1 Introduction
Following the end-Permian extinction (EPE) that eradicated more than 90% of marine species (e.g., Erwin, 1993), marine ecosystems underwent an important reorganization: Upper Permian benthic shelly communities dominating shallow marine carbonate environments were replaced during Earliest Triassic by widespread microbial communities (Baud et al., 1997). They were mainly located on the Palaeo-Tethys (Kershaw et al., 2007) and Neo-Tethys (Baud et al., 2005, 2007) margins with evidence of low-oxygen conditions (Bond and Wignall, 2010; Chen et al., 2011; Liao et al., 2010). They were traditionally considered as devoid of associated faunas, but discoveries of abundant ostracods (e.g., Crasquin-Soleau and Kershaw, 2005; Crasquin-Soleau et al., 2004a,b, 2006; Forel, 2012; Forel et al., 2009, 2013a,b), micro-gastropods, micro-brachiopods, foraminifers, bivalves, ammonoids and conodonts, contradict the idea of total anoxia (e.g., Baud et al., 1997; Brüwhiler et al., 2008; Ezaki et al., 2008; Frisk et al., 2012; Groves et al., 2005; Hautmann et al., 2011; Kaim et al., 2010; Richoz, 2006).
Ostracods are benthic micro-crustaceans present in all aquatic environments, from the Ordovician to the Recent. They were deeply affected by the EPE and the mechanisms of their survival and recovery are not yet characterised. Although some neritic species have been described in the Early Triassic, faunas in the early aftermath of the EPE are unknown. This lack of knowledge and the scarcity of the faunal record for this period highlight the importance of the ostracod faunas associated with microbialites. I describe here the peculiar characteristics of these unedited faunas. They are then used to depict environmental conditions associated with the development of this atypical microbial ecosystem. To finish, I introduce a new model of local oxygenation immediately after the EPE.
2 Biostratigraphic context
The Permian–Triassic boundary (PTB) Global Stratotype Section and Point (GSSP) is located at the Meishan section (Zhejiang province, China). It is defined at the base of bed 27c by the First Appearance Datum of the conodont Hindeodus parvus (Yin et al., 2001). The EPE is located slightly below it, at the base of bed 25 (Jin et al., 2000). Small structures of possible microbial origin in bed 27 (Cao and Zheng, 2009) were finally identified as micrite-filled burrows (Kershaw et al., 2012): there are no microbialites in Meishan. Biostratigraphic evidence is not yet sufficiently developed to fully constrain the stratigraphic range of microbialites following the EPE. However, they surround the PTB: they do not occur lower than the equivalent of bed 24 and may extend into the equivalents of beds 28/29 (Jiang et al., 2007). I therefore refer to them as Permian–Triassic boundary microbialites (PTBM; Kershaw et al., 2012). Based on their maximal extension and recent stratigraphical data on the Meishan section, the duration of their growth can be inferred to about 180 ± 80 kyr (Shen et al., 2011).
3 State of the art
3.1 Oxygen, ostracods and Permian–Triassic boundary microbialites
Because the most recent works on PTBM growth give high importance to marine water oxygenation, a consensus on the vocabulary is needed. For modern environments, several values of dissolved-oxygen (mL/L O2) for oxic, dysoxic and anoxic conditions are available. Despite slight differences, the following chart can be drawn:
- • oxic/suboxic: > 0.3 mL/L O2;
- • dysoxic: 0.1–0.3 mL/L O2;
- • anoxic: 0–0.1 mL/L O2 (e.g., Kaiho, 1994).
Microbialites are important parts of the Lower Proterozoic and Cambrian rocks, but are only episodic later during the Phanerozoic (Riding and Liang, 2005). Their abundance after the EPE is therefore considered as anomalous (Baud et al., 2007). Traditionally, two views of PTBM build-up are debated:
- • the first highlights the importance of carbonate saturation in their calcification (Riding, 2005);
- • the second suggests that they would respond to the extinction of metazoan as disaster biotas (Schubert and Bottjer, 1992) or anachronistic facies (Sepkoski et al., 1991).
