1 Introduction
Marine and estuarine harbour sediments are often subject to anthropogenic impact including elevated concentrations of potentially toxic metals such as Cu, Pb, and Zn. The degree of metal association with the distinct geochemical phases in sediment depends on the binding capacity and physicochemical characteristics of those phases. Mobility studies are conducted in order to estimate the mobile metal fraction considered as bioavailable. According to the literature (Semple et al., 2007), there are many definitions of ‘bioavailability’, a term used in many different scientific fields. Here the bioavailable portion is considered to be the amount of a chemical compound that could be mineralized from soil or sediment through desorption processes under various physiological conditions, then which could be transferred to biota (Baraud and Leleyter, 2012; Ehler and Luthy, 2003; Kramer and Ryan, 2000). Various chemical extractions are used to assess metal mobility, which is equated to the potential bioavailability of those metals (Da Silva et al., 2002; El-Azim and El-Moselhy, 2005; Giancoli Barreto et al., 2004; Gismera et al., 2004; Singh et al., 2005). Chemical extraction procedures follow two approaches: the thermodynamic approach and the kinetic approach. Unlike the thermodynamic approach, the kinetic approach uses different extraction times to assess the time frame of element mobilisation.
Extraction using a single reagent is a simple and cost-effective way to investigate the metals mobility in soils and sediments. Numerous reactants may be used for single extractions; these generally fall into three categories: acids, unbuffered salts, and complexing reagents. However, depending on the matrix and the reactant, the actual mobility can be over- or underestimated. Single or mixed diluted acids are often used to estimate the mobility of elements [e.g., 0.10 mol·L−1 CH3COOH (Lebourg et al., 1996), 1.00 mol·L−1 HCl or a mixture such as 0.05 mol·L−1 HCl and 0.0125 mol·L−1 H2SO4 (Mulchi et al., 1992)]. HCl is assumed to extract metals due to its acidic properties and the chelatant property of Cl−. HCl has been studied extensively in lability studies and is recommended by many authors (Doherty et al., 2000; Duinker et al., 1974; Scouller et al., 2006), a concentration of 1.00 mol·L−1 is suggested for harbour sediments (Burton et al., 2005; Larner et al., 2008; Leleyter et al., 2012; Santos et al., 2010; Szefer et al., 1995).
Sequential extractions are widely used to investigate the association between heavy metals and the different mineral and organic phases in sediments. Results can be used to predict the mobility and potential bioavailability of the metals. The technique uses reagents to carry out successive leaching of specific geochemical fractions and several different protocols are proposed in the literature. The first, developed by Tessier et al. (1979), proposed a five-step extraction to establish the different fractions to which the elements are sorbed. Subsequent authors, including Ure et al. (1993), Rauret et al. (1998) and Leleyter and Probst (1999), have adapted this protocol by using other reagents or by adding or reducing the number of steps in order to improve the efficiency and selectivity of the protocol. However, the sequential extraction protocols are criticized about the lack of selectivity and re-adsorption phenomena of the elements (Gleyzes et al., 2002; Gomez-Ariza et al., 1999). Moreover, the element mobility is assessed in specific physicochemical conditions imposed by the chemical reagents used.
The optimum contact time between sediment and reagent corresponds to the time taken for maximum extraction of the elements and is determined using kinetic extraction (Fangueiro et al., 2002; Gismera et al., 2004; Labanowski, 2004; Lin and Chen, 1998; Yu and Klarup, 1994). Single and sequential extractions assume that the reaction equilibrium is reached by the end of the extraction period; however, optimum contact times determined by kinetic extraction can be longer than those recommended for single and sequential extractions (Abi-Ghanem, 2008). Moreover, kinetic extractions allow fast and slow metal mobilisation to be distinguished (Bermond et al., 2005; Bordas and Bourg, 1998). Several kinetic models that differentiate this temporal mobilisation have been proposed in the literature, including the Elovich equation, the two-compartment model, the diffusion model and an equation with two constants (Abi-Ghanem, 2008; Abi-Ghanem et al., 2009). Fangueiro et al. (2005) assert that the two-compartment model has the advantage of separating the elements into three distinct categories; Q1: quickly mobilised, Q2: slowly mobilised and Q3: not mobilised. Gismera et al. (2004) and Labanowski (2004) use EDTA as an extractant because it is non-specific (only cations) and can therefore mobilise a large number of elements; it is also capable of extracting metal bound to organic matter, carbonate and Fe and Mn oxides providing good long-term prediction of metal bioaccessibility from these different sediment phases. EDTA is sometimes considered as overestimating bioavailability (Hooda, 2010). However, Leleyter et al. (2012) suggest that, in the case of marine sediment, EDTA is adapted to estimate the bioavailability of Cu, Pb and Zn.
