1 Introduction
Environmental changes occurring at present and predicted for the future have increased interest in paleoclimatic and paleoenvironmental studies (Becker et al., 2006). Records on paleoenvironments may elucidate the causes of climate change (Stern et al., 1997), which could be retained in stable isotopes. The oxygen and hydrogen isotopic signatures of naturally occurring kaolin minerals (kaolinite, halloysite, nacrite, and dickite) carry information about the climatic conditions under which these minerals formed (Savin and Hsieh, 1998).
Kaolinite is correlated with climatic factors, such as temperature and water availability in the soil, because these factors strongly affect the amount and effectiveness of chemical weathering from which they result (Sheldon and Tabor, 2009). Due to its small particle size and large surface area, kaolinite is essentially affected by the duration of weathering, the slope, the water–rock ratio, and the hydrochemistry (Fagel and Boës, 2007; Fürsich et al., 2005; Heckroodt, 1991; Moriarty, 1977). In addition, continental weathering rates and runoff are controlled by climatic changes, which also affect sediment formation and transport of these sediments into a Basin (Das et al., 2013). Hence, the stable isotopic compositions of kaolinite are potentially powerful proxies of paleoenvironmental and paleoclimatic conditions (Ortega et al., 1998; Tabor et al., 2002). However, some conditions need to be taken into consideration when using stable isotopic compositions of kaolinite as paleoenvironmental and paleoclimatic proxies. These include: known kaolinite–water equilibrium isotope fractionation factors, closed system conditions since mineral formation, water dominated environments of crystallisation, and information on the behaviour of the specific isotope in kaolinite and water (Sheldon and Tabor, 2009).
Several studies have applied stable isotope geochemistry of clay minerals to various disciplines, such as geothermometry; provenance of clays, diagenetic processes, origin and evolution of upper crustal fluids, hydrothermal systems, water-rock interaction and paleoclimates (Sheppard and Gilg, 1996). The application of the stable isotopes of oxygen and hydrogen to reconstruct paleoenvironments of formation of kaolins have also been used by several authors (Das et al., 2013; Ekosse, 2007; Elliot et al., 1997; Gilg et al., 1999, 2013; Gilg, 2000; Horbe, 2011; Martinez-Ruiz et al., 2014; Roseneau and Tabor, 2013; Savin and Hsieh, 1998; Tabor and Montanez, 2005; Tabor et al., 2002; Turc et al., 2013; Westermann et al., 2013).
Sheppard et al. (1969) developed the hypogene–supergene line, which is equivalent to kaolinite in equilibrium with meteoric waters at about 35 °C. This line allows the distinction between a supergene and a hypogene environment of formation of a kaolin deposit. Later studies by Savin and Epstein (1970) led to the development of the kaolinite line, which represents the location of isotopic data points for pure kaolinites from weathering zones formed in approximate equilibrium with meteoric water at about 20 °C.
On the one hand, knowledge on the environment of formation of kaolins can provide crucial information for their exploration; on the other hand, African paleoclimates have not been substantially addressed. This paper, therefore, provides information on the environment of formation of Cretaceous–Tertiary kaolins of the Douala Sub-Basin and it presents evidence of the Cretaceous–Tertiary climate in Douala and its environs, using the stable isotope composition of Cretaceous–Tertiary kaolins found in the Douala Sub-Basin.
2 Study area
2.1 Geology of the Douala Sub-Basin
The studied Cretaceous–Tertiary kaolins of the Douala Sub-Basin are found in five localities, namely, Bomkoul, Dibamba, Logbaba, Missole, and Yatchika kaolins (Fig. 1). The Douala Sub-Basin stretches on the southern coast of Cameroon, covering a total surface area of 19,000 km2 (Mbesse et al., 2012), and is subdivided into seven formations (Logmo et al., 2013).
