1 Introduction
Understanding how biodiversity responds to climate change is among the main issues of ecological studies. It is thus instructive to examine how organisms have responded to Quaternary climatic fluctuations because these responses are an important source of information that can be useful in predicting the future dynamics of ecological systems. Particularly instructive are the responses of organisms in the rain forests of western and central Africa. This is because these forests are among the World's major biodiversity hotspots (Myers et al., 2000). It is therefore probable that their fluctuations during the Quaternary induced huge changes in the distribution of organisms. During cold periods, montane forests extended to low altitudes. Connections then existed between montane forest patches that are now isolated (Maley, 1996; Plana, 2004). Such events are revealed by the current distribution of some species (Lachaise and Chassagnard, 2001; Lamotte and Petter, 1981; Missoup et al., 2012). Montane forests may thus constitute refugia for fauna and flora that predominated during the last glacial maximum of the Pleistocene. But with time, admixture and loss of species of different ecological affinities also happened in montane forest (Lézine et al., 2013). Today, drosophilids are one group whose biodiversity retains the signature of Quaternary changes. Among species related to Drosophila melanogaster (the melanogaster subgroup), these signatures are found in patterns of genetic diversity in the forest species Drosophila teissieri (Cobb et al., 2000; Lachaise et al., 1981), as well as in patterns of speciation (Lachaise et al., 1988) and in the presence of two endemic species, D. orena and D. santomea, along the Cameroon volcanic line (CVL), (Lachaise et al., 2000). Drosophilid species are abundant in African ecosystems. Most species are primary consumers, feeding on mushrooms, flowers and, the majority, on decaying fruits (Lachaise and Tsacas, 1983). Thus they are highly dependent on the vegetation and may constitute a valuable indicator of its history. Given their short generation time and their high reproductive potential, their populations are able to respond quickly to changes in the distribution of their resources.
The responses of African drosophilid species to climate can be examined by their responses to seasonal changes in climatic factors. However, despite the attention paid to African drosophilds, little attention has been given to the dynamics of drosophilid biodiversity through the entire year. Whatever the model community studied, temporal patterns of biodiversity have received much less attention than spatial ones (Magurran, 2011). Most studies have consisted of collecting trips in the most favourable season. It is nevertheless clear that the population dynamics of the drosophilid guild in Africa respond in complex ways to resource availability and seasonal climate changes. This complexity was revealed by the extensive pioneering work carried out by Lachaise (1974) in Ivory Coast. In order to improve assessments of the dependence of drosophilid populations on climatic factors, and of the variety of their responses, we studied the dynamics of populations along two environmental gradients. These were the succession of seasons over the annual cycle and the altitude between the lower and upper limits of the Mt Oku montane forest in the CVL.
2 Materials and Methods
2.1 Study site
The study site was in the Community Forest and the Plant life Sanctuary of Mt Kilum-Ijim in Oku. This mountain is one of the major peaks of the Cameroon Volcanic Line (CVL) in the Northwest Region of Cameroon. It is covered by montane forest extending from 2200 m to 2800 m asl between farmland (below 2200 m) and grassland (from 2800 m to 3000 m). The dominant tree species of the forest include Carapa grandiflora (Meliaceae), Nuxia congesta (Loganiaceae), Syzygium staudtii (Myrtaceae) and Arundinaria alpina (Poaceae) (Momo Solefack, 2009). Podocarpus milanjianus (Podocarpaceae) occurs above 2500 m. A number of endemic species are known from the mountain including plants, birds, amphibians and mammals (Cheek et al., 2000; Fotso, 2001; Maisels et al., 2000; Nussbaum, 1981; Petter, 1986). Oku village just below the study site (at 2000 m) but close to it provided a useful logistic base in a region where access to primary forests may be difficult during the rainy season. Rainfall and temperature data were obtained from the Oku village weather station where records are made at 07:00 every day. These records indicate the main seasonal changes on the mountain.
2.2 Collecting samples
Flies were collected using traps made from 1.5 L plastic bottles. A minute-hole was made that allowed drosophilid flies to enter but prevented entry by larger insects. The traps were baited with crushed soft banana and hung in the understorey vegetation. Three traps were used at each site: two of them being on the right side of the trail, approximately five meters away from each other, the third being about ten meters away on the left side of the trail. The traps were left for three days before collecting. There were seven trapping sites and they were spaced about every 100 m of elevation from 2200–2800 m along the KJ and the KA trails. The sites were thus at 2206, 2301, 2400, 2520, 2603, 2683, and 2804 m. All collections were made by SWM between 08:00 and 11:00 from the lowest to the highest collection site. The collection period began on 20 October 2008 and continued until 06 October 2009. The 29 collecting dates were as regularly spaced as possible given weather conditions.
