1 Introduction
There is much current interest in using geochemical and isotopic techniques to decipher and discriminate the geologic and/or geographic origin of gemstones (see, e.g., Giuliani et al., 2014 and references therein) and such work both informs, and is informed by, genetic ideas about gemstone formation. For example, in the case of red and pink spinels found in marbles worldwide, Giuliani et al. (2017) recently published a trace element and oxygen stable isotope ratio database that permits the useful characterization of the main currently productive and historical sources of gem spinel. The study included samples from classic sites of Mogok, in Myanmar, and Kuh-i-Lal, in Tajikistan, as well as material from Vietnam, Nepal, Pakistan, Tanzania, and Kenya.
At the local level, given the metamorphic origin of many of the spinels, the utility of δ18O decreases because the parental fluid of the minerals can be isotopically buffered by the host impure marble, and many marine carbonates and their marble counterparts have (in the absence of metasomatic alteration) a rather restricted range of depositional δ18O through geological time (see, e.g., the discussion in Veizer, 1992). Thus, in the specific case of spinels of a wide palette of colours from Vietnam, Pham et al. (2018) have pointed out that, whilst there is a considerable range in spinel δ18O (spanning 12.1 to 24.2‰ for 32 specimens), discrimination amongst geographically distinct deposits is not possible using oxygen isotopes alone, whereas it can be accomplished using selected trace elements (specifically Fe–Zn–Cr–V), whose concentrations are presumably locally controlled by the impure nature of the host marbles.
These considerations offer an opportunity to look a little more closely into the genetic model for the Vietnamese spinels, to investigate in greater detail the factors influencing their δ18O values and to enquire whether any conclusions reached might have more widespread utility and applications to metallogenesis. Within this overall framework, the present study investigates the oxygen isotope composition of calcite–spinel pairs of Vietnamese and other worldwide deposits in order to characterize the O-isotope fractionation between calcite and spinel and to estimate the temperature of formation for spinel. Discrepancies between the calculated temperatures by the increment method for oxygen isotope fractionation and those derived by phase diagram considerations are discussed, as well as the use of oxygen isotopes for deciphering the geographic origin of spinels.
2 Geological background of gem spinel deposits in marble with a special focus on Vietnamese mineralization
Gem spinels in marble have compositions close to MgAl2O4 sensu stricto and the most appreciated are the pink, orange, or red crystals (Fig. 1). World-economic Cr–V-bearing spinels occur in Himalayan and East Africa and were formed under high-degree metamorphic conditions in the amphibolite facies (Giuliani et al., 2017).
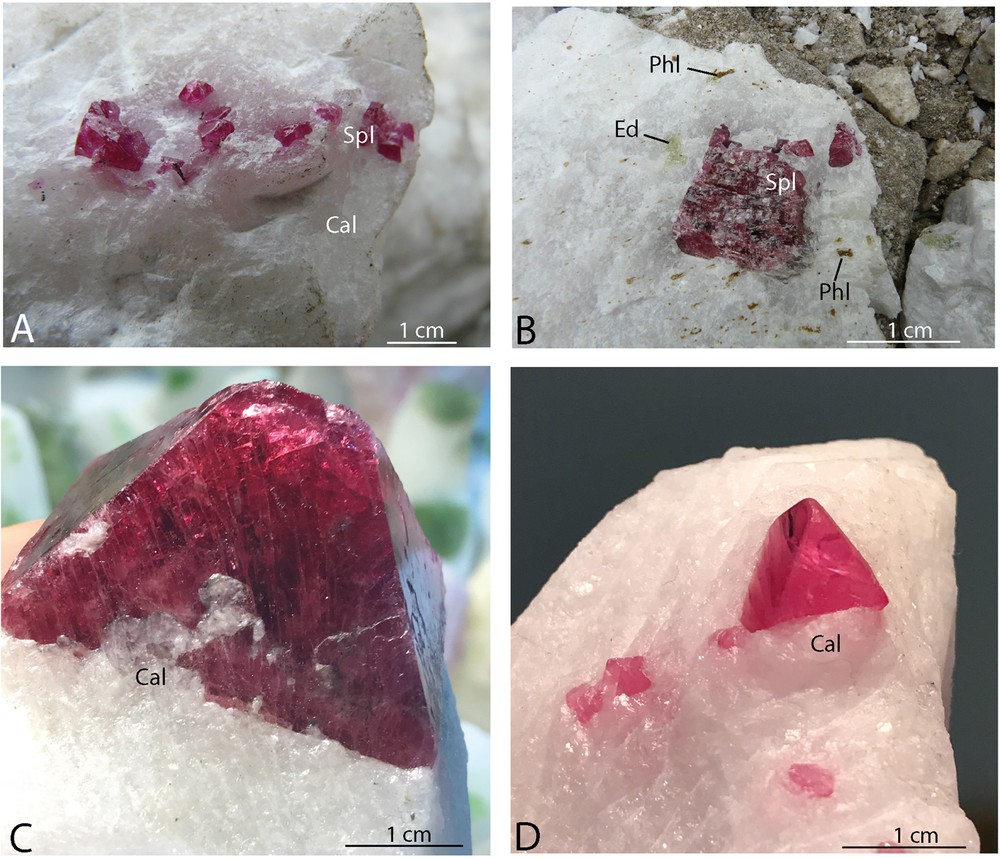
Vietnamese spinels from Luc Yen and An Phu mining districts. Spinels (Spl) in a calcitic (Cal) marble, Cong Troi deposit (A). Spinel associated with edenite (Ed) and phlogopite (Phl) in a marble from Cong Troi deposit (B). Octahedral spinel associated with calcite (Cal) (C). Gem crystals of pink spinel in a calcitic (Cal) marble (D). Photographs A–B: Giuliani G; C–D: Pardieu V.