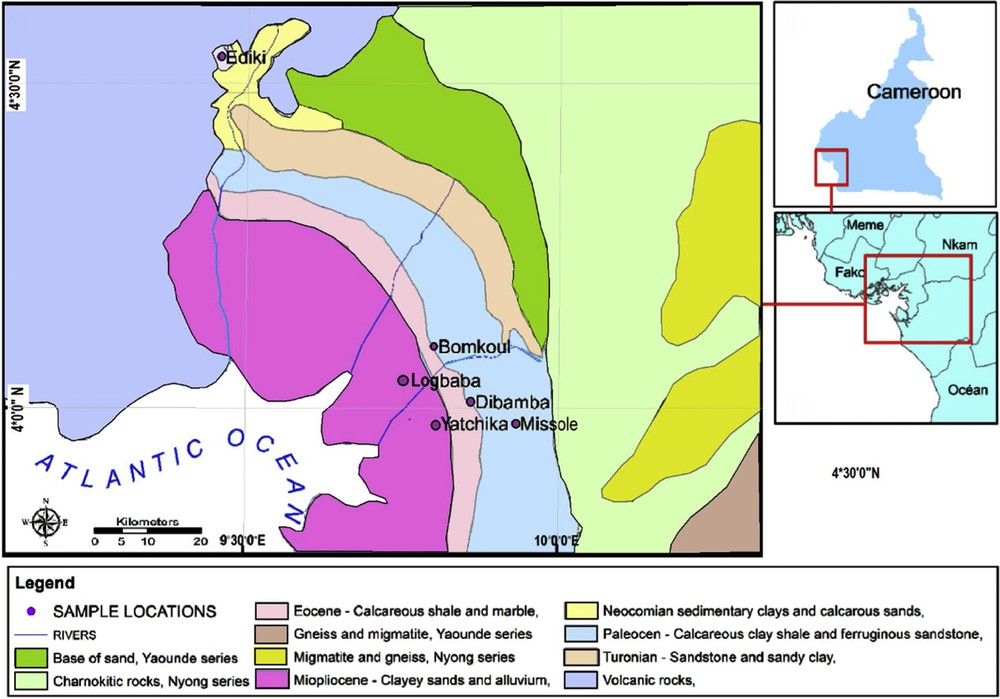
Geologic map of the Douala Sub-Basin (modified from SNH/UD, 2005).
The kaolin occurrences belong to four Cretaceous–Tertiary Formations of the Douala Sub-Basin: the Early Cretaceous Mundeck Formation made up of sandstones; the Late Cretaceous Logbaba Formation, made up of sandstones and clays with intercalations of sands and rare occurrence of limestones; the Paleocene to Middle Eocene Nkapa Formation composed of marls, shales, and calcareous sandstones; the Upper Eocene to Oligocene Souellaba Formation, composed of sandstones and marls, shales, clayey sands, sands, and gravels (Effoudou-Priso et al., 2014). The lithostratigraphy of the Douala Sub-Basin is shown in Fig. 2.

Lithostratigraphy of the Douala Sub-Basin (modified from Brownfield and Charpentier, 2006).
Sedimentation in the Sub-Basin was marked by (Kenfack et al., 2012a, 2012b): Jurassic continental sediments, which were deposited in an Afro-Brazilian depression; a listric faulting during the Jurassic–Barremian; an intensive erosion activity of the highlands and deposition in the previously formed graben; a salt deposition and transform direction resulting in a series of cross-faults that have segmented the rift(s structure during the Mid-Late Aptian; a marginal clastic sedimentation, with sporadic build-ups between the Albian and the Paleocene.
2.2 General description of studied kaolins
A general description of the studied kaolins is given in Table S1 (Ngon Ngon et al., 2012; Blackwatch, 2016).
2.3 Climate
The Douala Sub-Basin has the Guinean type of the equatorial climate, resulting from the combined effect of convergence of the tropical oceanic low-pressure zone and the inter-tropical front within the continent. There are two main seasons: namely, a long rainy season that lasts from April to September, and a short dry season, which generally stretches from October to March. Between these two seasons are transitional seasons: the wet-dry season (September to November) and the dry-wet season (March to May). As shown in Fig. 3, annual precipitation since 2006 is above 2500 mm, and annual temperatures fluctuate between 27 and 28 °C (IAEA/WMO, 2017).