2.3 Data analysis
Almost all the insects trapped were drosophilids. SRP classified the drosophilids into morphospecies under a binocular stereomicroscope with coupled numerical imaging. The classification was based on the external characteristics routinely used in drosophilid systematics (e.g. Prigent and Chen, 2008; Prigent and Toda, 2006). For taxonomical reference, we used the extensive collection of drosophilids in the Paris Museum (Muséum National d’Histoire Naturelle, MNHN). This collection includes a large number of specimens from central Africa, many of them being holotypes collected between 1970 and 2000 by L. Tsacas, D. Lachaise, S. McEvey, J. David and M.-T. Chassagnard.
For analysis, samples were pooled into 12-monthly periods each beginning on the fourteenth of the month and lasting until the thirteenth of the following month. We thus designated 14 July to 13 August as “July” and other periods similarly by the month in which they started. Only traps with at least one fly were considered when averaging by trap. Statistical analyses were run using R (R Development Core Team, 2010). We tested whether repeated collections at a given site could affect the local population abundances by correlation. Pairwise homogeneity tests across the seven altitudinal points of capture were run between the six most abundant species. One trap containing three individuals was discarded from the elevation analysis due to an unreadable label. The interaction between season and altitude was investigated using a two-way analysis of variance for the number of individuals caught, the number of species found, and for two diversity indices: equitability and Shannon's H index. To this end, the season factor was divided into twelve months, and altitude was divided into two classes by pooling the lower three altitudes and the higher four altitudes.
3 Results
3.1 Taxonomic diversity
Of the 29 × 7 × 3 = 609 traps used, 154 yielded no drosophilids. The 455 remaining traps yielded an average of 23.8 individuals (SD = 32.6), with a maximum of 177 individuals and a median of ten individuals per trap, suggesting that the size and the amount of bait were not limiting. Taxonomic diversity is shown in Table 1. A few specimens were damaged and could not be assigned to a morphospecies or to a sex. We were able to distinguish 62 morphospecies, most of which were represented by both sexes, while 14 species were represented by either only males or females. Most morphospecies collected belonged either to the subgenera Drosophila and Sophophora of the genus Drosophila or to the genus Zaprionus.
List of the drosophilid species determined in this study.
| Species name | 1 year | Male | Female | ? | ||
| Genus Drosophila Subgenus Dorsilopha |
1 | Drosophila busckii | 3 | 1 | 2 | – |
| Subgenus Drosophila immigrans group |
2 | Drosophila immigrans | 10 | 0 | 10 | – |
| loiciana complex | 3 | Drosophila allochroa | 11 | 5 | 6 | – |
| 4 | Drosophila aff. allochroa | 9 | 3 | 6 | – | |
| nutrita complex | 5 | Drosophila aff. nutrita | 2 | 1 | 1 | – |
| Ungrouped | 6 | Drosophila aff. nitida | 201 | 94 | 107 | – |
| 7 | Drosophila sp.1 | 4 | 1 | 3 | – | |
| “adamsi group” | 8 | Drosophila adamsi | 10 | 4 | 6 | – |
| 9 | Drosophila aff. adamsi | 10 | 3 | 7 | – | |
| 10 | Drosophila acanthomera | 2 | 1 | 1 | – | |
| “brachytarsa group” | 11 | Drosophila aff. brachytarsa | 1 | 0 | 1 | – |
| 12 | Drosophila sp.2 | 283 | 146 | 137 | – | |
| 13 | Drosophila sp.3 | 64 | 42 | 22 | – | |
| 14 | Drosophila sp.4 | 17 | 6 | 11 | – | |
| 15 | Drosophila sp.5 | 155 | 70 | 85 | – | |
| “dyaramankana group” | 16 | Drosophila dyaramankana | 22 | 14 | 8 | – |
| 17 | Drosophila aff. dyaramankana sp.1 | 43 | 3 | 40 | – | |
| 18 | Drosophila aff. dyaramankana sp.2 | 3 | 3 | 0 | – | |
| Subgenus Sophophora dentissima group |
19 | Drosophila lamottei | 9 | 8 | 1 | – |
| 20 | Drosophila aff. matilei | 62 | 41 | 31 | – | |
| Fima group | 21 | Drosophila aff. kulango | 1 | 1 | 0 | – |
| 22 | Drosophila microralis | 14 | 6 | 8 | – | |
