compositions isotopiques du Sr, (c) les concentrations en Nd et les rapports isotopiques du Nd et (d) les concentrations en Pb et les rapports isotopiques du Pb dans les cernes d’épicéas. On observe une évolution au cours du temps pour les concentrations en Ca, Sr et Nd en fonction des rapports isotopiques du Sr et du Nd mais pas pour les concentrations en Pb en fonction des rapports isotopiques du Pb. Masquer
Relations entre (a), (b) les concentrations en Ca et en Sr et les compositions isotopiques du Sr, (c) les concentrations en Nd et les rapports isotopiques du Nd et (d) les concentrations en Pb et les rapports isotopiques du Pb ... Lire la suite
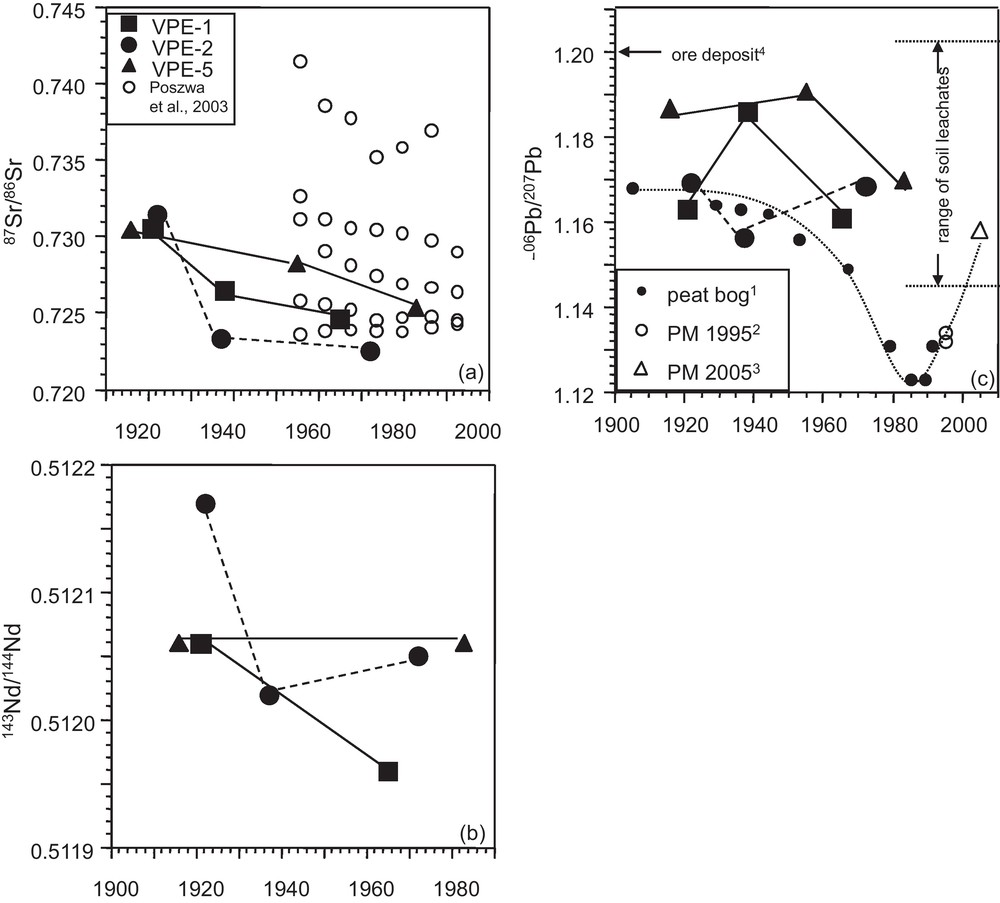
(a), (b) 87Sr/86Sr and 143Nd/144Nd vs. time for spruce growth rings, (c) 206Pb/207Pb vs. time for spruce growth rings; comparison with the evolution of the atmospheric Pb isotopic composition through the 20th century derived from peat bog and atmospheric particulate matter (PM). The uppermost 40 cm of the soils are strongly enriched in Pb compared to the bedrock. At close to the surface the corresponding soil leachates carry a present-day anthropogenic Pb isotopic signature (206Pb/207Pb: 1.148 and 1.167) and at 30 to 40 cm depth an old anthropogenic Pb isotopic signature derived from ore mining and smelting 200 to 300 years ago (206Pb/207Pb: 1.197 and 1.225; Stille et al., 2011) (1: Shotyk et al., 2001; 2: Monna et al., 1997; 3: Lahd Geagea et al., 2008a; 4: Marcoux, 1987). Masquer
(a), (b) 87Sr/86Sr and 143Nd/144Nd vs. time for spruce growth rings, (c) 206Pb/207Pb vs. time for spruce growth rings; comparison with the evolution of the atmospheric Pb isotopic composition through the 20th century derived from peat bog and atmospheric particulate ... Lire la suite
(a), (b) Évolution au cours du temps de 87Sr/86Sr et 143Nd/144Nd dans les cernes de croissance d’épicéas, (c) 206Pb/207Pb en fonction du temps pour les cernes d’épicéas ; comparaison avec l’évolution de la composition atmosphérique en Pb au cours du XXe siècle déterminée à partir de la composition isotopique en Pb de tourbières et de particules atmosphériques (PM). Les 40 cm supérieurs des sols sont fortement enrichis en Pb par rapport à la roche mère. Près de la surface les lessivats des sols montrent une signature isotopique en Pb similaire à la signature anthropique