Mean annual temperatures and precipitation in Douala between 2006 and 2015 (IAEA/WMO, 2017).
The IAEA/WMO (2017) recorded the stable isotope composition of meteoric water in Douala. It is shown in Table S2.
3 Materials and methods
Kaolins from five localities in the Douala Sub-Basin were studied: namely, Bomkoul, Dibamba, Logbaba, Missole II, and Yatchika (Table S1). In these localities, kaolin is generally hosted in sandstone deposits and occurs as beds less than 5 m in thickness. Samples were collected and were 1 to 5 m apart, depending on the extent of kaolin beds. Due to the low probability of obtaining a pure mineral fraction on the field, sample preparation and physical purification were carried out.
Organic matter was removed using hydrogen peroxide (H2O2) and the samples were dispersed using calgon (a mixture of 40 g of sodium hexametaphosphate (NaPO3)6 and 10 g of soda (Na2CO3)), according to the method described by van Reeuwijk (2002). After the dispersion of the samples, the sedimentation technique was used to remove sand and silt fraction, and clay fractions (< 2 μm fraction) were obtained with the use of a centrifuge, after removal of sand and silt fractions (Jackson, 1979; van Reeuwijk, 2002). Removal of carbonates, iron oxides and hydroxides was not carried out, because the presence of these minerals does not affect the stable isotopic compositions of clay minerals (Bird and Chivas, 1988, 1989).
The bulk and < 2 μm fractions of the kaolins were analysed for their mineralogy by X-ray diffractometry (XRD) using a Rigaku Ultima IV diffractometer working with 40 kV and 30 mA, and using a cobalt source (λ = 1.789 Å) and an alpha filter (Co Kα). Samples were scanned from 3o 2θ to 40o 2θ at a rate of 2o per minute. The results of this analysis are reported in Bukalo et al. (2018). Eight samples, representing the samples in each locality with the highest amounts of kaolinite, were selected based on their mineralogical compositions (Table S3). The samples selected for isotopic analyses were the < 2 μm fraction, enriched in kaolinite (> 80 wt%) and with lesser amounts of other minerals based, in order to avoid oxygen contamination from other minerals.
In the selected samples, the other minerals present were illite, smectite, quartz, anatase, rutile, goethite, and hematite. Based on a study by Hassanipak and Elsinger (1985), there is little or no correlation between iron oxide minerals and the stable isotopic composition of kaolinite. Since the stable isotopic fractionation factors of smectite and kaolinite are very similar under low-temperature weathering conditions, smectite will not be regarded as a potential contaminant on the stable isotopic composition of kaolins (Clauer et al., 2015). Clauer et al. (2015) also proved that the isotopic compositions of kaolinite-rich samples also containing quartz and illite fall in the analytical uncertainty of the stable isotopic compositions of pure kaolinite. Therefore, mineral phase separation was not carried out.
The method used for stable isotopes analyses was similar to the method described by Harris et al. (1999). Approximately 20 mg of each sample was placed in a glass container and attached to the vacuum line. The content was then frozen in liquid nitrogen. The air in the sample was then outgassed and the absorbed water was cryogenically distilled to a second vessel by heating the sample at 150 °C with an air gum. The mass of the original sample and the produced water were measured and used to determine the wt % of absorbed water.
3.1 Oxygen isotopes
The samples were outgassed under high vacuum at 200 °C for 2 h, after having been dried in an oven at 110 °C. Then, the samples were reacted with an excess of ClF3 at ∼560 °C for 12–16 h. The evolved O2 was quantitatively converted into CO2 using a hot platinised carbon rod. The δ18O of CO2 was then measured using the Finnegan DeltaXP mass spectrometer. Duplicate splits of the MQ quartz standard were analysed with each batch of eight samples. These values were used to normalise the raw δ18O to the SMOW scale, using δ18O = 10.1‰ for MQ. The precision of δ18O measurements was ±0.2 ‰.