The replacement of the complex ecosystems of Late Permian reefs by these microbial communities dominated by cyanobacteria may have been the consequence of the overturn of sluggish deep-water circulation, causing the upwelling of bicarbonate-rich, low-oxygen waters on the shelves. This super-saturation of surface waters would have caused calcium carbonate precipitation as PTBM (Kershaw et al., 1999, 2007).
Ostracods associated with PTBM were first noticed on a thin section slabs from the Jianshuigou section (Sichuan province, China; Fig. 5a in Kershaw et al., 1999) and from the Çürük Dağ section (Turkey, Plate 1 in Baud et al., 1997). The pioneer studies addressing ostracod faunas associated with PTBM were performed by Crasquin-Soleau et al., 2002, 2004a (Çürük Dağ section) and Crasquin-Soleau and Kershaw, 2005 (Laolongdong section). Of these, Crasquin-Soleau and Kershaw, 2005 compared ostracod faunas from two samples of the Laolongdong section (Sichuan province, China; Crasquin-Soleau and Kershaw, 2005): one was located near the top of the PTBM and the other one slightly above the microbial crust. Using ostracod faunas to estimate the oxygenation (Lethiers and Whatley, 1994; see below), they concluded that the PTBM fauna reflects dysoxia, whereas the post-PTBM one corresponds to oxic conditions. These conclusions were afterwards used as empirical arguments to justify the general view that PTBM deposited in low-oxygen waters (e.g., Kershaw et al., 2007).
3.2 The reconstruction of past oxygen levels: a controversial tool
To reconstruct the Palaeozoic oxygen levels in marine shallow waters, a model established by Lethiers and Whatley (1994) has been widely used in many studies of different ages (e.g., Devonian: Lethiers and Casier, 1996; Early Triassic: Crasquin-Soleau and Kershaw, 2005). It is directly issued from a similar model in recent environments reporting that nutrition and respiration are intimately linked in living ostracods (Whatley, 1990, 1991). The soft parts of these small crustaceans (∼ 1 mm long) are enclosed in bivalve calcified carapace. For most of them, the respiration is carried out by beating ventilatory plates that create and maintain a backwards water-current (Fig. 1). It provides O2 to the domicilium, where gas exchanges are achieved through the body regions where tegument is thin, particularly the inner lamella of the valves (e.g., Vannier and Abe, 1995). This water-current is also charged with nutritional particles that are caught and used according to the feeding strategy of the studied species. Filter-feeding ostracods (Platycopida) have numerous beating ventilatory plates bearing numerous setae and setules to extract the suspended alimentary particles that are then trapped and brought to the mouth (Cannon, 1926, 1933). On the opposite, deposit-feeders (mainly Podocopida) use their mandibles to gather and crush particles from the sediments. They are then brought to the mouth by the maxilla and the water-current generated by the ventilatory plates (Elofson, 1941). Whatley (1990, 1991) state that in low-O2 periods, filter-feeders are favoured by:
- • a higher volume of water passed over their respiratory surfaces, providing higher quantities of O2;
- • less oxidized organic particles;
- • the brooding of their eggs and instars that provide them the benefits of filtration.
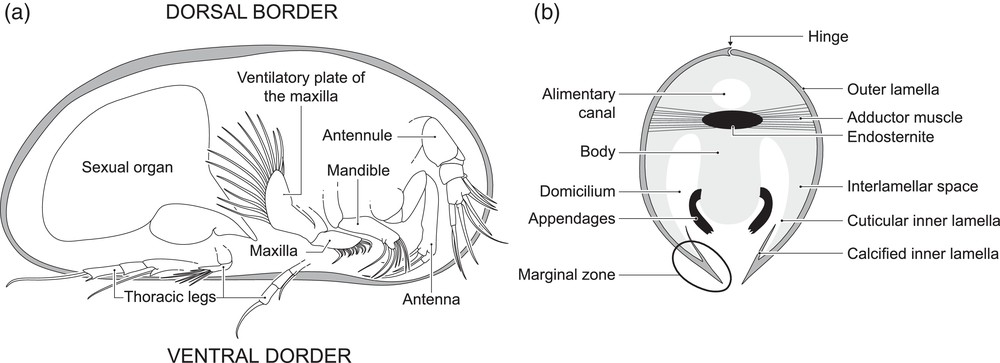
Internal anatomy of a male recent ostracod (Podocopida). a: right lateral view, right valve removed; b: schematic sagittal section.