actuelle (206Pb/207Pb : 1,148 et 1,167). Vers 30–40 cm de profondeur les signatures correspondent à d’anciennes signatures anthropiques, similaires aux mines et industries d’il y a 200 à 300 ans (206Pb/207Pb : 1.197 et 1.225; Stille et al., 2011). (1 : Shotyk et al., 2001 ; 2 : Monna et al., 1997 ; 3 : Lahd Geagea et al., 2008a ; 4 : Marcoux, 1987). Masquer
(a), (b) Évolution au cours du temps de 87Sr/86Sr et 143Nd/144Nd dans les cernes de croissance d’épicéas, (c) 206Pb/207Pb en fonction du temps pour les cernes d’épicéas ; comparaison avec l’évolution de la composition atmosphérique en Pb au cours du Lire la suite
The 143Nd/144Nd isotope ratios of the VPE-5 samples in the outermost (1983) and innermost (1916) segments are identical (Fig. 4b). However, innermost and outermost growth rings of VPE-1 and VPE-2 samples are significantly different with comparatively lower Nd concentrations and 143Nd/144Nd ratios in the outermost growth rings (Fig. 3c). Observable but less pronounced is the time dependent evolution of the 143Nd/144Nd ratios in the growth rings of trees VPE-1 and VPE-2 (Fig. 4b). Their outermost segments show lower Nd isotopic composition values than their innermost segments. The Sr and Nd isotopic compositions of atmospheric PM from urban environments and the catchment are significantly lower than those observed for tree-rings (Table 2).
In contrast to the Sr and Nd isotope ratios, the different segments do not allow us to recognize a time dependent evolution of the Pb isotope ratios (Fig. 4c). Similarly, Pb concentrations and Pb isotopic compositions show no covariation and high Pb concentrations and 208Pb/204Pb isotope ratios are observable for outermost and innermost segments (Fig. 3d). The 208Pb/204Pb and 206Pb/204Pb ratios of the growth rings are well correlated with each other and plot within the range of soil leachates from the uppermost 40 cm of the neighboured PP soil profile (Fig. 5) (Stille et al., 2011).
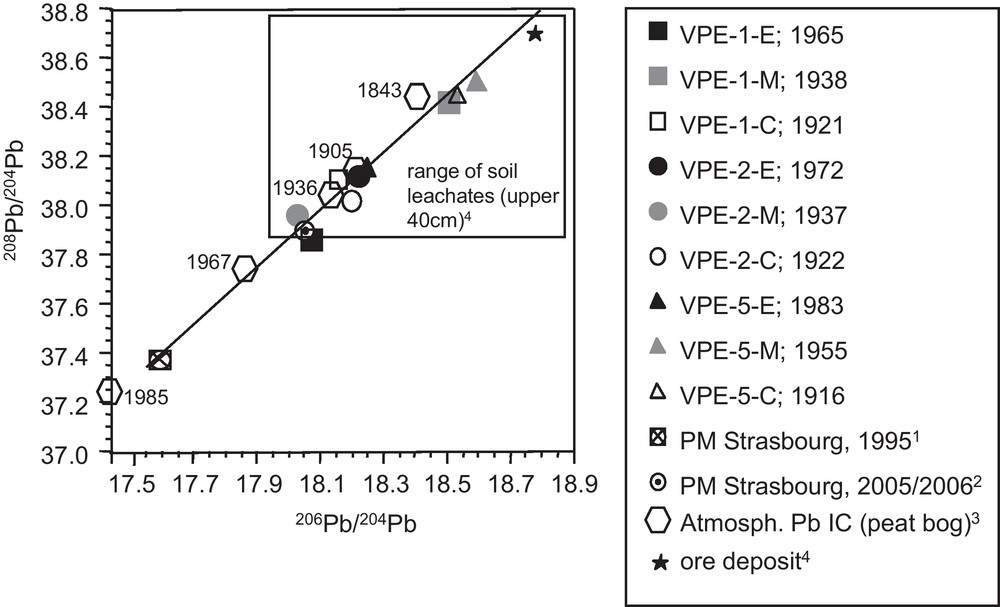
208Pb/204Pb vs. 206Pb/204Pb for spruce growth rings which show the same range of Pb isotopic compositions as the leachates from the uppermost soil compartments (Stille et al., 2011). Also shown are atmosperic Pb isotopic compositions derived from particulate matter from an urban environment (cities of Strasbourg and Kehl situated 80 km north of the catchment) (1: Monna et al., 1997; 2: Lahd Geagea et al., 2008a) and peat bog (3: Shotyk et al., 2001). Pb isotopic signature of ore deposit from 4: Marcoux (1987). Masquer