3.2 Hydrogen isotopes
Prior to the determination of δ2H, three replicate analyses of the kaolinite standard, Bulk Serina Kao (SB8) from Serina Mine near Cape Town, gave structural water contents of 12, 12 and 13 wt%, and a mean δ2H value of −57‰. The extracted absorbed water was converted to hydrogen gas using a variation of the closed tube Zn reduction (Harris et al., 1999). Hydrogen isotopic composition was then measured on the Finnegan DeltaXP mass spectrometer calibrated using SMOW. Internal standards (CTMP2010 and RMW, exactly 2 mg) were used to calibrate the raw data to the SMOW scale. This was done by correcting the raw data versus the reference gas value, then adjusted so that CTMP2010 = −7‰, then “stretched” so that RMW = −131‰. The silicates were then adjusted so that kaolinite = −57‰ (i.e. the δ2H value of SB8, used as kaolinite standard).
4 Results
The δ18O and δ2H of the samples are shown in Table S4 and plotted in Fig. 4. δ18O values varied between +18.2‰ (BKL 02) and +21.0‰ (DBB), whereas δ2H values varied between −69‰ (BKL 02) and −53‰ (MSL II 01 and MSL II 02). The means of δ18O and δ2H are +19.5 ± 1.1‰ and −61 ± 6‰, respectively, indicating that δ18O and δ2H have homogenous values. All the samples fall between the kaolinite line and supergene-hypogene line.

δ18O versus δ2H plot of clay fractions of selected samples. The LMWL, GMWL, S/H line and the kaolinite line are plotted for reference. Values of the Cretaceous–Tertiary Georgia kaolins in USA (Hassanipak and Elsinger, 1985), Cretaceous Rio Capim kaolins in Brazil (Santos et al., 2007), and Cretaceous Aswan kaolins in Egypt (Baouimy, 2013) are also included for comparison.
Dibamba and Logbaba samples (DBB 01, DBB 02 and LBB 01) plot very close to the kaolinite line. These samples all have a kaolinite content > 90.0 wt%. Missole II samples (MSL II 01 and MSL II 02) plot on the right side of the supergene–hypogene line, but closer to this line than other samples. They have an anatase content of 4 wt% and lower kaolinite contents, 87 and 86 wt%, for MSL II 01 and MSL II 02, respectively.
5 Discussion
5.1 Temperature of kaolinisation
For reference, the local meteoric water line (LMWL) for Douala (Wirmvem et al., 2016), the global meteoric water line (GMWL) (Craig, 1961), the supergene–hypogene line for kaolinite in equilibrium with meteoric waters at ≈35 °C (Sheppard et al., 1969) and the kaolinite line, for kaolinite in equilibrium with meteoric waters at ≈20 °C (Savin and Epstein, 1970) are used for comparison in Fig. 4, according to the equations below:
| (1) |
| (2) |
| (3) |
| (4) |
The stable isotopes composition of kaolins, as well as of other clay minerals, is a function of the isotopic composition of the water from which they formed. The equilibrium isotopic fractionation factors between kaolinite and water were developed by Gilg and Sheppard (1996) and Sheppard and Gilg (1996) (Eqs. (5) and (6)).
| (5) |
| (6) |
These fractionation factors are a function of the temperature of kaolinisation; therefore, the isotopic composition of kaolinite can provide information about its genesis (Fernández-Caliani et al., 2010). By combining Eqs. (2), (5), and (6), the kaolinisation temperature can be derived as follows (Clauer et al., 2015):
| (7) |
The temperatures of kaolinisation (TG) of the studied kaolins were computed and presented in Table S5. The kaolins found in Cretaceous Formations had a mean TG of 22 ± 2 °C, whereas those found in Tertiary Formations had a mean TG of 27 ± 6 °C. These temperatures suggest that kaolins hosted in Cretaceous Formations formed at temperatures about 5 °C cooler than those found in Tertiary Formations, and both Cretaceous and Tertiary temperatures are humid temperatures, favouring the formation of kaolins.