| 23 | Drosophila aff. microralis | 6 | 2 | 4 | – | |
| Melanogaster group melanogaster subgroup |
24 | Drosophila erecta | 4 | 1 | 3 | – |
| 25 | Drosophila aff. orena | 1 | 0 | 1 | – | |
| 26 | Drosophila teissieri | 4 | 1 | 3 | – | |
| 27 | Drosophila yakuba | 44 | 22 | 22 | – | |
| Montium subgroup | 28 | Drosophila bakoue | 15 | 12 | 3 | – |
| 29 | Drosophila bocqueti | 8 | 5 | 3 | – | |
| 30 | Drosophila aff. bocqueti | 12 | 11 | 1 | – | |
| 31 | Drosophila burlai | 29 | 23 | 6 | – | |
| 32 | Drosophila aff. chauvacae sp.1 | 62 | 25 | 37 | – | |
| 33 | Drosophila aff. chauvacae sp.2 | 10 | 10 | 0 | – | |
| 34 | Drosophila aff. megapyga sp.1 | 57 | 37 | 20 | – | |
| 35 | Drosophila aff. megapyga sp.2 | 20 | 15 | 5 | – | |
| 36 | Drosophila nikananu | 4 | 3 | 1 | – | |
| 37 | Drosophila seguyi | 9 | 7 | 2 | – | |
| Genus Lissocephala | 38 | Lissocephala aff. diola | 1 | 0 | 1 | – |
| Genus Microdrosophila | 39 | Microdrosophila aff. mamaru | 5 | 2 | 3 | – |
| Genus Scaptodrosophila | 40 | Scaptodrosophila latifasciaeformis | 1 | 1 | 0 | – |
| 41 | Scaptodrosophila aff. nicolae sp.1 | 79 | 55 | 24 | – | |
| 42 | Scaptodrosophila aff. nicolae sp.2 | 14 | 10 | 4 | – | |
| 43 | Scaptodrosophila sp.1 | 109 | 62 | 47 | – | |
| 44 | Scaptodrosophila sp.2 | 1 | 1 | 0 | – | |
| 45 | Scaptodrosophila sp.3 | 1 | 0 | 1 | – | |
| Genus Scaptomyza | 46 | Scaptomyza sp.1 | 1 | 1 | 0 | – |
| Genus Zaprionus Group armatus |
47 | Zaprionus spineus | 4 | 1 | 3 | – |
| Group inermis | 48 | Zaprionus badyi | 3 | 2 | 1 | – |
| 49 | Zaprionus inermis | 1 | 1 | 0 | – | |
| 50 | Zaprionus momorticus | 8 | 4 | 4 | – | |
| 51 | Zaprionus tuberculatus | 170 | 89 | 81 | – | |
| Group neglectus | 52 | Zaprionus neglectus | 1 | 1 | 0 | – |
| Group vittiger | 53 | Zaprionus aff. camerounensis | 1 | 0 | 1 | – |
| 54 | Zaprionus davidi | 20 | 9 | 11 | – | |
| 55 | Zaprionus indianus | 46 | 24 | 22 | – | |
| 56 | Zaprionus koroleu | 11 | 3 | 8 | – | |
| 57 | Zaprionus aff. lachaisei | 199 | 141 | 58 | – | |
| 58 | Zaprionus ornatus | 4 | 2 | 2 | – | |
| 59 | Zaprionus aff. ornatus | 8 | 3 | 5 | – | |
| 60 | Zaprionus taronus | 2 | 1 | 0 | 1 | |
| 61 | Zaprionus vittiger | 8818 | 5295 | 3506 | 17 | |
| 62 | Zaprionus aff. vittiger | 99 | 34 | 65 | – | |
| Undetermined | 1 | 0 | 0 | 1 | ||
| Total | 10829 | 6369 | 4451 | 19 |
Our sample includes only two Drosophila known to be invading species or human commensals (D. immigrans and D. busckii), and in very low numbers.
The number of individuals collected for each morphospecies is shown in Fig. 1 and Table 1. A single morphospecies, Zaprionus vittiger, made up 81% of the individuals collected. Eleven species were represented by only one specimen. Excluding Z. vittiger, the other species were represented by an average of 33 individuals. The sex-ratio shows a majority of males for Z. vittiger (M/F = 1.51) as well as for the other species (average M/F = 1.136).

Abundance (logarithmic scale) of drosophilid morphospecies from Mt Oku as a function of the rank of abundance.
3.2 Weather data
Rainfall and temperature fluctuations are shown in Fig. 2. The study began in October 2008, at the end of the rainy season. The dry season lasted from November to March (with some rain in early December), and the rainy season lasted from March to November. Heavy rain was recorded from July to October. This period is locally considered the main rainy season. Thus for the period covered by the study during this year, we divided the rainy season into three stages: early (March–July), main (July–October), and late (October–November) rainy season. Note that since our collection protocol began the 14th of the first month, these divisions connect in the middle of months (Materials and Methods). Temperatures fluctuated greatly over the year, reaching a maximum in May (17–18 °C), intermediate values during the main rainy season (13 °C in July) and with an abrupt decrease to its lowest value in the second half of December (8–9 °C).