In Asia, the main primary deposits (Fig. 2) are located in marble intercalated within the high-grade metamorphic gneisses of the Lo Gam zone in northern Vietnam (Giuliani et al., 2017), in Myanmar in the Mogok Metamorphic Belt exposed along the meridian trending Shan scarp and Sagain fault (Searle et al., 2007), and in Tajikistan in the metamorphic series of the southwestern Pamirs (Kievlenko, 2003), whereas minor occurrences are observed in Pakistan in the Hunza valley (Okrusch et al., 1976) as well as in Nepal in the Navakot series of the Ganesh Himal (Garnier et al., 2006).

Location of the main spinel deposits in southeastern Asia (modified from Mattauer et al., 1999).
In East Africa, the spinel deposits are located in the Neoproterozoic Metamorphic Mozambique Belt and were formed during the East African Orogeny (Giuliani et al., 2017). Gem spinels are related to marble at Pamreso in Kenya, in the Voi area, and in Tanzania, in the Mahenge and Uluguru mountains, respectively at Ipanko and Morogoro deposits (Balmer et al., 2017; Giuliani et al., 2017).
Gem-quality Vietnamese spinels have been mined since 1987 in the Luc Yen and An Phu mining districts (Yen Bai Province, Fig. 3). The primary deposits are linked with the formation of the sinistral Red River Shear Zone, during the Tertiary, when the north-moving Indian plate collided with the Eurasian plate to form the Himalayan mountain belt, with Indochina extruded to the southeast (Leloup et al., 1995; Tapponnier et al., 1990). Amphibolite facies metasedimentary sequences of Cambrian depositional age, formed of marble units and intercalated gneisses and schists, are situated in the Lo Gam Zone (Fig. 3), between two left-lateral faults of the mainly 10–15-km-wide Red River Shear Zone, and with intrusions of granitoids of Triassic age (Garnier, 2003). The spinel deposits extend between Luc Yen and An Phu localities, either in marbles or placers (Fig. 3). The Luc Yen mining district encompasses principally the mines of Nuoc Ngap, Khoan Thong, Luc Yen, and Minh Thien, as well as the An Phu mining district includes the well-known Cong Troi deposit. Spinel in primary deposits occurs either as crystals disseminated in marbles or associated mostly with calcite, dolomite, forsterite, clinohumite, pargasite-edenite, phlogopite, and clinochlore.

Geological map showing the major tectonic domains of the Red River Shear Zone in northern Vietnam with locations of the main spinel occurrences and deposits (modified from Garnier, 2003).
3 Material and methods
Spinels from Vietnamese primary and secondary deposits were sampled, between 1998 and 2013, in the field by Giuliani, Pardieu, and Pham. Oxygen isotopes analyses (n = 32) were performed at S.U.E.R.C. Glasgow (Scotland) using a modification of the laser-fluorination technique (Giuliani et al., 2005). Data are reported in the conventional δ18O notation as per mil (‰) relative to the Vienna Standard Mean Ocean Water (V-SMOW). The oxygen isotope database of spinel worldwide published by Giuliani et al. (2017) and Pham et al. (2018) is presented in a modified version in Supplementary Table 1.