208Pb/204Pb vs. 206Pb/204Pb for spruce growth rings which show the same range of Pb isotopic compositions as the leachates from the uppermost soil compartments (Stille et al., 2011). Also shown are atmosperic Pb isotopic compositions derived from particulate matter ... Lire la suite
208Pb/204Pb vs. 206Pb/204Pb pour des cernes d’épicéas qui présentent une amplitude de variation similaire à celle des compositions isotopiques en Pb des lessivats des compartiments superficiels des sols (Stille et al., 2011). Les compositions isotopiques en Pb dérivées de matières particulaires provenant d’un environnement urbain (villes de Strasbourg et de Kehl situées à 80 km au nord du bassin versant) (1 : Monna et al., 1997 ; 2 : Lahd Geagea et al., 2008a), ainsi que de tourbières (3 : Shotyk et al., 2001) sont également représentées. La signature isotopique en Pb des mines provient de 4 : Marcoux (1987). Masquer
208Pb/204Pb vs. 206Pb/204Pb pour des cernes d’épicéas qui présentent une amplitude de variation similaire à celle des compositions isotopiques en Pb des lessivats des compartiments superficiels des sols (Stille et al., 2011). Les compositions isotopiques en Pb dérivées de ... Lire la suite
δ44/40Ca values of the tree-rings are within error bars relatively homogenous between the three studied trees (Fig. 6). Their average value is equal to 0.42 ± 0.06 (n = 9, 2SE). Moreover, the 3 growth rings of each of the studied trees show rather small variations of their δ44/40Ca values (0.50 ± 0.07, 0.37 ± 0.06, 0.38 ± 0.13, N = 3, 2SE, for VPE-1, VPE-2, VPE-5, respectively).
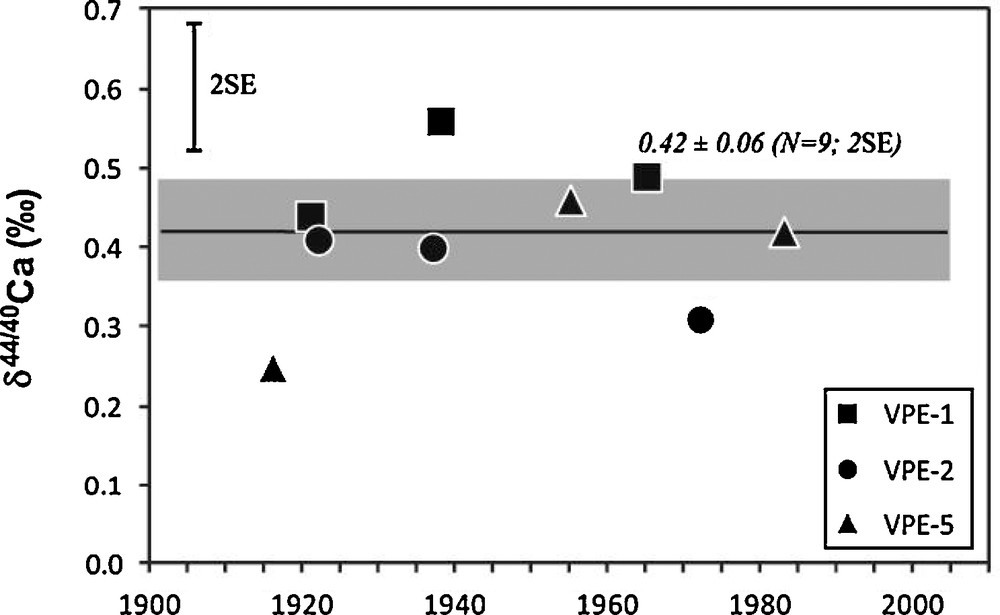
δ44/40Ca values determined on spruce growth rings. The 2SE amplitude of Ca isotopic replicate measurements is indicated in the top left corner. The black line and the shaded area correspond to the average value of the nine measured tree-rings and its corresponding 2SE.
Compositions isotopiques en Ca mesurées dans les cernes d’épicéas. La reproductibilité externe des mesures (2SE) est représentée dans le coin en haut à gauche. Le trait noir et le rectangle grisé correspondent respectivement à la valeur moyenne et au 2SE des neuf valeurs de cernes.