Rainfall and temperature during the study period; the four horizontal shaded bars identify the seasonal periods used in this study: dry season (white), early rainy season (darker grey), main rainy season (black) and late rainy season (lighter grey).
3.3 Seasonal fluctuations in population abundance
About 300 flies were caught per sampling day over the 21 traps, ranging from nine individuals (15 June 2009, rainy season, with only four successful traps) to 938 individuals (24 November 2008, beginning of the dry season, with 17 successful traps). The time lapse between collections ranged from five to 26 days. We found no significant negative correlation between the sampling lapse time and the change in the number sampled, suggesting that successive sampling did not affect trapping efficiency.
Fig. 3 shows patterns of fluctuation in abundance for the twenty most abundant species (n ≥ 20 individuals). None of them showed a stable population for the whole annual cycle. The species most constantly present, Drosophila sp.5, showed a large stable population for only half the year at the boundary, across the limit between the end of the main rainy season and the dry season (Fig. 3t).

Annual population fluctuation in the 20 most abundant drosophilid species from Mt Oku; the four seasonal periods are as defined in Fig. 2; n: total sample size for each morphological species; vertical bars are proportional to the relative contribution of each month (sum = 1.00); species: a: Z. aff. lachaisei, b: Z. indianus, c: D. aff. nitida, d: Z. vittiger, e: D. aff. matilei, f: D. aff. dyaramankana sp.1, g: Drosophila sp.3, h: Scaptodrosophila aff. nicolae sp.1, i: Z. aff. vittiger, j: Scaptodrosophila sp.1, k: Z. davidi, l: D. yakuba, m: Z. tuberculatus, n: D. dyaramankana, o: D. aff. chauvacae sp.1, p: D. burlai, q: D. aff. megapyga sp.2, r: D. aff. megapyga sp.1, s: Drosophila sp.2, t: Drosophila sp.5.
The period of abundance varied greatly across species. The most common species, Z. vittiger fluctuated in number, with a peak in November-December and a period of low capture from April to September (Fig. 3d). The most important change was a sudden drop in abundance corresponding to the onset of the early rainy season in April 2009. Some species were found year round with wide fluctuations whereas others occurred for a very limited period of time, only one or two months. Consequently some species were caught during only one season and some species were found for each of the four seasons.
Owing to the small number of individuals of some species captured, a convenient way to compare them was to pool the samples obtained for one species within each of the four seasonal periods defined above. This is shown in Fig. 4, which summarizes the same data as Fig. 3 in a way which is no longer proportional to time, since in this classification the seasonal periods are not of equal duration, but which makes it easier to understand the global response of the drosophilid guild to seasonal factors. It appears that groups of species are characteristic of a given part of the year, being found during one period or two successive periods. A few species occurred over three or four periods. In Fig. 4, species are placed in chronological order of their occurrence to smooth the transition from one species to the next. They thus follow a kind of succession around the year. A smooth graduation was observed when considering species mainly trapped during the late rainy and the dry seasons. Indeed, between the species with the highest abundance in the late rainy season and the one with the highest number in the dry season we observed several species with intermediate distribution over both seasons. More abrupt transitions are observed between the other seasons. Species that were the most abundant during the early rainy season were much less abundant in the dry season. Similarly the most common species during the main rainy season are rather rare in both the preceding and following seasons.
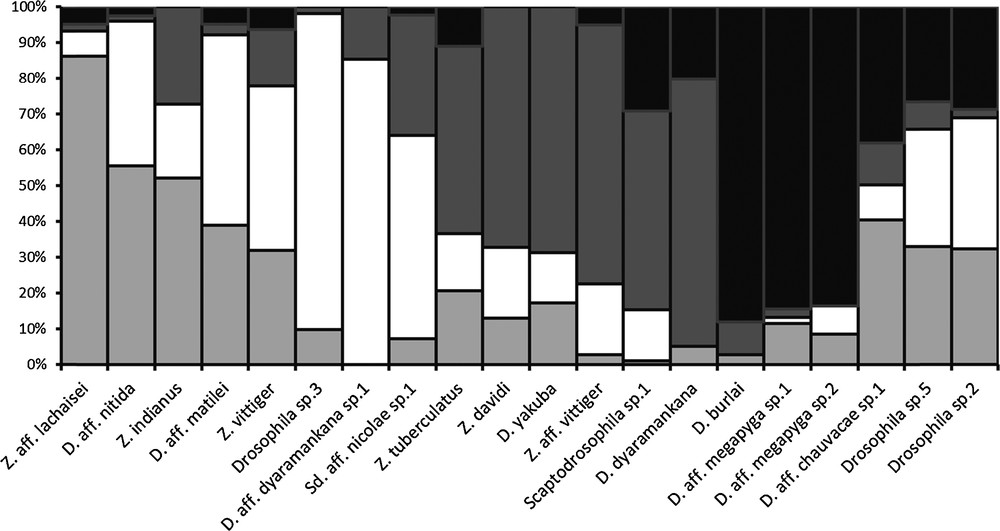
Relative abundance of the 20 most abundant drosophilid species from Mt Oku during the four seasonal periods defined in Fig. 2.