The local meteoric water line of Douala was developed for the years 2013 and 2014 by Wirmvem et al. (2016) (Eq. (1)). Using the same method and based on the same assumptions as in Delgado and Reyes (1996) and Clauer et al. (2015), the temperature of kaolinisation in equilibrium with the local meteoric water line of Douala is derived as follows:
| (8) |
Hence, the temperatures of kaolinisation (TL) of the studied kaolins in equilibrium with the local meteoric water line were computed and presented in Table S5. The TL values were slightly lower than the TG values, with Cretaceous kaolins having a mean of 20 ± 2 °C, and Tertiary kaolins having a mean of 25 ± 6 °C. These values also show that kaolins hosted in Cretaceous Formations formed at temperatures about 5 °C cooler than those found in Tertiary formations. Moreover, in both cases (kaolinites in equilibrium with the global meteoric water line or with the local meteoric water line), kaolinisation temperatures in Tertiary kaolins (the warmest) are slightly higher than present-day mean annual temperatures in Douala (27 °C) (IAEA/WMO, 2017).
The studied kaolins are exposed occurrences that might have been formed through interactions with modern local meteoric water. Knowing the present-day mean isotopic composition of meteoric water in Douala, −3.1 and −10‰ for δ18OW and δ2HW, respectively (Wirmvem et al., 2016) and present-day mean annual temperature (27 °C in 2015) (IAEA/WMO, 2017), it is possible to evaluate the influence of modern local meteoric water on the kaolinisation of Cretaceous–Tertiary sediments in the Douala Sub-Basin. This could be done by computing the isotopic composition of Cretaceous–Tertiary kaolins in the Douala Sub-Basin using the modern mean annual temperature (27 °C in 2015) (IAEA/WMO, 2017), as in Eqs. (5) and (6) (Santos et al., 2007). From these calculations, kaolinites that formed under modern mean annual temperature should have an isotopic composition of +20.8 and −40‰ for δ18OK and δ2HK, respectively. Though DBB has a slightly heavier δ18OK, Cretaceous–Tertiary kaolins have lighter δ18OK and δ2HK than computed modern kaolins. Thus, it can be concluded that Cretaceous–Tertiary kaolins of the Douala Sub-Basin are not in equilibrium with modern meteoric water.
5.2 Stable isotope composition of the meteoric water in equilibrium with Cretaceous–Tertiary kaolinites in the Douala Sub-Basin and equilibrium fractionation factors
Mean temperatures of kaolinisation determined using the GMWL and LMWL were used to determine the mean stable isotope composition of the meteoric water in equilibrium with Tertiary kaolins (300 and 298 K) and Cretaceous kaolins (295 and 293 K) using Eqs. (5)–(8). The results are shown in Table S6. Using the GMWL equation, the mean isotopic composition of the meteoric water in equilibrium with Cretaceous kaolins is −5.3 and −32‰, for δ18OW and δ2HW, respectively, whereas its mean isotopic composition when in equilibrium with Tertiary kaolins is −4.5 and −26‰, for δ18OW and δ2HW, respectively (Table S6 and Fig. 5).