Calcite and spinel pairs have been chosen after close examination by microscopy of the mineral assemblages. They comprise mineral pairs originating from the deposits of An Phu (n = 3) and Cong Troi (n = 3) in Vietnam, Paigutan (n = 2) in Nepal, Ipanko (n = 1) in Tanzania, and Mogok in Myanmar (n = 2 from Yui et al., 2008).
Carbon and oxygen isotopic compositions of calcite were determined by using an auto sampler Gasbench coupled with a Thermo Scientific MAT253 isotope ratio mass spectrometer (IRMS) at the CRPG UMR 7358 CNRS-UL, Vandœuvre-lès-Nancy (France). For each sample, an aliquot between 250 to 300 μg of carbonate was reacted with 2 mL of supersaturated orthophosphoric acid at 70 °C for at least 5 h under a He atmosphere. The carbon and oxygen isotopic compositions of the produced CO2 were then measured with a Thermo Scientific MAT 253 continuous flow isotope ratio mass spectrometer. Values are quoted in the delta notation in ‰ relative to V-PeeDeeBelemnite (V-PDB) for carbon and to V-SMOW for oxygen. All sample measurements were adjusted to the internal reference calibrated on the international standards IAEA CO-1, IAEA CO-8 and NBS 19. Stable isotope compositions of calcite and spinel are presented in Table 1. The O-isotope fractionation between calcite and spinel is expressed as Δ18Occ–sp.
C–O isotopic compositions of calcite and O composition of coexisting spinel in marbles from northern Vietnam, Nepal, Tanzania, and Myanmar.
| Country | Deposit | Sample | Colour | Mineral | ||||||
| sample | Calcite | Spinel | Calcite–spinel | References | ||||||
| δ13C (‰ V-PDB) |
δ18O (‰ V-SMOW) |
% calcite | δ18O (‰ V-SMOW) |
Δ18Occ-sp | Mean Δ18Occ-sp | |||||
| Vietnam | An Phu | AP2 | Pale purple | 0.8 | 18.1 | 99.7 | 14.2 | 3.9 | 3.73 ± 0.15 | This work |
| An Phu | AP4 | Red | 2.7 | 20.5 | 99.8 | 16.8 | 3.7 | This work | ||
| An Phu | Apa | Deep purple | 0.4 | 18.5 | 97.7 | 14.9 | 3.6 | This work | ||
| Cong Troi | VIET 31 | Pale pink | 0.6 | 18.2 | 99.9 | 14.7 | 3.46 | 3.64 ± 0.16 | This work | |
| Cong Troi | VIET 32 | Dark brown | 0.9 | 20.8 | 81.7 | 17.0 | 3.75 | This work | ||
| Cong Troi | VIET 33 | Red | 2.1 | 18.5 | 100 | 14.8 | 3.71 | This work | ||
| Nepal | Paigutan | G85-1 | Reddish | 2.7 | 23.4 | 100 | 19.8 | 3.56 | 3.45 ± 0.18 | This work |
| Paigutan | G85-2 | Pink | 2.7 | 23.2 | 100 | 19.9 | 3.34 | This work | ||
| Tanzania | Ipanko | IPAN2 | Pink | 3.9 | 26.7 | 100 | 23.3 | 3.39 | 3.39 | This work |
| Myanmar | Mogok | K-4 | Red | 2.3 | 25.3 | ? | 22.2 | 3.1 | 3.55 ± 0.64 | Yui et al. (2008) |
| K-5 | Red | 2.1 | 23.7 | ? | 19.7 | 4.0 | Yui et al. (2008) |
4 Vietnamese spinel δ18O
The oxygen isotope database for the current discussion comprises the 32 analyses previously documented (Giuliani et al., 2017; Pham et al., 2018). The specimens came from three localities, An Phu, Cong Troi, and Bai Son, separated from each other by a few kilometres; primary, placer, and alluvium occurrences are represented (Table SM1). A very wide range of colours are exhibited and the mineral parageneses are varied. Previously, Hauzenberger et al. (2003) reported a single δ18O of 14.3‰ for a purple spinel from Luc Yen, also in the Lo Gam Zone, but cautioned that the silicate minerals in the sample were not in oxygen isotope equilibrium. Fig. 4 plots the 32 spinel δ18O (‰, V-SMOW) data as a quasi-histogram with datapoints identified by host deposit and spinel colour range. It is readily apparent that two narrow intervals (approximately 14.2 to14.9‰ and 22.4 to 22.9‰) spanning only one tenth of the total range contain almost half (15 out of 32) of the samples, with the remainder being spread with low frequency over the rest of the entire range (of 12 to 24‰). The value of 14.3‰ from Hauzenberger et al. (2003) falls in the first interval. Each of the observed δ18O modes (at ∼14 and ∼22‰) contains specimens from at least two occurrences and with a wide range of colours indicating a rather wide range of trace element concentrations (Pham et al., 2018), and each includes at least one primary deposit.