3.4 Patterns in distribution and abundance across an altitudinal gradient
Pairwise homogenity tests across the seven altitudinal locations were run for the six most abundant species. They were highly significant in all cases (p-values < 2.10−7), showing that all the species which were abundant enough for this comparison to be made varied in their altitudinal distribution. Among them, Z. aff. lachaisei and Z. vittiger occurred at the lowest elevations of the study, whereas D. aff. nitida occurred at the highest elevations (Fig. 5). The other three species occurred at intermediate altitudes. For Z. tuberculatus, we found a peak at two distinct altitudes: 2300 m and 2700 m. Since we could not differentiate this species from its sibling species Z. sepsoides, which can be identified only by dissection of male gonads, this finding may result from the presence of the two species, combined with environmental heterogeneity.
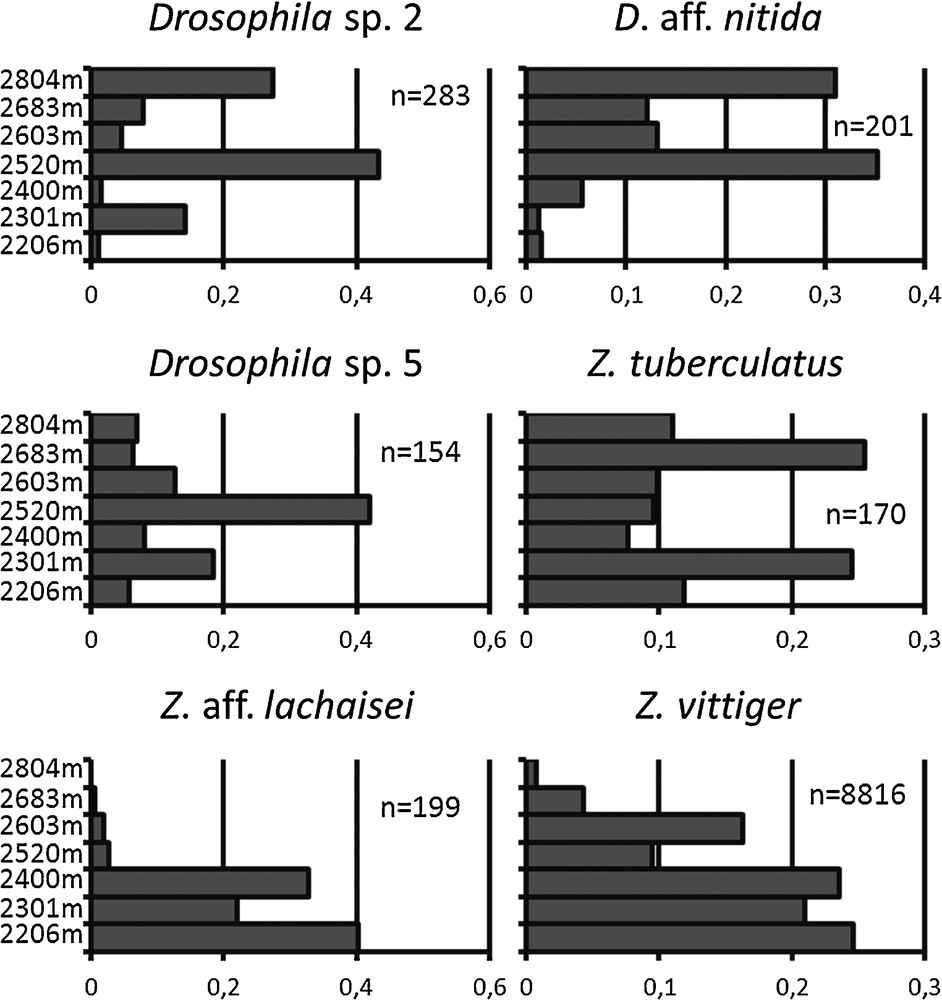
Altitudinal distribution of the six most abundant drosophilid species of Mt Oku from 2200 m to 2800 m.