5 Discussion
5.1 Biological effects on the tree-rings’ chemical and isotopical composition
A key question is to what extent growth rings record changing environmental conditions. It is furthermore not clear whether growth rings are closed systems with no chemical exchange with the adjacent rings. A cross-section through a trunk might help to answer this question. The wood rays are indeed composed of rows of elongated living cells in the radial direction (Fig. 7). They provide a living link between the center and the periphery of the trunk. Parenchymal cells of wood rays are able to transfer substances, among others nutrients, from the center of the trunk to its living peripheral part or to store substances taken from the peripheral part in the older parts of the trunk. Some wood rays are continuous from the periphery of the wood to the center of the trunk, others are shorter and do not reach the center. Phloem rays have for their part the same structure as the rays of the wood. They connect the phloem to the outer part of the bark and thus allow the transfer of material from the cambium to the bark and vice versa. Radial-transport and re-equilibration of base-cations in growth rings have been discussed by several authors (Feretti et al., 2002; Drouet et al., 2005, and cit. therein). The study of Drouet et al. (2005) on beech from a limed and a not limed beech stand shows that the dendro-isotopical Sr pattern of the beech from the limed stand has been significantly influenced: the tree-rings foredate the liming application date by about 50 years. These experiments confirm on the one hand that growth rings are environmental archives, but on the other hand that radial transport might to some extent cause some isotopic re-equilibration between older and young growth rings. Thus, isotope ratios do not always allow a precise timing record of a polluting event. Similarly, the Ca, Sr and Nd concentration decrease from the inner to the outer part of the trees growth rings (Figs. 2a–c) might not only be or not at all linked with an increasing cation depletion of the soils. Several studies have shown that outwardly decreasing Ca concentrations in oak and beech wood is not related to environmental changes, but to endogenous parameters such as wood binding capacity (cation exchange capacity) which decreases from pith to bark (Herbauts et al., 2002, and cit. therein). Other research on red spruce stemwood, however, showed that relative to the binding capacities increasing or decreasing Ca concentrations might occur in tree-rings, pointing to increasing cation mobilization, respectively decreasing availability of nutrient cations, in soils (Bondietti et al., 1990; Momoshima and Bondietti, 1990). Our study does not allow us to decide how far the Sr, Ca and Nd concentrations are really related to environmental changes and, therefore, shall not be included in the following discussions.
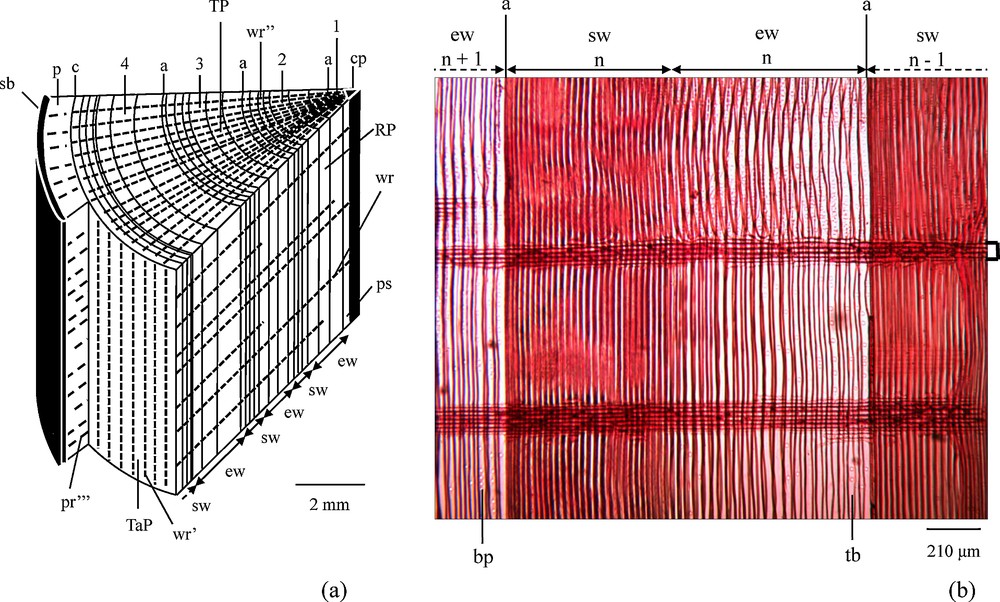
(a) Simplified block diagram of a portion of the trunk of a conifer in the late summer of the fourth year, of growth, and (b) radial cut in the trunk of a conifer (adapted from Bresinsky et al., 2008). Microscopic preparation: Turtox No BW 1.1, Gen. Biol. Sup. House Chicago, Illinois, USA, safranin coloration; Microscope: Olympus CH-BI45-T-2; Shooting: caméra DCM 130E, 1.3 Mpixels. Note that the wood rays are continuous from year to year in both parts of the Figure. TP: transverse plane; RP: radial plane; TaP: tangential plane; 1, 2, 3, 4: successive years; n–1, n, n + 1: successive years; ew: earlywood; sw: summer wood; cp: pith; ps: primary and secondary tissues of the first year; a: delineation of a growth ring; wr: wood ray (parenchyma cells) seen in the radial plane; wr’: wood ray (parenchyma cells) seen in the tangential plane; wr”: wood ray (parenchyma cells) seen in the transverse plane; c: cambium; p: phloem; pr”’: phloem ray; sb: suberized bark; tb: tracheid with bordered pits; bp: bordered pits. Masquer
(a) Simplified block diagram of a portion of the trunk of a conifer in the late summer of the fourth year, of growth, and (b) radial cut in the trunk of a conifer (adapted from Bresinsky et al., 2008). ... Lire la suite