Quasi-histogram of δ18O (‰ VSMOW) for 32 specimens from three deposits, respectively An Phu, Cong Troi and Bai Son, in northern Vietnam with a wide range of colours (see ESM Table 1 for data and details). Note the wide spread of values (range of δ18O > 12‰) and the two strong and narrow modes, at ∼14‰ (where are located the six spinels originating from primary deposits), and ∼22‰ (where almost half of the data (15/32) occupy only one tenth of the entire δ18O range). Each symbol represents one δ18O value of spinel.
These observations reasonably preclude an explanation of the data distribution as being an artefact of very close sampling of spatially restricted, but spinel-rich areas of the marble hosts. Rather, it seems more likely that there is a range in the δ18O of host marbles for all three occurrences, and that locally two marble zones differing in δ18O by ∼8‰ are especially productive. Mixing (e.g., near marble zone boundaries), Rayleigh decarbonation isotope effects and perhaps even metasomatism (as discussed in Hauzenberger et al. (2003) for their spinel-bearing sample) might all also be locally influential in affecting marble and so spinel, δ18O. However the 8‰ δ18O difference is far too large to result from isochemical decarbonatation (Valley, 1986).
This discussion helps to elucidate why spinel δ18O is not geographically diagnostic for these Vietnamese deposits, as has been previously pointed out by Pham et al. (2018). At each occurrence, there are the same two influential marbles with different oxygen isotopic compositions. Additionally, it may be of economic exploration interest that spinels from the high (∼22‰) δ18O mode have all been recovered from placers: the primary deposit has not been sampled for the spinels discussed here.
5 Further considerations and O-isotope data of calcite associated with spinel
An intriguing feature of the data distribution, illustrated in Fig. 4 is how narrow the two modes are: for the lower 14.2 ± 0.2‰ (1σ, n = 8) and for the higher 22.7 ± 0.2‰ (1σ, n = 7). The analytical precision of the laser fluorination method for determining δ18O of high-Mg minerals is likely 0.1 to 0.2‰ at 1σ, indicating very little geological scatter in the data from the two or three occurrences within each mode. A metamorphic spinel (sp) precipitating in oxygen isotopic equilibrium from a fluid (f) at temperature T (K) will have an oxygen isotopic composition that can be represented by a polynomial in 1/T and, frequently, to reasonable approximation (O’Neil, 1986), expressed as:
| (1) |
where Asp, Bsp are mineral-specific numerical constants (which, to our knowledge, have not been empirically evaluated for spinel). If, for a suite of spinels, δ18Of is buffered by the host marble and is uniform, then a constant δ18Osp implies a constant spinel precipitation temperature, and this argument applies equally to any other mineral co-crystallized with the spinel (and in oxygen isotopic equilibrium with it), which does not subsequently exchange oxygen isotopes.
Spinels in marble are markers of the high temperature metamorphism that affected the marbles (Garnier et al., 2008; Hauzenberger et al., 2001; Okrusch et al., 1976). In the amphibolite facies conditions, spinel, sapphirine and corundum are the main stable mineral phases and the main equilibrium reaction between corundum and spinel during prograde metamorphism is the following:
| (2) |
and then calcite coexisting (ideally in textural equilibrium) with spinel is of especial interest since combining by subtraction (1) with the equivalent equation for calcite (cc) readily gives:
| (3) |
so that, at constant T, the oxygen isotopic difference between the calcite and spinel should be a constant.