3.5 Interaction between seasons and altitude
The interaction between season and altitude was assessed using a two-way analysis of variance for four variables relating to community structure: the number of individuals caught, the number of species found, equitablity and Shannon's H index. Since biotic heterogeneity probably occurs at each elevation, data were pooled into two classes of elevation: below 2450 m and above 2450 m, thus considering the different observations from the same class to be repetitions of the same protocol. This analysis was irrelevant for the number of species found and for Shannon's H, since only one of the two factors showed significantly different classes (Table 2). The two factors did interact for the number of individuals caught and for the equitability index. Inspection of the data shows that equitability is low and the number of individuals caught high from November to April, the reverse being true the rest of the year. Likewise, equitability is low and the number of individuals caught high for the lower five altitudes, the reverse being true for the higher two.
Two-ways analysis of variance between season and altitude for four community variables.
| Season (df = 11) | Altitude (df = 1) | Interaction (df = 11) | |
| Number of species | < 10−8 | NS | NS |
| Number of individuals | < 10−14 | < 10−6 | < 10−6 |
| Equitability | < 10−3 | < 10−2 | < 10−2 |
| Shannon's H | NS | < 10−3 | NS |
4 Discussion
Our study is the first ecological survey of the drosophilid guild in one of the richest ecosystems on Earth, the montane rain forest of central Africa. It is perhaps the most extensive study of its kind in a species-rich animal group in this area, together with a study of Coleoptera by Muafor et al. (2011). Our results reveal a level of complexity much more difficult to describe than would be possible using only simple terms based on the mere opposition between the dry and the rainy seasons, or with reference to a uniform montane forest. We are conscious that this study might well have been conducted over several years, and averaged over a larger number of collecting sites. We are also conscious that this study raises new issues, including the description of new species. Nevertheless, our results lead to conclusions sufficiently firm to merit discussion.
4.1 Species diversity in Mt Oku drosophilids
Since this study was essentially ecological, we relied on the identification of morphospecies. A full determination of drosophilid species requires, for each male individual, a dissection of genitalia. This cannot be carried out routinely in the framework of a quantitative analysis. Thus the level of taxonomic precision of our study varied across species, from the extensively studied melanogaster subgroup to more poorly documented species which systematists have not yet put into “subgroups” or “species complexes”, and for which we only mention apparent affinities. In regard to the morphospecies at hand, we suggest for instance the creation of the three new groups of species, adamsi, brachytarsa and dyaramankana in the subgenus Drosophila (Table 1).
The presence of clear-cut distinct types in a species group, occurring both in males and females and in similar numbers across samples, may be used as an indication of which members of either sex correspond to the same species. However this method has limitations in drosophila systematics, including the differential distribution of the darkening of abdominal segments in the two sexes for some species, and the occurrence of two phases, dark and light, in females from the montium subgroup (Burla, 1954). Moreover, several complexes of cryptic species have been described in Zaprionus from male genitalia and from crossing experiments conducted in the laboratory (Chassagnard and Tsacas, 1993, Yassin, 2008; Yassin et al., 2008b). Our ongoing molecular study on the Zaprionus genus in central Africa confirms the existence of a number of cryptic species which cannot be distinguished on the sole basis of their external morphology, especially in three groups including Z. tuberculatus, Z. indianus, and Z. davidi (Suwalski et al., in preparation). In consequence, we possibly pooled some species, which are biologically distinct. However, grouping biological species into morphospecies is conservative, meaning that when significant differences in distribution were observed between morphospecies, they were necessarily indicative of underlying differences which were larger than or equal to the differences observed. Discrepancies in the altitudinal distribution of Z. tuberculatus with separate peaks at 2300 and 2700 m (Fig. 5) and for two distinct seasonal peaks in Z. indianus in the early and late rainy seasons (Fig. 3b), might result from such cryptic species admixture. A companion study on the barcoding of our sample is therefore underway to confirm species identity. However the level of taxonomic determination of our sample cannot be lower than that of the extensive ecological study of African drosophilids by Lachaise (Lachaise, 1974, Lachaise and Tsacas, 1983) to which our study can be compared, and which predates the most recent taxonomic revisions (e.g. Chassagnard et al., 1997, Lachaise and Chassagnard, 2001, Tsacas, 1990).