Cornu et al. (2004) and Gismera et al. (2004) consider that the kinetic approach is complementary to sequential extractions and helps to expand understanding of the geochemical speciation of elements.
The objective of this study is to compare different procedures in order to assess the mobility of Cu, Pb, and Zn in marine harbour sediments collected in the English Channel. These metals are often present at elevated levels in harbour sediments due to pre-industrial deposition (Chiffoleau et al., 1999; Hamdoun, 2013; Pirrie et al., 2002). The mobility was studied using thermodynamic and kinetic extraction techniques.
2 Material and methods
2.1 Sample collection
Marine sediment samples were taken from the harbours of Ouistreham, France (S1), Concarneau, France (S2) and Pool, UK (S3) between March 2010 and June 2011. Each harbour was sampled at one GPS point using a grab or a suction dredger, water depths ranged from 4 to 11 m. On return to the laboratory, samples were homogenised, air-dried for 4 days, sieved at 500 μm using a nylon sieve and ground with an agate pestle and a mortar. The granulometric fraction (< 500 μm) was chosen according studies on harbour sediments (Sorensen and Milne, 2009; Townsed et al., 2009).
2.2 Total concentrations
The total metal contents in the samples are determined after a microwave-assisted (Berghof Speedwave MWS-2) aqua regia acid digestion was performed on 0.2 g of dry sediment (3.33 mL of HNO3 NORMAPUR 65% and 6.66 mL of HCl technical 35% VWR) (Alloway, 1995). Each acid digestion was performed in triplicate. For the three elements of interest, the metal contents then determined were similar to those measured after alkaline fusion (Hamdoun, 2013), for any tested sample. Moreover, the procedure (Table 1) was applied to a standard certified material HR-1 (Canada Centre for Inland Waters National Laboratory for Environmental). As noticed in Table 2, which compares the measured values to the certified ones, satisfactory recoveries (considering uncertainties), for the three elements of interest, validated the acid digestion procedure.
Program mineralization (power: 1000 w).
| Step | Power (%) | Temperature (°C) | Time (min) |
| 1 | 80 | 175 | 20 |
| 2 | 40 | 100 | 20 |
| 3 | 40 | 80 | 10 |
Element recovery for certified sediment HR-1 after microwave-assisted (Berghof Speedwave MWS-2) acid digestion (Cu, Pb and Zn in mg·kg−1).
| Cu | Pb | Zn | |
| HR-1 certified values | 80 ± 11 | 139 ± 37 | 1105 ± 173 |
| Acid digestion (three replicates) | 68 ± 13 | 114 ± 9 | 1056 ± 75 |
2.3 Other parameters
Other physicochemical measurements were realized on the sediments. In this paper are only reported the results regarding the CaCO3 content, assessed by Bernard calcimetry, of the total organic carbon (TOC), measured according ISO 14235, 1998, and the sediments pH (according ISO 10390) (Hamdoun, 2013).
2.4 Mobility determination
2.4.1 Single extractions
The single extractions were performed as batch extractions; 1 mol·L−1 HCl was used with a ratio of 10:1 (liquid: solid) (Leleyter et al., 2012), shaken for 1 h at room temperature. After filtration (0.45 μm HVLP with syringes), the solution was stored at 4 °C (acidified with 5% of HNO3) until chemical analyses. Each extraction step was performed in triplicate.
2.4.2 Sequential extraction
The procedure used (Leleyter and Probst, 1999; Leleyter and Baraud, 2005) allows seven mineralogical fractions to be distinguished successively. The sum of these seven fractions represents the operationally defined “labile fraction” for this technique. Operating conditions for sequential extraction are summarised in Table 3. Each extraction step was performed in triplicate.