In a study of stable isotope ratios of Mogok ruby from Myanmar, Yui et al. (2008) reported two spinel δ18O values of 22.2 ± 0.1‰ (n = 2) and 19.7 ± 0.0‰ (n = 2) associated with closely adjacent calcites of respective δ18O of 25.3‰ and 23.7‰, giving Δ18Occ–sp values of 3.1 and 4.0‰. They noted that geologically unreasonable (because too high, approaching 1000 °C) equilibrium temperatures were deduced if the isotope fractionation equations (essentially the A and B values) theoretically calculated by Zheng (1991) by the increment method were adopted. The “isotopic inheritance” mechanism of Zheng (2011) was discussed, but discarded.
The calcite–spinel mineral pair approach was largely restricted to carefully examined primary specimens, and six such samples were identified from the seven primary specimens from An Phu and Cong Troi deposits, and δ18O in calcite was measured. The data are given in Table 1 and the Δ18Occ–sp values range from 3.5 to 3.9 (mean 3.73 ± 0.15‰, n = 3 for An Phu, and 3.67 ± 0.15‰, n = 3 for Cong Troi) with overall mean 3.7 ± 0.1‰, n = 6. For the samples from Vietnam, therefore, there certainly appears to be a very consistent relationship of constant Δ18Occ–sp independent of the actual δ18Osp value. This conclusion raises the question of whether the observed relationship might be of more than local significance: certainly δ18Occ–sp ∼ 3.7‰ for Vietnam is remarkably similar to the Yui et al. (2008) values of 3.1 and 4.0‰ from Myanmar, suggesting at least a regional character.
Accordingly, additional appropriate calcite–spinel mineral pairs were sought for primary deposit specimens from amongst the worldwide database reported by Giuliani et al. (2017), and suitable material was available from Tanzania (sample IPAN2) and Nepal (samples G85-1 and G85-2). The data for these, together with the Myanmar data of Yui et al. (2008) are included in Table 1. The δ18O data for the two Nepal samples are analytically indistinguishable and give Δ18Occ–sp values of 3.6 and 3.3‰, whilst for IPAN 2 from Tanzania, the value is 3.4‰. We note that Hauzenberger et al. (2003) reported a spinel δ18O of 14.3‰ from Luc Yen in Vietnam, but only presented a range of host rock (not necessarily equilibrium assemblage) calcite δ18O values (25.65 to 26.16‰) and, as noted previously, claimed that the various silicate minerals present were “…not in equilibrium”. Therefore, we have not included these data in the current analysis.
For the nine pairs of new data reported in this study, the Δ18Occ–sp values range from 3.3 to 3.9‰ with mean 3.6 ± 0.18‰ (n = 9). Including the Myanmar data from Yui et al. (2008) expands the range slightly 3.1 to 4.0‰ with overall mean 3.6 ± 0.3‰ (1σ, n = 11). δ18Occ is plotted against δ18Osp in Fig. 5, and the best-fit line for all 11 samples is δ18Occ = 0.96 δ18Osp + 4.4, with r = 0.9974, which is significant above the 99.9% level for n = 11. Clearly, there is very little geological ‘noise’ in the spinel–calcite δ18O relationship, despite their worldwide geographical spread and a range of almost 10‰ in δ18O.

Plot of spinel δ18Osp versus calcite δ18Occ for two sets (each n = 3) of primary spinel samples from An Phu and Cong Troi deposits in Vietnam, two specimens from Nepal and one from Tanzania; also plotted are the two data from Myanmar of Yui et al. (2008). For the Vietnamese material, the best-fit line (which is that shown in the Figure) is δ18Occ = 1.01 δ18Osp +3.6, n = 6, r = 0.9925 (> 99.9%) and for the entire dataset δ18Occ = 0.96 δ18Osp + 4.4, n = 11, r = 0.9974 (> 99.9%). The mean difference between the calcite and spinel δ18O data is Δ18Occ–sp = 3.6 ± 0.3‰ (n = 11).
6 Discussion
We are not aware of any empirical calibration of equilibrium oxygen isotope partitioning between spinel and another mineral phase as a function of temperature. Accordingly we first investigated the efficacy of the increment-method theoretical approach of Zheng (1991), and for the calcite–spinel pair calculated 1647 K (T = 1374 °C) for Δcc–sp of 3.6‰, and so concur with Yui et al. (2008) that this approach generates temperatures too high to be geologically reasonable. Zheng (1995) has claimed that, for magnetite, different structural forms have different oxygen isotope partitioning behaviour, and that this can on occasion lead to an oxygen isotope composition inherited from a precursor phase of different structure. However, we further concur with Yui et al. (2008) that such a mechanism does not seem likely in the case of the spinels discussed here.