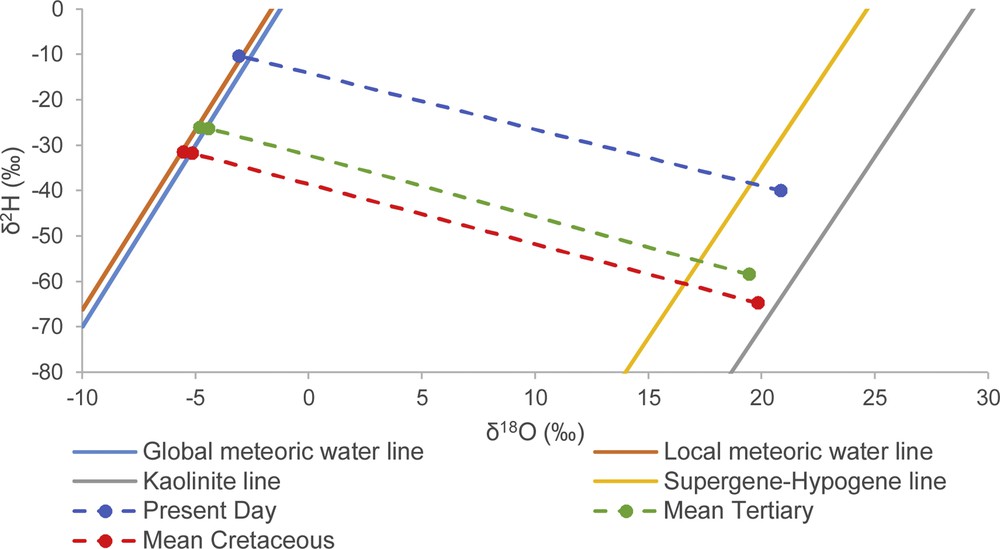
Plot showing the mean isotopic composition of meteoric water in equilibrium with Cretaceous and Tertiary kaolins.
Using the LMWL equation, the mean isotopic compositions of Tertiary meteoric waters (in equilibrium with Tertiary kaolins) are −4.8 and −26‰, for δ18OW and δ2HW, respectively, whereas the mean isotopic compositions of Cretaceous meteoric waters (in equilibrium with Cretaceous kaolins) is −5.7 and −31‰, for δ18OW and δ2HW, respectively (Table S6 and Fig. 5). These results, as well as the temperature of kaolinisation, are slightly lower than those obtained when using the GMWL equation. However, the equilibrium fractionation factor of 2H between kaolinite and water (αK–W 2H) using the GMWL or the LMWL has remained constant from the Cretaceous to the Present (αK–W 2H = 0.97), whereas the equilibrium fractionation factor of 18O between kaolinite and water (αK–W 18O) has decreased from 1.025 or 1.026, during the Cretaceous, to 1.024 since the Tertiary.
5.3 Paleoenvironmental reconstruction
The low temperatures of kaolinisation (20 °C ≤ TG ≤ 33 °C) and the positioning of the samples between the kaolinite and supergene/hypogene lines imply that kaolinisation took place in a supergene environment; therefore, Cretaceous–Tertiary kaolins of the Doula Sub-Basin are of weathering origin. As indicated by Bird and Chivas (1988), kaolins in a supergene environment generally have δ18O values between +17 and +23‰, and δ2H values between −80 and −40‰. This is sustained by the isotopic compositions of other kaolins formed by weathering, amongst which are the Cretaceous–Tertiary Georgia kaolins in the USA (Hassanipak and Elsinger, 1985), the Tertiary Lastarria kaolins (Gilg et al., 1999), the Cretaceous–Tertiary Rio Capim semi-flint kaolins in Brazil (Santos et al., 2007), the Permian Variscan kaolins in Spain (Fernández-Caliani et al., 2010; Clauer et al., 2015), the Cambrian Burela kaolin deposit in Spain (Gálan et al., 2016), and the Cretaceous–Tertiary Patagonia kaolins in Argentina (Domínguez et al., 2016).
Since kaolinite mostly preserves its original δ18O compositions, isotopic exchange after deposition is unlikely (Boulvais et al., 2000). However, the little oxygen isotopic exchange between kaolinite and water reported by several authors was exhibited in the studied kaolins from the Cretaceous (+19 ‰) to the Present (+21‰). An increase in δ18O values with time was also observed in Australian kaolinites (Bird and Chivas, 1989). Conversely, hydrogen isotopic exchange between kaolinite and water occurs at a more rapid rate and is responsible for the shift of samples from the kaolinite line (Bird and Chivas, 1989). Since their formation, these kaolins have been undergoing continual low-temperature isotopic exchange with meteoric waters of successively heavier isotopic compositions.