Sequential extractions procedure (order: F1 to F5).
| Fraction | Reagent | pH | Time (min) |
| F1: water-soluble | Water | 5.7 | 30 |
| F2: exchangeable | 1 M Mg(NO3)2 | 5.0 | 120 |
| F3: acid-soluble | 1 M NaOAc/HOAc | 4.5 | 300 |
| F4: reducible | |||
| Manganese oxides | 0.1 M NH2OH HCl | 3.5 | 30 |
| Amorphous iron oxides | 0.2 M (NH4)2C2O4 + M H2C2O4 | 3.0 | 240 |
| Crystalline iron oxides | 0.2 M (NH4)2C2O4 + M H2C2O4 + 0.1 M C6H8O6 | 2.3 | 30 |
| F5: oxidizable | 35% H2O2/0,02 M HNO3 (8 mL/3 mL), then 3.2 M NH4OAc | 2.0 | 300 |
2.4.3 Kinetic extraction
Kinetic extraction was achieved using a 0.05 mol·L−1 EDTA (Abi-Ghanem, 2008; Abi-Ghanem et al., 2009; Bermond et al., 2005; Bordas and Bourg, 1998) solution at 13 contact times (using 13 sacrificial batches) ranging from 15 min to 24 h and a solid/liquid ratio of 1:10. After filtration (0.45 μm HVLP), the solutions were stored at 4 °C (addition of 5% of HNO3) until chemical analysis. Experimental kinetic curves resulting from extraction with EDTA were modelled using two first-order reaction models as recommended by Cornu et al. (2004), Gismera et al. (2004), and Abi-Ghanem et al. (2009). The two first-order extraction reactions may take place simultaneously, having rates that are assumed to be independent of each other. This allowed three compartments to be defined: quickly mobilised, slowly mobilised, and not mobilised. Each extraction was performed in triplicate.
3 Chemical analysis
Reagents were used in all experiments. Deionised water with a resistivity of 18.2 MΩ·cm produced by a Milli-Q water system (MAXIMA, Millipore) was used throughout. Standard stock solutions of 1000 mg·L−1 for major metallic elements and 100 mg·L−1 for trace metallic elements (VARIAN, PLASMACAL, ULTRA scientific) were used for calibration. All glassware and plastic materials were soaked for 24 h in 10% nitric acid and rinsed with deionised water prior to use. Fifty-millilitre polyethylene vessels were used for the storage of leachates. All leachate solutions were analysed using ICP-AES (inductively coupled plasma-atomic emission Spectrometry, Varian, Vista MPX).
4 Results and discussion
4.1 Total concentrations
Characteristics of the three sediments are presented in Table 4. Metal concentrations are reported as mg·kg−1 of dry sediment (complementary results are detailed in Hamdoun, 2013).
Sediment characteristics.
| S1 | S2 | S3 | English Channel sedimentsa | French harbour sedimentsb | |
| pH | 7.2 ± 0.1 | 8.1 ± 0.5 | 8.1 ± 0.2 | 6.8 to 8.2 | – |
| CaCO3 (%) | 15.0 ± 1.3 | 11.5 ± 1.1 | 19.2 ± 2.1 | 11.5 to 25.1 | |
| TOC (%) | 3.8 ± 0.3 | 6.0 ± 0.2 | 1.9 ± 0.2 | 1.4 to 6.0 | |
| Cu (mg·kg−1) | 59 ± 6 | 60 ± 8 | 62 ± 29 | 12 to 393 | 41 |
| Pb (mg·kg−1) | 133 ± 17 | 135 ± 6 | 42 ± 6 | 11 to 190 | 41 |
| Zn (mg·kg−1) | 286 ± 21 | 290 ± 19 | 233 ± 18 | 53 to 1226 | 1500 |
Sediments S1 and S2 have similar concentrations of Cu, Pb and Zn. Likewise, S3 has similar concentrations of Cu and Zn to S1 and S2; however it has a lower concentration of Pb. The metal concentrations in all three sediments are within ranges previously reported for harbour sediments in the English Channel area (Dubrulle, 2007; Hamdoun, 2013; Pirrie et al., 2002). However, it should be noted that this area is submitted to a strong anthropological pressure (Hamdoun, 2013). Indeed, many authors (Chiffoleau et al., 2001; Cundy and Croudace, 1995; Ifremer, 2011) report anthropic contributions of Cu, Pb, Zn due to the mining industry and riverine inputs. Thus the presence of these three metals in the sediments probably results from both natural occurrence and anthropogenic sources. The anthropological origin elements are suspected to be more leachable than natural origin elements (Gabelle et al., 2012; Tolu et al., 2014).