(a) Bloc-diagramme simplifié d’une partie de tronc de conifère en fin d’été de quatrième année ; et (b) coupe radiale dans un tronc de conifère (adapté de Bresinsky et al., 2008). Préparation microscopique : Turtox No BW 1.1, Gen. Biol. Sup. House Chicago, Illinois, USA, coloration à la safranine ; Microscope : Olympus CH-BI45-T-2 ; Shooting : caméra DCM 130E, 1,3 Mpixels. Noter que les rayons ligneux sont continus d’une année à l’autre dans les deux parties de la figure. TP : plan transversal ; RP : plan radial ; TaP : plan tangentiel ; 1, 2, 3, 4 : années successives ; n –1, n, n + 1 : années successives ; ew : bois de printemps ; sw : bois d’été ; cp : moelle centrale ; ps : tissus primaires et secondaires de la première année ; a : delimitation d’un cerne annuel ; wr : rayon ligneux (cellule parenchyme) vu dans le plan radial ; wr’ : rayon ligneux (cellule parenchyme) vu dans le plan tangentiel ; wr” : rayon ligneux (cellule parenchyme) vu dans le plan transverse ; c : cambium ; p : liber ; pr”’ : rayon phloémien ; sb : écorce subérifiée ; tb : trachéide à ponctuation aréolée ; bp : ponctuation aérolée. Masquer
(a) Bloc-diagramme simplifié d’une partie de tronc de conifère en fin d’été de quatrième année ; et (b) coupe radiale dans un tronc de conifère (adapté de Bresinsky et al., 2008). Préparation microscopique : Turtox No BW 1.1, Gen. Biol. Sup. ... Lire la suite
5.2 The Sr-Nd isotope record of the growth rings
The changing Sr and Nd isotopic compositions through time can be explained by an overall replacement of pedogenic elements by needle litter and atmosphere-derived Sr and Nd as a consequence of acidification. A similar model has been proposed by Drouet et al. (2005) who observed for forest sites of High Belgium with very acid soils and low concentrations of exchangeable Ca “a decrease of the 87Sr/86Sr ratio in growth rings from inner to outer wood, for beech and oak, suggesting that these forest ecosystems were abruptly affected by atmospheric inputs of strong acids around the 1920”.
In both cases, it is the atmospheric contribution causing the shift toward less radiogenic isotopic composition values as emphasized in the Sr-Nd isotope diagram (Fig. 8), which allows one to distinguish between atmosphere- and bedrock-derived contributions (Aubert et al., 2001; Stille et al., 2009). Pure soil-derived materials plot upon or to the upper right of this mixing curve. In contrast, Sr-Nd isotopic compositions to the lower left of the curve are indicative for the presence of only atmospheric contributions (Stille et al., 2009). The Sr-Nd isotopic compositions of our spruce trunk samples, that plot to the lower left of the curve of bedrock alteration products (apatite-plagioclase mixing curve; Aubert et al., 2001), thus demonstrate that they do not only contain bedrock-derived Sr and Nd, but important quantities of atmospheric compounds. They are isotopically similar to those of lichen, tree bark, root and leaf samples from beech, leaf litter and throughfall. This interpretation is in accord with observations that show that soil acidification induces a rise of the fine root system of spruce towards the topsoil (Poszwa et al., 2003, 2004). Poszwa et al. (2004) have especially shown that the average depth of Sr and probably Ca absorption for spruces in boreal forest ecosystems is about 20 cm. Similar depths have been found by Ladouche (1997) for spruce from the Strengbach catchment using oxygen isotopes. The rise of the root system allows spruces in Ca-depleted soils to mobilize Ca accumulated in the organic matter-rich topsoil (Berger et al., 2006; Berner et al., 2007; Poszwa et al., 2000). This is certainly also the case for other trace elements such as Nd and Pb.
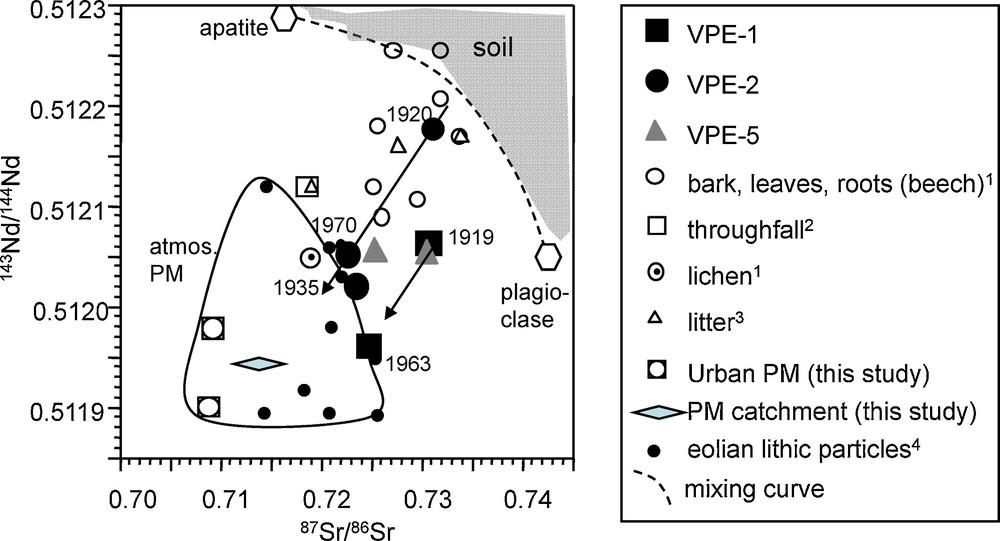
Comparison of Sr and Nd isotope data of spruce growth rings with soil, North African dust and particulate matter from an urban environment (cities of Strasbourg and Kehl) and from the Strengbach catchment (Table 2). Also given is the mixing curve of alteration products defined by apatite and plagioclase separating possible granite derived soil mineral contributions (to the right of the mixing curve) (Aubert et al., 2001) from atmospheric contributions (field below curve). (1: Stille et al., 2006; 2: Aubert et al., 2002a; 3: Stille et al., 2009; 4: Grousset and Biscaye, 2005). PM refers to Particulate Matter. Masquer
Comparison of Sr and Nd isotope data of spruce growth rings with soil, North African dust and particulate matter from an urban environment (cities of Strasbourg and Kehl) and from the Strengbach catchment (Table 2). Also given is the ... Lire la suite