The situation is not improved by using only the spinel-water calibration of Zheng (1991) together with an empirical calcite–water calibration: adopting the classic (O’Neil et al., 1969; and see also Kim and O’Neil, 1997) calcite–water equation gives a similarly unreasonable temperature of over 1000 °C. It would seem that the problem lies with the increment-method approach to spinel. One indirect (though less than ideal) approach is to examine the isotopic data for spinel-bearing mineral assemblages which likely preserve high-temperature oxygen isotope equilibrium fractionations with little likelihood of post-formational fluid-rock interaction (metasomatism). We can then use the relationship between spinel and other mineral δ18O values to indirectly constrain Δ18Occ-sp. Plausible candidate mineral assemblages are relatively anhydrous spinel lherzolites from the continental mantle. For the Vitim suite in southern Siberia, argued to reflect the bulk O-isotope character of the Earth's upper mantle (at 5.6 ± 0.1‰), Ionov et al. (1994) reported a relatively constant oxygen isotope fractionation between clinopyroxene (cpx) and spinel (sp) with Δ18Ocpx-sp of 0.6 ± 0.35‰ where the analytical precision for δ18O determination was stated to be “…ca. 0.2 – 0.3‰” and the petrological equilibration temperatures for the entire spinel peridotite suite spanned 270̊C. They noted that the spinel peridotite xenoliths from Tariat in Mongolia discussed by Harmon et al. (1987) had similar oxygen isotope characteristics to those at Vitim, albeit no spinel data were reported, presumably because of the low abundance of spinel at this location.
Chiba et al. (1989) have provided an empirical calibration of oxygen isotope partitioning between calcite and the pyroxene group mineral diopside:
Also,
and taking Δ18Ocpx-sp as 0.6‰ from Ionov et al. (1994) and Δ18Osp-cc as –3.6 (± 0.3‰) from the eleven spinel-calcite pairs reported above gives:
There will be error in this estimate associated with the temperature sensitivity of the value assumed for Δ18Ocpx-sp, and also with the uncertainty in Δ18Osp-cc. The 1σ standard deviation on the latter of ± 0.3‰ corresponds to a minimum temperature uncertainty of approximately ± 40 ̊C (calculated by inserting the mean ±1σ estimates into the equation for T).
This temperature estimate of 616 ± 40 °C seems geologically more plausible than the earlier estimates based on the increment-method oxygen isotope fractionation factor for spinel–water. Because of unquantified imprecision and inaccuracy in the overall approach adopted here, the main point is not to derive a robust temperature estimate together with an assessment of the associated error, but rather to suggest that equilibrium oxygen isotope fractionation at a constant and geologically reasonable temperature can explain the remarkably consistent difference in δ18O between calcite and spinel on local and global scales.
General estimates of pressure-temperature (P–T) conditions of peak metamorphism for the Day Nui Con Voi Range (Fig. 3) are constrained by biotite–garnet–sillimanite paragneisses at 7 kbar and 780 °C (Leloup et al., 1995). In the Lo Gam Zone where are located the ruby and spinel deposits in marble, these P–T conditions correspond to the presence of the mineral assemblage clinohumite–calcite–forsterite–dolomite–spinel (Hauzenberger et al., 2014) following the reaction:
| (4) |
Garnier et al. (2008) using mineral assemblages and fluid inclusions data in ruby deposits from southeastern Asia suggested the formation of ruby during the prograde and retrograde paths in the range 610 < T < 670 °C and 3 < P < 6 kbar. Ruby is found sometimes with spinel, but generally spinel formed by the disappearance of ruby following reaction (2) and in the absence of sapphirine. The estimate metamorphic P–T conditions for spinel formation are 3 < P < 6.5 kbar and 610 < T < 750 °C (Fig. 6). Yui et al. (2008) estimated temperatures of 607 and < 710 °C as upper limits for spinel and ruby at Mogok in Myanmar, based on the Raman spectra of graphite, with the higher of these temperatures being for a spinel-bearing assemblage.