In recent environments, filter-feeders would thus better survive low oxygenation than deposivores: this hypothesis is termed the “platycopid signal” (Whatley, 1991; Whatley et al., 2003). Morphological criteria were defined to differentiate filter from deposit-feeders in the fossil record, and led to the classical view that the fossil Palaeocopida, Platycopida and Metacopina were filter-feeders (Adamczak, 1969). Based on this distinction and sedimentological and climatic data, Lethiers and Whatley (1994) recognized a relationship between the level of O2 and the percentage of filter-feeder species per fauna in the Palaeozoic, termed the Lethiers and Whatley model (Fig. 2).
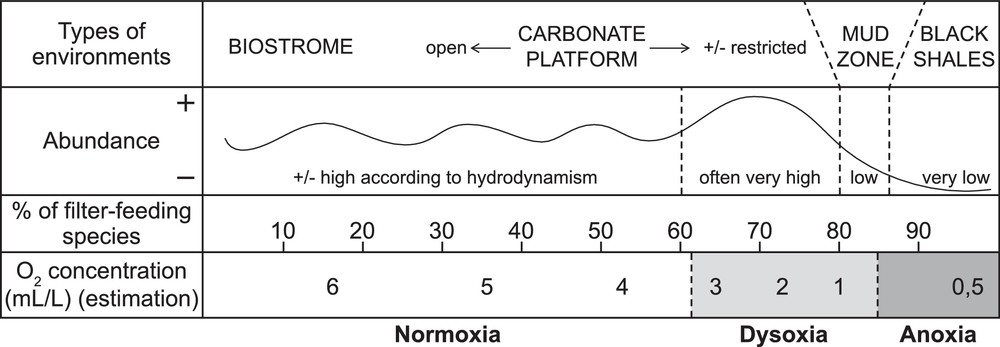
Lethiers and Whatley model of oxygen level reconstruction based on ostracod faunas (after Lethiers and Whatley, 1994).
The validity of these models is now highly questioned because of the re-study of initial data at the origin of the Platycopid Signal and recent discoveries on living/fossil ostracods (e.g., Brandão and Horne, 2009; Corbari et al., 2004, 2005; Olempska, 2008). For the time being, I consider that the use of the Lethiers and Whatley model in the PTBM debate is precluded. Nevertheless, an interesting specificity of the PTBM ostracods is helpful to overcome this difficulty. Indeed, the overall dominance of a still extant group makes it possible to draw some characteristics of environmental parameters, such as O2 levels, based on uniformitarianism.
4 What's new about PTBM ostracods?
4.1 An unexpected refuge
To clarify and eventually reconsider the information and conclusions of Crasquin-Soleau and Kershaw (2005), data were collected from different palaeo-geographical contexts (Fig. 3): the oriental border of the South China Block (SCB) opened on the Panthalassa Ocean (Dajiang and Runbao, Guizhou Province; Forel, 2012; Forel et al., 2009), the western border of the SCB on the Palaeo-Tethys Ocean (Laolongdong, Sichuan Province), the southwestern Taurus bathed by the Neo-Tethys Ocean (Çürük dağ, Turkey: Crasquin-Soleau et al., 2002, 2004a,b) and the northern margin of the Palaeo-Tethys Ocean (Bálvány North, Bükk Mountains, Hungary; Forel et al., 2013a). The list of ostracods species associated with the PTBM of each studied section is provided in Appendix 1; the reader is referred to the above references for more details. This large-scale study revealed that the presence of associated fauna is a general characteristic of PTBM whatever the locality, de facto precluding anoxia. In terms of abundance, the faunas are always dominated by ostracods, other organisms are anecdotal (micro-gastropods, micro-brachiopods, foraminifers, conodonts). The dominance of different fossil groups has been documented in other works, but this has not been observed here (e.g., bivalves: Ezaki et al., 2008; Hautmann et al., 2011; Frisk et al., 2012; Foraminifera: Baud et al., 1997; Richoz, 2006). The evolution of the ostracod biodiversity through the PTB shows striking similarities and three phases can be distinguished (Fig. 4):
- • low abundance, high species richness faunas in Upper Permian skeletal limestones;
- • high/very high abundance, low species richness in PTBM;
- • absence of ostracods above the PTBM (oolitic grainstone–packstone in Çürük dağ [Kershaw et al., 2010], mudstone in Dajiang [Lehrmann et al., 2003]).