Comparaison des signatures isotopiques en Sr et en Nd des cernes d’épicéas avec des valeurs de sols, des poussières provenant d’Afrique du Nord et des matières particulaires provenant d’un environnement urbain (villes de Strasbourg et Kehl) et du bassin versant du Strengbach (Tableau 2). La droite de mélange correspondant aux produits d’altération définis par l’apatite et le plagioclase et séparant la contribution des minéraux issus de l’altération du granite (à la droite de la courbe de mélange) (Aubert et al., 2001) des contributions atmosphériques (domaine en-dessous de la courbe) est également représentée. (1 : Stille et al., 2006 ; 2 : Aubert et al., 2002a ; 3 : Stille et al., 2009 ; 4 : Grousset and Biscaye, 2005). PM est l’abréviation de matière particulaire. Masquer
Comparaison des signatures isotopiques en Sr et en Nd des cernes d’épicéas avec des valeurs de sols, des poussières provenant d’Afrique du Nord et des matières particulaires provenant d’un environnement urbain (villes de Strasbourg et Kehl) et du bassin versant ... Lire la suite
The 87Sr/86Sr and most of the 143Nd/144Nd ratios of the outermost growth rings of VPE-1 and VPE-2 samples are significantly lower than those of the innermost ones. However, most significant is the change in Sr and Nd isotopic compositions between the innermost and intermediate segments. Thus, similar to the study of Drouet et al., 2005, one might suggest that the greatest rate of acidification occurred in the period between 1922 and 1938. This has to be tested in another, more detailed study. The low Nd-Sr isotopic compositions of the youngest growth rings of spruce thus reflect a dominating atmospheric contribution. They are comparable with isotopic compositions of urban PM and PM collected in the catchment, with those of industrial emissions from the Rhine valley (not shown; Lahd Geagea et al., 2007, 2008a,b) and with those of North African dusts (Fig. 8) (Grousset and Biscaye, 2005). In this scenario, atmospheric acid deposition would have led to a general cation depletion of the soil due to desorption and to dissolution of soil minerals, resulting in nutritional deficiencies for vegetation (Probst et al., 1990). This causes the progressive substitution of pedogenic cations by atmospheric inputs.
Thus, our study confirms for the first time that not only the Sr but also the Nd isotopic compositions of tree-rings record the chemical and isotopical changes in the soil system and, therefore, allow one to recognize the principal sources of Sr and Nd in the wood.
5.3 The origin and the behaviour of Ca isotopes in the trees growth rings
In contrast to Sr and Nd isotopes, the Ca isotopic homogeneity of the three growth segments of each of the trees ranging from 1916 to 1983 (δ44/40Ca: 0.42 ± 0.06, n = 9, 2SE) might suggest that the Ca sources remained the same or isotopically similar during this timespan (Fig. 6). The important nutrient environment for trees are soil solutions which carry pedogenic and needle litter derived cations. The needle litter itself contains important quantities of cations derived from nutrient cycling through vegetation and atmospheric deposition. Current atmospheric inputs such as rainwater and snow, analysed at the Strengbach catchment, have δ44/40Ca values scattering largely between 0.57‰ and 1.29‰ (Cenki Tok et al., 2009; Schmitt and Stille, 2005). This local scale scattering is consistent with atmospheric rain and snow deposits sampled all over the world (Ewing et al., 2008; Hindshaw et al., 2011; Holmden and Bélanger, 2010; Schmitt and Stille, 2005). We also note that the Ca taken up by roots from soil solutions at the spruce plot has a δ44/40Ca value similar to that of atmospheric deposits (0.82 ± 0.19; 2SE, n = 9) (Table 3) (Cenki Tok et al., 2009). Thus, the tree-rings are enriched in the light isotope compared to current soil solutions. This is consistent with other studies which have observed that the Ca uptake by vegetation causes the enrichment in the heavy 44Ca isotope in soil solutions and in the light 40Ca isotope in the vegetation (Cenki Tok et al., 2009; Cobert et al., 2011; Hindshaw et al., 2011; Holmden and Bélanger, 2010; Page et al., 2008; Platzner and Degani, 1990; Schmitt and Stille, 2005; Schmitt et al., 2003; Wiegand et al., 2005). The geogenic Ca source is for its part mainly controlled by apatite dissolution (δ44/40Ca = 0.4‰, Schmitt et al., 2003). Consequently, the rise of the fine root system of spruce towards the topsoil due to soil acidification (Poszwa et al., 2003, 2004) and the herewith induced change of Ca source from primary minerals (e.g. apatite; δ44/40Ca∼0.4‰) to top soil-derived Ca (δ44/40Ca∼0.8‰) has apparently no significant influence on the δ44/40Ca values of the trees growth rings.