Inorganic carbon and total organic carbon (TOC) are reported as percentage of dry sediment. The TOC contents measured in these sediments range from 1.9 to 6.0%; these values are within the range of 2 and 10%, as previously reported in marine sediments (Hamdoun, 2013; Isaure, 2001; Schneider, 2008; Tack and Verloo, 1999). The CaCO3 values for the three sediments in this study range from 11 and 20%, also falling within the previously reported range of 10% to 40% (Dubrulle, 2007; Hamdoun, 2013). The pH measured for the three sediments are within the pH range usually reported for such marine sediments.
4.2 Single extraction
The metal mobility expressed as the percentage of the total content of a metal leached by 1 mol·L−1 HCl is displayed for each sediment on Fig. 1.

Percentage of Cu, Pb and Zn mobilised by 1 mol·L−1 HCl for sediment samples S1, S2 and S3.
The mobilities of Cu and Pb are similar in S1 and S3, and are both significantly reduced in S2. For instance, the mobility of Cu is only 24% in S2 compared with 48% in S1, despite the fact that Cu total contents are virtually identical in all three sediments, ranging from 59 to 62 mg·kg−1. It can also be noted that the mobile fraction of Pb in S1 is close to its value in S3, despite the very different total content measured (Table 2). The lack of correlation between element mobility and total metal concentration emphasises the inadequacy of using total metal concentrations in risk assessment.
The sediment matrix plays an important role in binding and immobilising contaminants; these functions are affected by both the geochemical composition and the local environmental conditions. Extraction using 1 M HCl demonstrates here that S2 has a greater capacity to bind Cu and Pb than S1 and S3, which might be correlated with its higher TOC content (6%), suggesting that the part of Cu and Pb bound to the organic fraction is not easily mobilised by HCl. The Zn mobility is quite similar in the three sediments, with light variations in the order S2 < S1 < S3, similar to the trend observed for the CaCO3 content. This might suggest that the mobile Zn could bind to this fraction, which is known to be solubilised by HCl.
4.3 Sequential extractions
The metal distribution, expressed as the percentage of total metal that is present in each of the five sequential extraction fractions (F1–F5), is displayed on Fig. 2.

Percentages of Cu (a), Pb (b) and Zn (c) mobilised by sequential extractions for the three sediment samples.
The geochemical distribution of Cu, Pb and Zn in the three samples showed some differences:
- • Cu is mainly located in the acid-soluble fraction for S2 and S3 (35 and 49%, respectively), however in S1 it is mainly associated with the reducible and oxidizable fractions (32 and 10%, respectively). Thus, S1 and S3 both have a large proportion of Cu associated with the reducible fraction (32 and 20%, respectively). This distribution of Cu is unexpected, as Cu is often reported to be associated with the oxidizable fraction (Algan et al., 2004; Azzaoui et al., 1998; Ramos et al., 1994; Span, 1984);
- • Pb is mainly associated with the reducible fraction in all three sediments. The oxidizable fraction, which is the fraction thought to contain organic matter, does not have a high affinity with Pb in these samples (1 to 8%). Similar observations were made by Span (1984), Illou (1999) and Morillo et al. (2004) for marine sediments;
- • Zn in sediment S1 is distributed in the acid-soluble, reducible and oxidizable fractions (18, 12 and 15% respectively). Zn in S2, which has the lowest percentage of CaCO3, has a similar distribution to that of Cu; i.e. it is mainly located in the acid-soluble fraction (37%), whereas in S3 Zn is mainly associated with the reducible fraction (25%). Rousseau et al. (2009) have noted that in natural marine sediments from English Channel, zinc is mainly scavenged in reducible and in acid-soluble fractions, whereas Pempkowiak et al. (1999), Baize and Tercé (2002) have previously also demonstrated the important role of oxidizable and reducible fractions, in the retention of zinc in sediments.