Subsequently, the kaolinisation temperatures of Cretaceous–Tertiary kaolins in the Douala Sub-Basin (< 35 °C) reflect supergene environments, with Tertiary temperatures being higher than Cretaceous temperatures. This suggests a cooler climate during the Cretaceous than during the Tertiary. An increase in precipitation usually corresponds to an increase in δ2H, either in kaolinite or its corresponding meteoric water (Sheppard and Gilg, 1996). Therefore, the increasing mean δ2H values of kaolins in the Douala Sub-Basin means that there has been an increase in precipitation in the study area since the Cretaceous, favouring the formation of kaolins.
The Douala Sub-Basin is found in the Equatorial domain, which has precipitations ranging above 2000 mm per year, and mean temperatures around 25 °C (Pamo, 2008). In west and Central Africa, this Equatorial domain has a humid tropical climate, due to their low altitudes (Tardy et al., 1991). Regarding temperatures, Tardy et al. (1991) argued that western and central Africa have higher temperatures at the equator (> 5–6 °C) and at the tropics (> 20 °C) than at the same latitudes in South America (Brazil) and East Africa. Hence, when moving northwards from the equator to the Tropic of Cancer, temperatures increase, whereas when moving southwards from the equator to the Tropic of Capricorn, temperatures decrease (Tardy et al., 1991).
Using the paleolatitude calculator for paleoclimatic studies developed by van Hinsbergen et al. (2015) and the reference frame for paleoclimate studies proposed by Torsvik and Van der Voo (2002), the paleolatitude of the Douala Sub-Basin has been moving northwards, towards the Tropic of Cancer from the Cretaceous to the Present (Fig. 6).
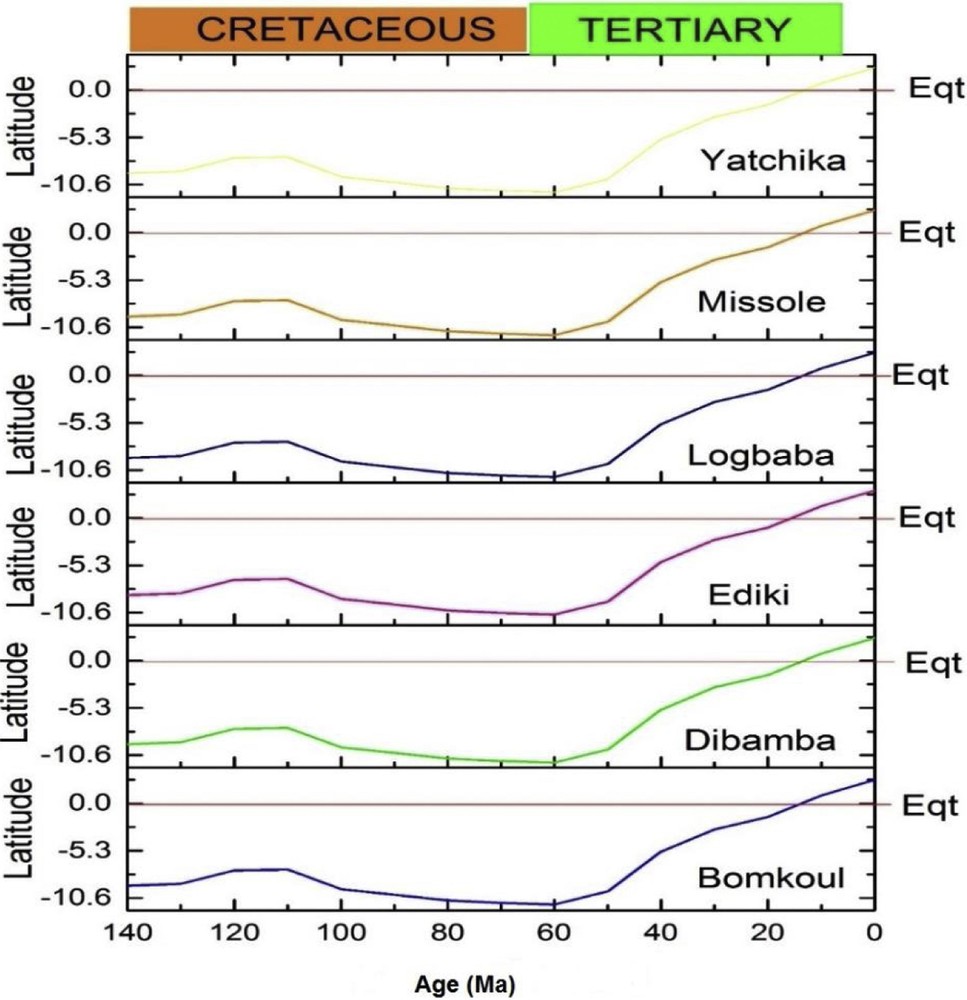
Paleolatitudinal evolution of the Douala Sub-Basin from the Cretaceous to the Present (van Hinsbergen et al., 2015). Eqt: equator.
According to the argument of Tardy et al. (1991), the climate in the Douala Sub-Basin has become warmer from the Cretaceous to the Present. The results obtained in the frame of this research converge towards this conclusion, showing that the Cretaceous had cooler temperatures than the Tertiary in the Douala Sub-Basin (20 ± 2 °C and 25 ± 6 °C, respectively), with mean annual temperatures between 2006 and 2015 of 27 ± 0.35 °C.