Flux annuels de Ca et de Sr et compositions isotopiques associées pour le site d’échantillonnage VP (épicéas).
| Sample | Ca | δ44/40Ca | Sr | 87Sr/86Sr |
| kg/ha/yr | g/ha/yr | |||
| Soil sol. 5 cm (F1) | 7.95a | 0.85a | 36d | 0.7227d |
| Soil sol. 10 cm | 5.59a | 0.77a | 35d | 0.7286d |
| Soil sol. 30 cm (F2) | 4.73a | 0.82a | 30d | 0.7237d |
| Soil sol. 60 cm | 3.34a | 0.82a | 33d | 0.7241d |
| Modelled flux of weathering (MFalt) | 0.2b | 0.4e | ||
| Tree uptake | 12c | 18c | ||
| Litter recycling | 7c | 7c | ||
| Immobilization (Ftree) | 5c | 0.42a/0.41 | 11c | 0.7252/0.7415 |
| Calculated weathering flux (Falt) | 1.78 | 5 | 0.77b | |
| Calculated supplementary flux (Fex) | 1.58 | –0.54 |
b Fichter et al. (1998b) and Dambrine et al. (1998).
d Aubert (2001) and Aubert et al. (2002b), values with no sign refer to calculated value.
This is confirmed by two mass balance equations. In the first case, it is assumed that all Ca loss between soil solutions F-5 cm (F1) and F-30 cm (F2) results from plant immobilization (Ftree) and that the weathering flux brought from primary soil minerals to soil solution (Falt) is still operating; in this case the soil solutions at 5 cm and 30 cm depths obey the following mass balance equation:
| (1) |
Using the Ca fluxes (Table 3) yields a weathering flux (Falt) of 1.78 kg ha−1 yr−1 which is high compared to the model estimations of Fichter et al. (1998b) and Dambrine et al. (1998). Using their weathering flux (MFalt) of 0.2 kg ha−1 yr−1 (Table 3) one observes that an additional Ca flux (Fex) of 1.58 kg ha−1 yr−1 is necessary to equilibrate the Ca budget of the soil solutions. Including the Ca isotopic compositions in the mass balance equations yields:
| (2) |
Using the flux and the corresponding δ44/40Ca values (Table 3) yields a δ44/40Ca value of –0.54 for the supplementary flux (Fex). This flux is strongly enriched in the light 40Ca and might be linked to a Ca desorption flux e.g. from colloidal phases. Preliminary data of a study actually running in our laboratory on soils and soil solutions from the same VP soil profile indicate that the colloïdal phases appear to be strongly enriched in the light 40Ca. In the second simplified case we assume that the weathering flux stopped and all Ca originates from the top soil (needle litter, atmosphere) and from the supplementary flux (Fex):
| (3) |
The Sr isotope system, however, behaves differently. Using the Sr fluxes of soil solutions and tree uptake (Table 3), one derives a weathering flux of 5 g ha−1 yr−1 for Sr from Eq. (1). These fluxes and the corresponding Sr isotopic compositions allow one to calculate a tree 87Sr/86Sr ratio of 0.7415 from Eq. (4) and values from Table 3.
| (4) |
The Pb isotopic composition of the atmosphere has dramatically changed during the past 100 years as deduced among others from Pb isotope determinations on ombrotrophic peat bogs from the Jura mountains, about 80 km south of the catchment (Rosman et al., 2000; Shotyk et al., 2001; Vallelonga et al., 2002). The atmospheric 206Pb/207Pb composition was about 1.17 in 1900 and decreased to 1.12 in 1985 (Fig. 4c). After 1995 the values increased again, mainly due to the diminution of industrial emissions and to the introduction of unleaded gasoline. Today's urban particulate matter (PM) from the cities of Strasbourg and Kehl (80 km north of the Strengbach catchment) yield an average 206Pb/207Pb ratio of 1.16 (Lahd Geagea et al., 2008a), which is clearly above PM values from 1985 (1.13; Monna et al., 1997). The Pb isotope data of the growth rings from the Strengbach catchment plot with a few exceptions above the peat bog-derived evolution line of the atmospheric Pb isotopic composition.