The geochemical distribution of Cu, Pb and Zn varies greatly between the samples reflecting the natural difference, structure, physicochemical conditions of the sediments and the complexity of the various parameters involved. We note a lack of correlation between the CaCO3 percentage (S2, S1 and S3 present respectively S3 11.5%, 15.0% and 19.2% in CaCO3) and the importance of the acid-soluble fraction (F3) in the Cu and Zn scavenging. This lack of correlation can be explained by the presence of other acid-soluble compounds in sediments (some other carbonates, such as dolomite or some phosphates, such as apatites, as reported by Leleyter and Baraud, 2006).
In the same way, we can notice a lack of correlation between TOC values (respectively 1.9, 3.8 and 6 for S3, S1 and S2) and the amount of Cu, Zn or Pb scavenged in the oxidizable fraction (F5). This result can be explained by the importance of sulphides, which can partially be oxidized during this step.
4.4 Kinetic extraction
Kinetic extraction has the advantage of allowing differentiation between the quickly and slowly mobilized fractions of an element present in the sediment. Preliminary tests showed that 24 h were sufficient to reach the equilibrium. Fig. 3 presents the metal mobility expressed as the percentage of element leached quickly (Q1) or slowly (Q2) by 0.05 mol·L−1 EDTA relative to its total content.
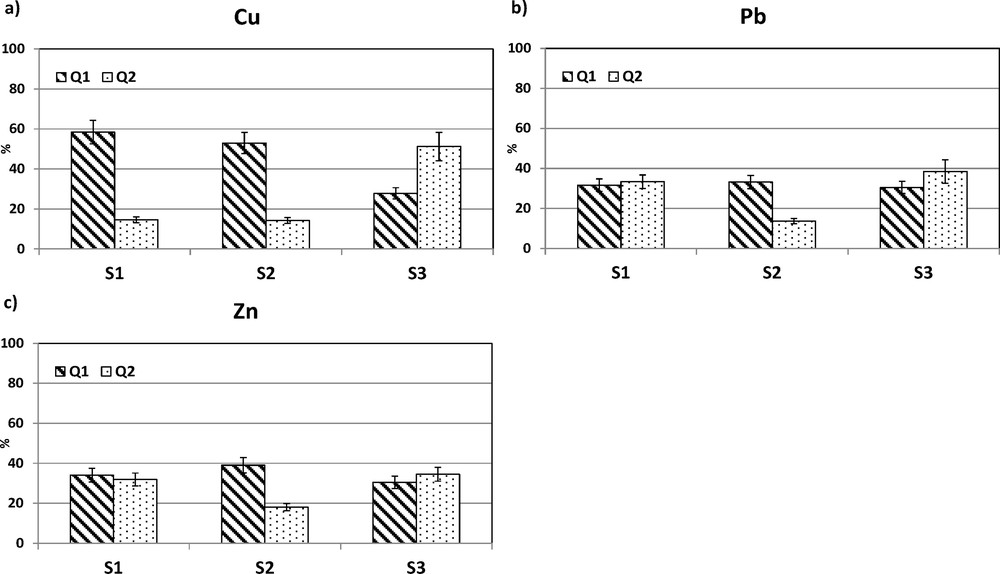
Percentage of Cu (a), Pb (b) and Zn (c) mobilized by kinetic extraction for the three samples (three replicates were analysed for each extraction) in the two compartments Q1 and Q2.
The amount of element associated with the Q1 or Q2 compartment is linked to the interaction of the sedimentary matrix with elements. Kinetic fractionation shows the strength binding of the sediment matrix for each of the elements and provides information on the time frame of the environmental risk posed by elevated metal concentrations, i.e. Q1 is thought to represent the highest potential environmental risk (Abi-Ghanem et al., 2009).