Our collection allowed the identification of 62 morphospecies. But there are reasons to believe that we underestimate true drosophilid diversity in Mt Oku. Eleven species were represented by a singleton, suggesting that our collection was not exhaustive enough. Moreover the heterogeneity of the forest ecosystem may accommodate a number of species with patchy distribution, thus our linear trapping protocol along an altitudinal gradient may not grasp the whole biodiversity at each level. In drosophilids, this survey focuses on the Drosophilinae subfamily, which is mostly represented by three genera: Drosophila, Zaprionus and Scaptodrosophila (Table 1). Species of the Steganinae subfamily are wholly absent from our samples. This bias is probably due to the selectivity of the trapping protocol which excludes most species from the Leucophenga, Stegana and Scaptomyza genera. Moreover, specialist taxa like the mycophagous and floricolous species and some drosophilids parasitizing insect larvae are usually caught by sweeping over the vegetation with insect-nets and are not attracted to banana traps. Another bias was an excess of males in the sex ratio of most samples. This is in agreement with observations showing that male drosophilids are more active in the morning (Lachaise, 1974).
African montane ecosystems are known to be home to drosophilid faunas including a number of endemic species (Lachaise and Chassagnard, 2001; Tsacas et al., 1985), especially in the loiciana complex, the megapyga complex of the montium subgroup and the dentissima group (Lachaise and Chassagnard, 2001, Tsacas, 1980; Tsacas and Chassagnard, 2000). Two morphospecies from each of these groups were found in Mt Oku (Table 1). A questionable female is possibly related to D. orena, another montane species, but no male specimen to support this species identification was found in the ∼ 11,000 individuals collected. A member of the melanogaster subgroup, this species is probably endemic to Mt Lefo, another summit of the Cameroon volcanic line, which stands about 50 km to the southwest of Mt Oku. It is known from a few specimens caught in the wild in 1975 (Tsacas and David, 1978), and from a laboratory line derived from a single female. This species was never found again in Mt Lefo, which has since been highly affected by agricultural development. Several other species are putatively new, in particular within the subgenus Drosophila, and are potentially endemic to Mt Oku. Our data suggest that the drosophilid community of Mt Oku includes a typical montane fauna component. Eastern African mountains contain species that are phylogenetically close to those on Mt Oku. This relationship suggests that the montane forests of Mt Oku and eastern Africa were connected in a not too distant geological epoch.
4.2 Marked fluctuations in the annual and spatial dynamics of species
In our analyses we only considered traps yielding flies. Missing data for some traps may result from causes which are difficult to identify and may not pertain to population abundance: destruction of the bait by the arboreal fauna (including rodents), invasion by ants, drying of the bait, heating of the trap by sunlight through the canopy, wind and storm perturbing fly activity.
The distribution of the twenty most abundant species over seasons (pooling data from several altitudes) showed evidence of contrasted patterns of abundance around the year. In temperate regions, populations of short-lived animals such as insects fluctuate widely. This is the case in Drosophila, where populations annually go through narrow bottlenecks, which can be measured genetically (Gravot et al., 2004). In tropical areas, Lachaise (1974) found that drosophilid populations from Ivory Coast followed large annual fluctuations in the local ecosystems: savannas, forested savannas and gallery forests. Such temporal fluctuations are common in other insects. Species richness and abundance of nymphalid butterflies in Ecuador are low in the dry season and high in the rainy season (DeVries et al., 1997). Similarly species diversity of adult butterflies in primary forests of Borneo was significantly higher during the dry season than during the wetter monsoon season (Hamer et al., 2005).
The distribution of species at seven altitudes (pooling data over a year) showed that the six most abundant species all significantly differed in their patterns of abundance, which may be caused by either altitude itself or the environmental heterogeneity at each study site. According to Rahbek (1995) about half of the studies on species richness patterns along elevational gradients show a steady decrease in species number with increasing altitude, whereas the other half detect a peak at intermediate elevations. Interestingly, our study showed no significant effect of altitude on species richness, but a strong effect was found on abundance. A striking change was observed in species composition. Such gradients in taxon composition are also reported e.g. in termites from Thailand, where Macrotermitinae decrease and Nasutitermitinae increase in abundance with altitude (Inoue et al., 2006). Dramatic changes in species composition are also reported in hymenoptera between the dry and rainy seasons and across habitats (Tylianakis et al., 2005). Lachaise (1974) found that all drosophilid species from Ivory Coast do not respond similarly to yearly climatic cycles, and show evidence of species succession. Our results show evidence of an annual succession of drosophilid species in a different kind of tropical ecosystem: the montane rain forest of central Africa, based on a very different set of species from the drosophilid taxonomic diversity. In the Zaprionus genus, species showed a variety of responses to ecological conditions. A characteristic Zaprionus species is observed for nearly every season. The Zaprionus genus underwent a recent and extensive radiation in Africa (Yassin et al., 2008a), suggesting successful ecological diversification in this continent, which may account for this diversity of responses. In contrast, most species from the Drosophila subgenus of Drosophila peaked in abundance in the dry season. Similarly the four species of the montium subgroup, within the Sophophora subgenus of Drosophila, peaked altogether at the rainy season. Interestingly, it is also at the rainy season that another member of the montium subgroup (the forest species D. bocqueti) was the most abundant in Ivory Coast (Lachaise, 1974).