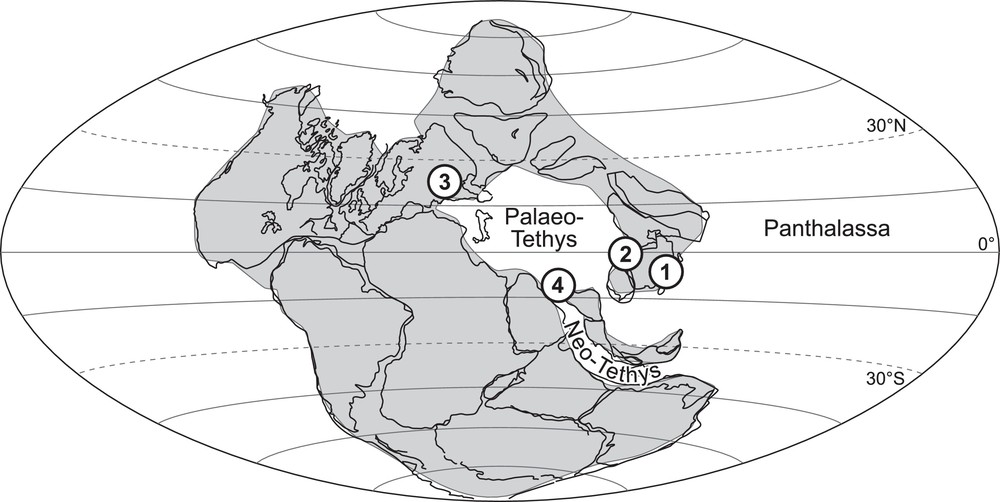
Upper Permian palaeogeographic map showing the location of studied localities. 1. Dajiang, South China. 2. Laolongdong, South China. 3. Bálvány North, Hungary. 4. Çürük dağ, Turkey (base map modified from Crasquin-Soleau et al., 2001).
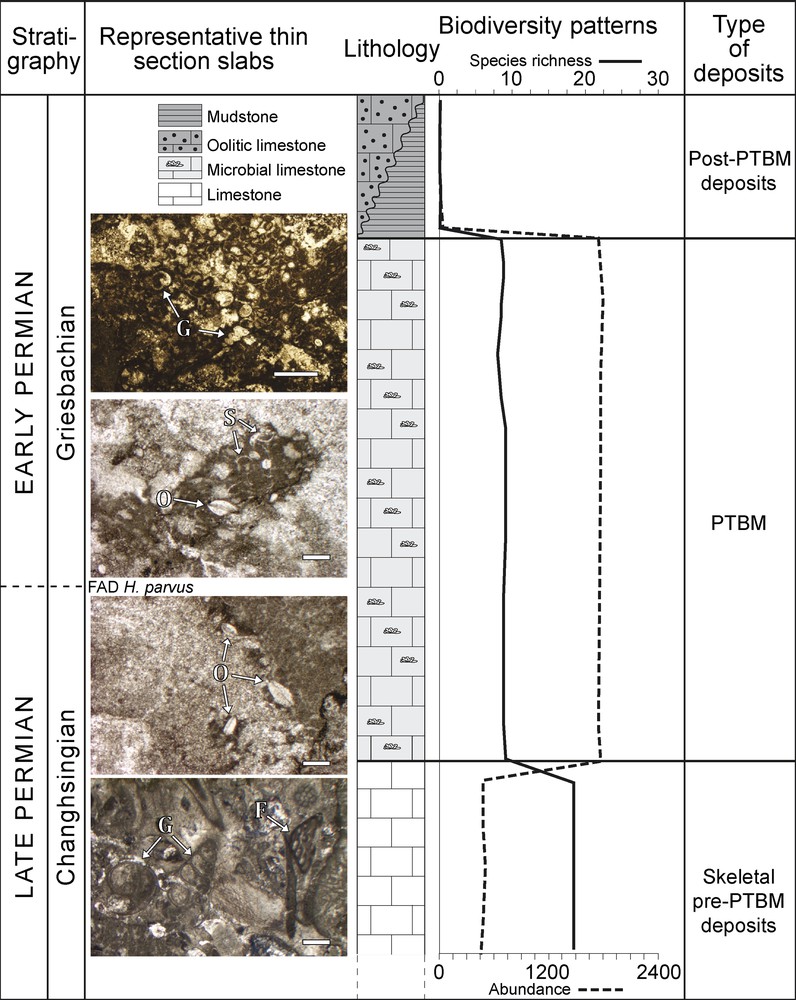
Synthetic evolution of ostracod abundance and specific richness through the Permian–Triassic boundary in Permian–Triassic boundary microbialites-bearing sections (Fig. 3). FAD: First Appearance Datum; F: foraminifers; G: gastropods; O: ostracods; S: undetermined shells.