P–T metamorphic conditions of stability for mineral associations of ruby and/or spinel-bearing marbles from the Luc Yen–An Phu area in northern Vietnam (modified from Garnier et al., 2008). The domain of stability of spinel is drawn in red. It is limited by the equilibrium: corundum + dolomite ↔ spinel + calcite + CO2, the equilibrium: 2spinel + 6corundum + clinochlore ↔ 2sapphirine + 4H2O, and the isochores of the fluids calculated for the CO2-bearing fluids trapped by gem ruby during the retrograde stage. Abbreviations: An = anorthite; And = andalusite; Cc = calcite; Clin = clinochlore; Co = corundum; Do = dolomite; Dsp = diaspore; Ksp = K-feldspar; Ky = kyanite; Ma = margarite; Mu = muscovite; Sill = sillimanite; spr7 = sapphirine; Sp = spinel; Zo = zoisite. Diagrams calculated with TWEEQU (Berman, 1991).
During subsequent cooling, any free fluid phase would have its oxygen isotopic composition buffered by the relatively great volume of host marble, and so we do not expect any oxygen isotope closure issue (as in Jenkin et al., 1991) to affect the measured calcite δ18O.
The δ18O values of Vietnamese spinels (An Phu and Luc Yen area) defined two narrow intervals, respectively, 14.2 to 14.9‰ and 22.4 to 22.9‰ (Fig. 4), and the calcite–spinel pairs have a remarkably constant Δ18Occ–sp of 3.7 ± 0.1 (n = 6). The excellent correlation with other worldwide cc–sp pairs (Δ18Occ–sp of 3.6 ± 0.3, n = 11; Fig. 5) signifies that the oxygen isotope fractionation factor is applicable to all calcite and spinel association that grew in equilibrium during metamorphism. Garnier (2003) has shown that, in the Luc Yen and An Phu mining districts, the O-isotopic compositions of calcite and dolomite in marbles vary from one district to another, but were homogeneous in each district. The δ18O values of these carbonates, between 18 and 29‰ (n = 22; Table SM 2), define two ranges, respectively 18 to 21.5‰ and 24 to 28.9‰ (Fig. 7). The first δ18O range of marble fits with the δ18O values of calcite in equilibrium with spinel (this study), corroborating the calculated Δ18Occ–sp of around 4.0. The existence of δ18O values between 21 and 24‰ for spinels from placers of both An Phu and Luc Yen opens the question of the isotopic composition of calcite of the spinel-bearing marble. Taking in account the Δ18Occ–sp ∼4.0, the δ18O values of calcite from marble must be within the range ∼24–25 and 29‰. Fig. 7 shows that such a range of δ18O values is found for both marbles from Luc Yen and An Phu mining districts. This result indicates that a great part of the spinels found in placers originates from high-δ18O carbonates mother rock that could have value as a parameter used for geochemical prospecting of spinel occurrences in northern Vietnam.

Oxygen isotopic signatures of marbles from the Luc Yen and An Phu mining areas (from Garnier, 2003; Garnier et al., 2008), and those from the calcite–spinel pairs from the An Phu and Cong Troi deposits (this study).
7 Conclusions and perspectives
The main conclusions of this study can be summarized as follows:
- • For gem spinels associated with marbles from a variety of locations in Vietnam, there is a remarkably constant oxygen isotope fractionation between the oxygen in the spinel oxide structure and that in the carbonate of genetically-associated calcite with Δ18Occ–sp = 3.7 ± 0.1‰ (n = 6);
- • A similar relationship has been observed for material from Myanmar, with values of 3.1 and 4.0‰ (Yui et al., 2008). New data reported here from Nepal (3.1 and 3.3‰) and Tanzania (3.4‰) are also consistent, with an overall Δ18Occ–sp of 3.6 ± 0.3‰ for all the spinel samples (n = 11). We hypothesise that the observed relationship has a global character. There is no evidence of any strong influence of other parameters (such as pressure or fluid salinity);
- • The constant oxygen isotope fractionation observed between coexisting spinel and calcite reflects a constant temperature, whose current best estimate is ∼620 ± 40 ̊C, but unquantified uncertainties associated with various steps in the argument preclude robust estimate of the accuracy of this estimation;
- • These observations support the emerging genetic model (Giuliani et al., 2017) for economically gem spinels, including an explanation of why spinel δ18O is not, on its own, locally a discriminant of geographic origin (Pham et al., 2018).
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-profit sectors.