Rainfall and solar intensity were reported by Lachaise to be the main variables acting upon drosophilid population dynamics in Ivory Coast savannas. In forests, the temporal and spatial distribution of species is unlikely to change in response to only two physical factors. In the tropical forest of Panama the higher abundance of caterpillars during the wet season is probably driven by the increase in food availability (Connahs et al., 2011). Similarly the increase in Hymenoptera diversity during the rainy season is thought to result from higher flowering herb diversity (Tylianakis et al., 2005). Species composition in beetle communities varies significantly between trees (Gering et al., 2003). Abundance in 19 arthropod groups, either herbivores or predators, is higher in forest gaps during the rainy season and higher in the understorey during the dry season (Richards and Windsor, 2007). Gaps offer more food resources due to higher plant density and more young leaves in the rainy season. But exposure of gaps to higher temperatures and lower humidity in the dry season affects insect populations.
We lack information on food preferences among drosophilids from Mt Oku, however we cannot exclude that variability in food resource is also involved in the diversity of observed patterns of population dynamics. Forest gaps are also present in the Oku forest and could contribute to local increase in biodiversity. This could explain the short population burst of D. yakuba in the early rainy season in the Mt Oku forest. In Ivory Coast, this member of the melanogaster subgroup is known to be more abundant in savannas than in forests, and its population number also increases during the dry season (Lachaise, 1974). Its occurrence in the montane forest for a short time could result from migration from open areas. Migration between different types of savannas has been suggested for S. latifasciaeformis (Lachaise, 1974). It is noticeable that all D. yakuba samples were caught between 2600 and 2800 m, suggesting that these individuals may have come from the summit grassland.
5 Conclusion
The drosophilid fauna of the montane forest of Mt Oku is exceptionally diverse and probably contains a number of endemic species. A similar conclusion has been reached for other groups including amphibians (Nussbaum, 1981), birds (Fotso, 2001), rodents (Petter, 1986) and plants (Cheek et al., 2000; Maisels et al., 2000), emphasizing the uniqueness of this site and the need to act for its preservation. Three conclusions emerge from our ecological survey.
Firstly, with their short generation time, drosophilid flies are highly sensitive to environmental changes in space and time, and potentially constitute good indicators of different kinds of ecosystems and of changes in different kinds of physical conditions, including weather factors (rainfall and temperature) and altitude. Similarly, drosophilids may have responded to past climatic changes, and for this reason may also constitute a good indicator of the Quaternary history of forest ecosystems in central Africa, as confirmed by demographic studies derived from patterns of genetic diversity (Bouiges et al., 2013). Moreover, as all species do not respond to the same environmental factor or in the same way, studies of different species are complementary.
Second, as already pointed out for other insects (Summerville and Crist, 2005; Tylianakis et al., 2005) faunistic studies tend to be biased if they do not cover a comprehensive annual survey. In particular, species showing a demographic peak in the rainy season will generally be under-represented, since weather conditions interfere with field studies and few studies are thus carried out in this period. This means that studies covering several periods of the year in each of several environments would achieve a better record of taxonomic diversity. However all species may not be equally detectable over time. This will generate artifactual records of seemingly rare species. For this reason alternative sampling procedures are recommended (Magurran, 2011; Summerville and Crist, 2005).
Third, in biogeographical studies involving sampling campaigns carried out at the same time over widely dispersed collecting sites, for instance, with stations located at different latitudes, attention should be paid to the fact that the different regions may not be strictly comparable since seasonal factors are not simultaneous in each of them. For each collecting site it is necessary to take into account the local characteristics of seasons.
Acknowledgments
This study was supported by the Agence Nationale de la Recherche (ANR) grant IFORA to Michel Veuille and Philippe Le Gall, and by a two-year ATER fellowship of Ecole Pratique des Hautes Etudes (EPHE) to Stéphane Prigent. We thank the Ministère de la Recherche Scientifique of Cameroon (MINRESI) for issuing research permits, and Xavier Garde, of the Institut de Recherche pour le Développement (IRD), for the logistical help of the Yaoundé Delegation. We thank Leonidas Tsacas for the list of drosophilids from Cameroun and for access to the original drawings of the types of many species. We thank Andrew Davis for reviewing English and for fruitful suggestions on the manuscript. This work would not have been possible without the encouragement and help of His Royal Highness the Fon of Oku Sentieh II, who offered us all available local facilities for our fieldwork.