Even though warm temperatures are required for kaolin formation, precipitation and leaching are the main factors influencing the formation of kaolins (Bird and Chivas, 1988). According to the rainfall patterns during the Mesozoic to Cenozoic derived by Parrish et al. (1982), Douala and its environs fall in the high rainfall domain since the Cretaceous. However, the wettest months (June–October) also correspond to the coolest ones. Therefore, the formation of Cretaceous–Tertiary kaolins in the Douala Sub-Basin was more influenced by high precipitation than by high temperatures, and seasonal characteristics show that these kaolins were formed in a humid tropical climate. It implies that Douala had a humid climate during the Cretaceous, and the climate is gradually becoming hotter as concluded by Tardy et al. (1991), regarding the climate in West and Central Africa; but more humid.
6 Conclusion
Cretaceous–Tertiary kaolins in Douala Sub-Basin were formed in a weathering (supergene) environment. The temperatures of kaolinisation (assuming equilibrium with the global meteoric water line) were 22 ± 2 °C for Cretaceous kaolins and 27 ± 6 °C for Tertiary kaolins. Assuming equilibrium with the local (Douala) meteoric water line, the temperatures of kaolinisation were 20 ± 2 °C for Cretaceous kaolins and 25 ± 6 °C for Tertiary ones. The mean isotopic composition of the meteoric water in equilibrium with Tertiary kaolins (300 K and 299 K) and Cretaceous kaolins (295 and 293 K), using the GWML, is −5.3 and −32‰, for δ18OW and δ2HW, respectively during the Cretaceous and −4.5 and −26‰, for δ18OW and δ2HW, respectively during the Tertiary, whereas present-day mean isotopic composition of meteoric water in Douala is −3.1 and −10‰ for δ18OW and δ2HW, respectively. Douala had a cooler and rainy climate during the Cretaceous, and the climate is gradually becoming hotter and more humid, favouring refinement of the existing kaolins and the kaolinisation of kaolin-forming minerals in the Sub-Basin.
Acknowledgements
This research was sponsored by the National Research Foundation (NRF) through grants NRF CPRR (UID 91559), and NRF IMGR (UID 102226). The financial assistance of the NRF towards this research is hereby acknowledged. Opinions expressed and conclusions reached are those of the authors and are not necessarily attributable to the NRF.