This discrepancy between Pb isotope data from growth rings and atmospheric composition is, for different reasons, conceivable:
- • Above ground tree organs such as foliage or bark are indeed important sinks for airborne particles and have successfully been used as biomonitors (Lahd Geagea et al., 2007, 2008a). However, Pb is accumulated for more than 10 years in the bark (Guéguen et al., 2012) and the Pb, if some of it is transferred from the bark to the outermost growth ring, will therefore have a mixed isotopic signature. This is illustrated by tree barks collected recently in an urban environment (Strasbourg, France; Kehl, Germany), which furnished intermediate Pb isotopic compositions between those of actual airborne PM and those from 1995 (Grobéty et al., 2010);
- • The Pb isotope data of the growth rings plot with a few exceptions above the peat bog-derived evolution line of the atmospheric Pb isotopic composition (Shotyk et al., 2001). However, their isotope ratios are in the range of soil leachates from the uppermost 40 cm of the corresponding acid soils (Figs. 4c and 5; Stille et al., 2011), demonstrating that the Pb isotopic composition of the growth rings is significantly influenced by root absorption. This is supported by the fact that the uppermost 40 cm of the soils in the Strengbach catchment are strongly enriched in Pb compared to the bedrock. Pb isotope ratios of these leachates indicate that the Pb from close to the surface corresponds to a very recent isotopic signature of the atmosphere and that the Pb from 30 to 40 cm depth corresponds to Pb airborne PM derived from historical mining activities 100 to 150 years ago (Stille et al., 2011). The soils are very acid with pH values of 3.5, 3.9 and 4.2 at 0–10 cm depth, 10–30 cm depth, and 30–50 cm depths, respectively (http://ohge.u-strasbg.fr). Likewise it has previously been shown that Pb in oak tree-rings seem to be more related to soil pH than bedrock type and that soils with low pH have higher tree-ring Pb concentrations than soils with higher pH (Bukata and Kyser, 2008). Therefore, some of the Pb is easily mobilized in these soils and bioavailable (Stille et al., 2011). Accordingly, it is still likely, as suggested by Watmough and Hutchinson, 2002, that tree-rings of Picea abies L. growing on a soil with higher pH than that observed for the VP soils in the catchment and with much less bioavailable Pb monitor historical changes in atmospheric Pb deposition.
Consequently, our current observations do not confirm that the Pb of growth rings originates entirely from absorption of airborne particles at aboveground parts of the tree as suggested by Novak et al. (2010). On the contrary, we suggest, like Bindler et al. (2004), that growth rings integrate mixed isotopic signals resulting from absorption of atmospheric Pb through aboveground tree organs and/or from root uptake of atmosphere-derived Pb accumulated over years in the topsoil. Similarly Bellis et al. (2004) found that beech trees accumulate Pb from soils via roots in the annual growth rings and Watmough and Hutchinson (2002) suggest, based on their Pb isotope study on tree-rings from Acer pseudoplatanus, “that soil Pb accumulates within rings of diffuse porous wood over a number of years” and, therefore, that the dendroisotopical Pb record is not a reliable archive to reconstruct past atmospheric Pb pollution. Likewise, in their study on Acer pseudoplatanus, Patrick and Farmer (2006) conclude that “in areas with no local point source of lead, accurate records of changes in atmospheric lead concentration and isotopic composition are not preserved in the annual growth rings of sycamore trees”.
6 Conclusion
Growth rings of spruce can be to a certain extent archives for the reconstruction of the chemical evolution of the uppermost soil compartments which are accessible for root uptake. The innermost rings show Nd and Sr isotopic compositions closer to those of soils and soil minerals whereas outermost growth rings have significantly lower Sr and Nd isotopic compositions and approach values similar to those of industrial or pre-industrial “natural” aerosols like Saharan dust. Thus, both the Sr and Nd isotopic compositions of spruce growth rings record the chemical and isotopical changes in the soil and, therefore, allow one to identify the principal sources of Sr and Nd in the wood. However, due to re-equilibration processes, isotope ratios do not always allow a precise timing record of a polluting event. Mass balance calculations clearly indicate that decreasing alteration flux causes a decrease of the 87Sr/86Sr ratio in the trees growth rings.
In contrast, mass balance calculations for Ca indicate that the alteration flux alone does not allow one to equilibrate the Ca budget of the soil solutions. An additional 40Ca enriched flux of 1.6 kg ha−1 yr−1 (δ44/40Ca:–0.54) is necessary; it might be linked to a Ca desorption flux from an instable pool (e.g. colloidal phases) in the soil. However, the rise of the fine root system of spruce toward the topsoil due to soil acidification had no impact on the Ca isotopic composition of the growth rings, suggesting that still enough Ca was bio-available, and that during past century Ca was taken up from the upper soils rich in organic matter.
Compared with Sr, Nd and Ca, the Pb isotope ratios and concentrations behave differently. The Pb isotope data of the trees growth rings show that Pb carries a mixed isotopic signal which resulted from: (1) absorption of atmospheric Pb through direct aerial atmospheric interception and/or through root uptake from a large pool of atmosphere-derived; and (2) isotopically variable Pb accumulated over years in the uppermost part of the soil profile. Therefore, we suggest that the dendroisotopical Pb record is not suitable for the study of past atmospheric Pb pollution in the Strengbach catchment.
Acknowledgements
We thank Th. Perrone and R. Boutin for their technical assistance. This study has been financially supported by the EU soil project SoilTrEC, and by the French CNRS INSU/EC2CO program. The hospitality at the branch of Isotope Geology of the University of Berne and the great help of Th. Nägler during the Nd isotope measurements on the MC-ICPMS is greatly acknowledged.



Vous devez vous connecter pour continuer.
S'authentifier