In S1 and S3, mobile Pb and Zn are evenly divided in the “rapid Q1” and “slow Q2” compartments. By contrast, in S2, Q1 is more important than Q2 for both elements, meaning that most of the mobile Zn and Pb are rapidly mobilized from this sediment. This could be linked to the natural or anthropogenic origin of these elements in S2. Indeed, Abi-Ghanem et al. (2009) concluded from isotopic analysis of their EDTA kinetic extracts that the anthropogenic Pb was more extracted than the residual lithogenic Pb.
The behaviour of Cu is different from that of Pb and Zn. For Cu, the results of kinetic extraction show differences in the time frame of the potential environmental risk. The global mobility of Cu (Q1 + Q2) remains close for S1, S2 and S3: 73, 67 and 79%, respectively. However, the kinetic mobilisation of Cu in S3 is very different: 28% in Q1 (58 and 53% for S1 and S2) and 51% in Q2 (15 and 14% for S1 and S2). This indicates that the mobile Cu is rapidly mobilised (mainly present in Q1) for both S1 and S2, whereas most of the mobile Cu present in S3 is slowly mobilised, so that the short-term risk due to mobile Cu in S3 is much lower than in S1 and S2. This phenomenon is probably linked to the strength of the interaction between the sediment matrix and the elements concerned.
These results show the importance of assessing short- (45 min) and long-term (1440 min) metal mobility. In a scenario where the sample was exposed to elevated concentrations of complexing ligands, long contact times will cause maximum dissolution of the elements. However, the Q1 compartment helps to differentiate samples. Moreover, a feature of the EDTA is an ability to dissolve some iron oxides (Sigg et al., 2000). This slow reaction may explain somehow the element mobility in the Q2 compartment for S3.
4.5 Comparison of three approaches
The global mobility (%) estimated by sequential (F6: sum of fractions F1 to F5), single and kinetic (Q1 + Q2) extractions is shown on Fig. 4.
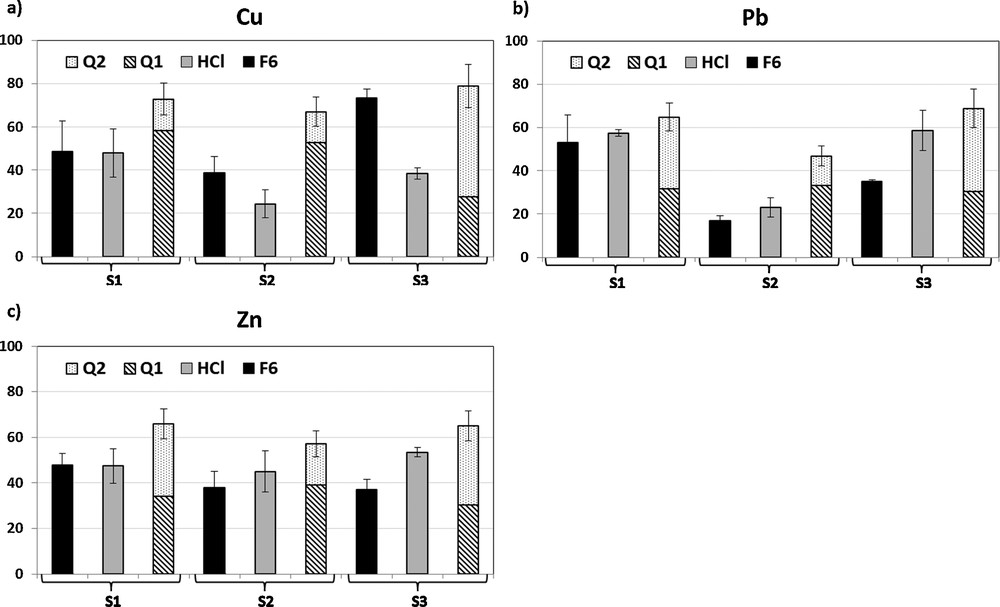
Percentage of Cu (a), Pb (b) and Zn (c) mobilised by sequential (F1 to F5), HCl and kinetic (Q1 + Q2) extraction for the three sediments.