Ostracods have been reported above the PTBM in Çürük dağ (Richoz, 2006) and in South China (Ezaki et al., 2008), but this has not been verified in the present work. These patterns are not recognized in localities of deeper settings lacking PTBM: two poor faunas are described in the Early Triassic of Meishan (Hindeodus parvus zone; Crasquin et al., 2010; Forel and Crasquin, 2011a). More than 100 samples spanning the Induan–Olenekian interval in West Pingdingshan (China; Tong and Zhao, 2005) were processed but are barren. Krystyn et al. (2003) documented abundant diversified faunas, among which ostracods, in the very Early Griesbachian of the Wasit block (Oman), lacking PTBM. However, they record an exceptionally rapid recovery of the ecosystems, contrary to the present faunas documenting survival phenomenon. Data from the Griesbachian–Smithian interval from the Guangxi province (China, Crasquin-Soleau et al., 2006) report one fauna associated with PTBM, followed by discontinuous occurrences in the Dienerian and Smithian (two productive samples among 17). Poorly-oxygenated bottom waters may have invaded the platform, interrupted by oxygenated events leading to temporary ostracod faunas. It suggests that these abundant ostracods are intimately linked to the presence of PTBM:
- • in space, areas lacking PTBM are devoid of ostracods;
- • in time, faunas temporarily disappear with PTBM.
Whatever the locality, faunas are dominated by species of the still extant Bairdioidea (Podocopida). Their long-lasting history from the Early Ordovician to the Recent (Moore, 1961) documents that they are epibenthic and associated with stable open marine settings, in well-oxygenated waters (Pr R. Maddocks, Univ. of Houston, pers. commun.). Their dominance in PTBM faunas consequently indicates that waters were neither anoxic nor dysoxic and that dissolved O2 was present in the water column. Nevertheless, such abundant faunas are hardly conceivable in the devastated environments of the aftermath of the EPE, where evidence of low-oxygenation is growing in deep-waters (e.g., Grice et al., 2005; Isozaki, 1997), on the platform (Algeo et al., 2007) and in PTBM (Bond and Wignall, 2010; Chen et al., 2011; Liao et al., 2010). Two important observations lead to an alternative explanation for the co-existence of low-O2 markers and normoxic ostracods. First, a biological hypothesis states that bacteria are the most digest food resource to all deposit-feeding organisms (the microbial stripping theory, see Lopez and Levinton, 1987). Living deposit-feeding ostracods are important grazers on extant cyanobacteria and algae (e.g., Wickstrom and Castenholz, 1985). As a consequence, PTBM ostracods dominated by deposit-feeding Bairdioidea were adapted to feed on bacteria and may have grazed microbes from the mats. Until now, possible traces of ostracod grazing activity on recent bacterial and/or algal mats are unknown. Such mats are frequently gelatinous (e.g., Defarge et al., 1994) and the probability to find tiny grazing traces preserved is quite null. The second important observation comes from debates on the nature of microbes at the origin of microbialites. Several groups of microorganisms are the framework of reefal formations, among them renalcids are calcimicrobes known from the Neoproterozoic to the Oxfordian (Fischer et al., 2007; Turner et al., 2000). They are interpreted as calcified cyanobacteria in PTBM (Lehrmann, 1999) or fossilized biofilm clusters in the Late Devonian (Stephens and Sumner, 2002). Molecular records from another Chinese PTBM document the contribution of autotrophs such as cyanobacteria to the microbial community (Chen et al., 2011), and calcified coccoid structures were related to cyanobacterial remains (although did not include renalcids; Adachi et al., 2004; Ezaki et al., 2008). Therefore, I consider that cyanobacteria were part of the PTBM builders. Renalcids in PTBM vary in preservation and the best quality was observed in the Baizhuyuan section (Sichuan Province, China; some work in progress shows that ostracods are similar to Dajiang section). The Baizhuyuan PTBM show renalcid-group microorganisms with shelly fossils in cavities, close to food and dissolved O2 injected to ambient waters by photosynthesis (Forel et al., 2013b).