Comparison of the two thermodynamic approaches (HCl and F6), indicates that the global mobilities (as estimated by HCl or F6) of Cu, Pb and Zn is similar for S1, but are quite different for S2 and S3. Considering Pb and Zn, HCl extraction seems to be a little more aggressive than the sequential extraction. This is surprising, as sequential extractions are usually expected to be more aggressive than single reagent extraction using HCl (Larner et al., 2008). It is possible that a potentially labile fraction (e.g., sulphides) was not leached during the sequential extraction. Indeed Aranguren (2008) suggests to use a solution of 8 mol L−1 nitric acid to extract the trace metals bound to the recalcitrant sulphide phase in the sediment in order to modify the Leleyter and Probst (1999) sequential extraction procedure. Thus, we can suppose (due to the relatively short extraction of the oxidizable phases) that Cl− and EDTA ligands were able to desorb Pb and Zn from this fraction by complexation. Pb and Zn are chalcophile (Goldschmidt, 1954); therefore in an anoxic environment it is possible that Pb and Zn are trapped in sulphide mineral phases (Morin, 2010). However, in this study, the oxidation state of the sediments was changed by removing them from the anoxic environment on the sea floor to the oxic environment in the laboratory. This may have influenced the speciation of the metals with a redistribution into the geochemical fractions.
The comparison between the three extractions shows a higher percentage of Cu, Pb and Zn mobilised by the kinetic extraction for the three samples studied. This result suggests that the thermodynamic approaches do not reach reaction equilibrium:
- • it is generally accepted that the metals associated with the water-soluble, exchangeable and acid-soluble fractions are easily mobilised (Sundaray et al., 2011). For Cu, Pb and Zn, there is no obvious relationship between Q1 (“rapid” compartment) and the sum of the first three fractions of the sequential extraction analysis (F1 + F2 + F3);
- • the sequential and single extractions do not extract the entire labile fraction from the sediment. EDTA is a stronger ligand than the chloride ion and as such may complex metals hosted in the sulphide phases.
Cornu et al. (2004) and Gismera et al. (2004) show that certain sequential and kinetic extractions are consistent and complementary for Cu, Pb and Zn. For example, in river sediments, Gismera et al. (2004) found correlations between the mobility of Cu, Pb and Zn between the exchangeable and carbonate-bound fraction, and the fraction that was quickly leached by EDTA.
For improved assessment of the environmental risk associated with the sediment, a kinetic approach should be considered.
5 Conclusion
The aim of this work was to compare several procedures for the environmental risk assessment. Metal mobility was scrutinized using single, sequential and kinetic extractions; each method provided different information on the sediment. Key findings of this research are that:
- • the mobility of Cu, Pb and Zn varied from sediment sample to another and was dependent on the binding nature of phases present in the sediment matrix;
- • of the five sediment fractions distinguished by the sequential extraction, only the acid-soluble, oxidizable and reducible fractions appeared to be involved in the process of retention and release of metals, suggesting that the remobilization of elements in marine sediments will be highly dependent on local pH and redox conditions;
- • simplification of the sequential extraction method by starting at step 3 is justified in marine sediments since the first two fractions, water-soluble and exchangeable, will have already been dissolved in situ;
- • a specific step in the sequential extraction procedure to estimate the sulphide fraction would provide further information on the labile metals present in the sediments. This would involve a modification of the current extraction for the oxidizable fraction;
- • the time frame of one hour for single extractions may be too short. The results presented here for kinetic extraction using EDTA raise the possibility that risk is underestimated (for a time contact exceeding 60 min) if only the quickly mobilised fraction is considered;
- • kinetic fractionation provides additional information showing a possible underestimation of the quantities of element potentially released and thus of the potentially elevated long-term risk. The mobility of Cu, Pb and Zn assessed by kinetic extraction were higher than by thermodynamic approaches (single and sequential), although a different extractant was used in each case.
Numerous studies conducted on metal mobility from harbour sediments focus on the thermodynamic aspect of mobility and ignore the kinetic aspect. However, even if results comparing the three protocols: single, sequential and kinetics are not always consistent, it is important to consider kinetic extraction in addition to methods with a fixed extraction time to ensure that the equilibrium has been achieved and long-term risk can be effectively predicted.