To sum up:
- • bacterial photosynthesis may have engendered relatively better-oxygenated conditions in the close neighbourhood of the PTBM;
- • bacteria may have been an unlimited food pool for the abundant deposit-feeding ostracods;
- • the very low predation pressure in the absence of ostracod predators (e.g., fishes, larger Crustacean) allowed their thriving.
Based on these characteristics, the PTBM were recently defined as refuge zones in the aftermath of the EPE (Forel et al., 2013b). However, some other parameters of communities indicate that conditions stayed slightly harmful, despite these refuge advantages. Firstly, such high abundance/low species richness faunas are generally related to stressed and restricted environments (e.g., Sageman and Bina, 1997). Secondly, important reduction of the ostracods’ size is observed in PTBM faunas, whatever the locality. This phenomenon is abundantly described for other organisms in the lead-up of the EPE (He et al., 2007) and more typically in its aftermath (e.g., Payne, 2005) and is generally related to shifting environment to inhospitable conditions (e.g., Harries and Knorr, 2009; see Brayard et al., 2010 for a discussion). Thirdly, all faunas show high intraspecific variability that hindered the differentiation between species. For both recent and fossil ostracods, modifications of the carapace such as the apparition/modification/strengthening of ornamentation or noding have been reported mainly in relation with salinity variations (e.g., Keyser, 2005). PTBM ostracods do not show such patterns, but variations of the general shape of the valves (e.g., anterior and posterior angles, position of the maximum and minimum height): I exclude that salinity was at the origin of the observed morphological modifications. I rather suggest that O2 from cyanobacteria did not completely annihilate the low-O2 context and did not fully satisfy their respiratory needs. Consequently, two buffer mechanisms interfered between external mean and PTBM ostracods, allowing their survival:
- • the PTBM refuge, providing food and O2;
- • the anatomical and population characteristics.
A two-step oxygenation process explaining the existence of the peculiar ecosystem associated with the PTBM has been proposed (Forel et al., 2013b). In the first phase, the development of microbial communities may have been triggered by an invasion of relatively low-O2 waters on the platform. Indeed, these micro-palaeontological data are a snapshot of mean temporal events, so the time resolution is not fine enough to exclude this kind of temporary events. In the second phase, the functioning of the microbial ecosystem produced secondary O2 in the low-O2 setting.
It appears that the unusual faunas I report from the PTBM not only bring elements on this microbial growth episode, but highlight the lack of knowledge on fossil and recent ostracods requirements, behaviour and adaptive potential regarding O2 levels. Particularly, I obviously face the necessity to reconsider the traditional view of Bairdioidea only adapted to stable and normal marine conditions: my data de facto reveal that they abundantly survived in relative harmful conditions.
4.2 Survival or early recovery?
The PTB marks the major turnover in ostracod history, with the replacement of Palaeozoic representatives by fauna of Meso-Cainozoic affinities. PTBM faunas occur during the ostracods survival interval and show a mixture of typical Palaeozoic, Meso-Cainozoic and relatively panchronic genera (Crasquin and Forel, 2013; Crasquin-Soleau et al., 2007). They clearly represent a transitional state between fully Palaeozoic and fully Modern faunas. However, at the generic level, higher surviving lineages (Palaeozoic) are still dominant compared to newly evolved ones (Meso-Cainozoic), indicating a survival phenomenon linked to the refuge zone rather than an early recovery (see Crasquin and Forel, 2013, for details). This interpretation is reinforced by their limited stratigraphical extension that highlights their transitory aspect. Similar dominance of Palaeozoic forms is observed later in the Griesbachian (Meishan, Forel and Crasquin, 2011a), whereas younger faunas from the Spathian onwards are dominated by Meso-Cainozoic genera (Tulong section, Tibet, China; Forel and Crasquin, 2011b; Forel et al., 2011). The evolutive bridge between survival and recovery for ostracods appears to occur during the poverty phase recorded in Dienerian–Smithian interval (Crasquin and Forel, 2013; Crasquin-Soleau et al., 2007).
Nevertheless, the type of survival I present here is somewhat unusual, because it does not imply low extinction/turnover rates. On the contrary, most of the Upper Permian species do not cross the PTB: most PTBM species are new-comers, leading to a high specific turnover (Crasquin and Forel, 2013). Based on this observation, one could be tempted to define these faunas as reflecting opportunistic or disaster behaviour (Table 1). First of all, the long-lasting history of the dominating Bairdioidea is contradictory to the alleged absence of disaster taxa in fossil record before the extinction level. Bairdioidea and their genera have been major components of marine faunas throughout their history (e.g., Chitnarin et al., 2008). Although some characteristics of the PTBM ostracods reflect the ability of Bairdioidea to cope with somewhat pervasive conditions, they are not known for thriving in devastated environments and do not possess any special adaptations to such settings. Bairdioidea cannot be considered either as opportunist or as disaster taxa. The phenomenon observed here is no more that the empirical demonstration that in a relatively unstable and stressed context, the stable and normal marine Bairdioidea have broader faculties of adaptation, which require to be characterised. The thriving phenomenon is strictly related to the characteristics of PTBM and to the absence of competition pressure.
Main characteristics of disaster and opportunist taxa in equilibrium faunas, during biological crisis and in the fossil record (Kauffman and Erwin, 1995; Kauffman and Harries, 1996).
| Disaster taxa | Opportunist taxa | |
| During equilibrium phases | Excluded by competition | Minor ecological role |
| Return when competitor taxa is decimated | Small communities when opportunism is low | |
| During crisis phases | Special adaptations to high stress | Blooms during biological stress, loss of population, disappearance of equilibrium species (r strategist) |
| Secondary elements of impoverished biotas during rapid changes in final phases of biological crisis | ||
| Blooms in the early survival (r strategist) | ||
| In the fossil record | Normally absent | Populations abundant |
| Rare temporal and spatial occurrences | Restricted temporal distribution | |
| Limited to important ecological perturbations | Short time span but constant presence |
5 Conclusions
Until recently, PTBM were considered as growing in anoxic setting and so devoid of any associated organisms. This large-scale analysis reveals that ostracods are rare or absent in sections lacking PTBM. Their occurrence is strictly associated with these peculiar formations of microbial origin and clearly evidence the absence of true anoxia. These exceptional faunas show important survival in the immediate aftermath of the most devastating extinction, in an unexpected refuge. Although the PTBM were thought to be inhospitable to metazoans, the abundant microbes did serve as a major food source for ostracods. Moreover, the emission of dissolved O2 in impoverished ambient waters may have enhanced the hospitality of this ecosystem as refuges for ostracods. Although PTBM may have grown under the influence of dysoxic waters, the microenvironments created in their surroundings could have allowed the survival of important micro-faunas. These surviving ecosystems were unique and complex because of their trophic chain microbially-driven and lacking a well-developed hierarchy.
Acknowledgments
This work is part of the IGCP 572 “Restoration of marine ecosystems following the Permian–Triassic mass extinction: lessons for the present” and was funded by Actions transversales du museum (ATM) Biodiversités actuelles et fossiles. It has been realised at the UMR 7207 Centre de recherche sur la paléobiodiversité et les paléoenvironnements (CR2P), Paris. I thank Martine Fordant (UPMC) for the processing of samples and preparation of ostracods and Alexandre Lethiers (UPMC) for his help with the drawings. I am more than grateful to Dr. Sylvie Crasquin (CNRS, UPMC) for her fundamental opinion about this manuscript, patience and trust. I am deeply indebted to the Académie des sciences for the attribution of the “Grand Prix Louis-Gentil-Jacques-Bourcart 2011”. I am very indebted to the two anonymous reviewers, whose comments greatly improved an earlier version of this article.


