1. Introduction
Prokaryotes form an invisible yet major part of Earth’s biosphere. Although easy to overlook due to their small sizes, bacteria and archaea contribute to a significant fraction of modern biodiversity in terms of species abundance, total biomass, and capacity to thrive in habitats inhospitable to more conspicuous forms of life [Bar-On et al. 2018]. Moreover, due to their impressively diverse metabolic capabilities, prokaryotes participate in transformations and fluxes of most elements present at the surface of the Earth, and hence are important drivers of geochemical cycles [Falkowski et al. 2008]. Since the origin of life about 4 billion years ago, microorganisms have been influencing their geochemical environment, and have in turn been influenced by it. Throughout this long co-evolutionary history, some prokaryotes have acquired the capability to form minerals, a process called biomineralization [Frankel and Bazylinski 2003]. Prokaryotic biomineralization mirrors Eukaryotes’ capacity to form mineral skeletons, shells, or teeth, and has been evidenced as early as the 19th century, with, for instance, the discoveries of Fe-oxide biomineralization by Gallionella [Ehrenberg 1836] or Ca-carbonate biomineralization by Achromatium oxaliferum [Schewiakoff 1893].
Under given conditions, certain prokaryotes biomineralize more intensively than others, while many do not biomineralize at all, and thus we can define this capability as a biological trait*, i.e. a phenotypic* characteristic that can be measured. In this review, we focus on an often-overlooked aspect of this trait: its potential biological functions. Much progress has been made in recent years on the molecular-level mechanisms of microbial mineral formation, due to combined advances in genomic approaches [e.g., Koeksoy et al. 2021; UebeSchüler 2016; Zhang et al. 2020] and microscopy techniques [e.g., Blondeau et al. 2018b; Comolli et al. 2011; Li et al. 2017]. However, the question of the biological functions of biomineralization is often relegated to the discussion section of articles on the topic—when not completely ignored. As theorized by the Nobel Prize laureate Nikolaas Tinbergen, ultimate causes (“why?”) need to be addressed along proximate ones (“how?”) if a comprehensive understanding of any biological phenomenon is to be achieved [Tinbergen 1963]. There is now a need to shed light on the ultimate causes of prokaryotic biomineralization, including its potential functional and adaptive* aspects. After presenting the classical framework used to conceptualize prokaryotic biominerals and their potential functions, we describe recent results and hypotheses about the biological functions of prokaryotic biomineralization by focussing on a few selected examples (elemental sulfur, extracellular Fe(III) minerals, and intracellular carbonates). We then propose experimental approaches that may allow future progress on these “ultimate” questions, before focussing on two emerging roles for microbial biomineralization: cooperative interactions and biofilm* architectures. The examples and concepts developed in this review finally allow us to propose a new framework to classify biomineralization processes based on their adaptive value.
Note that we will use the terms “microbes” and “microbial” interchangeably with “prokaryotes” and “prokaryotic” (respectively) in this review, leaving aside biomineralization by microbial Eukaryotes. Terms specific to microbiology or evolutionary biology rarely used in the Earth science literature are defined in Table 1 and are indicated by an asterisk on their first occurrence. Also note that the term ‘biological function’ can have diverse meanings depending on authors, serving different explanatory schemes [Donovan 2019]. A function may relate to how a trait contributes to the functioning of a biological system, without discriminating adaptations* from “fortuitous effects”. This is called a “causal role” function* and can be determined for example by observing how a microbial system responds to the removal or alteration of the trait [Klassen 2018]. In a more teleological way, a function may be seen as the effect of a trait which has been responsible for its selection upon evolution (i.e. referring to its causal history). This is called a “selected effect” function*. Last, the “fitness contribution” function* of a trait may be seen as the effect it presently contributes to the fitness* (or reproductive success) of the organism, regardless of its history. The last two views imply that the trait is adaptive. In this review, we will discuss these different views when referring to potential functions of biominerals, while carefully avoiding the Panglossian paradigm as defined by Gould and Lewontin [1979], in which all traits taken separately are considered as adaptive.
Definitions of some technical terms specific to microbiology and evolutionary biology
| Term | Definition |
|---|---|
| Adaptation | Any change in the structure or functioning of successive generations of a population that makes it better suited to its environment. |
| Adaptive | Generally, providing, contributing to, or arising as a result to adaptation. More specifically, refers to a heritable trait that serves a specific function and improves an organism’s fitness or survival. |
| Aerotaxis | The movement of a cell or microorganism driven by a dioxygen gradient. |
| Antagonistic pleiotropy | Phenomenon in which versions of genes that are detrimental under certain conditions (e.g., late in life) improve fitness under other conditions (e.g., earlier in life). |
| Autotroph(ic)* | Refers to an organism that can build its own macromolecules from inorganic molecules, such as carbon dioxide (carbon autotrophy) or ammonia (nitrogen autotrophy). |
| Biofilm | A complex aggregation of bacteria and other microorganisms, that adheres to a substrate and is encased within a matrix of extracellular polymeric substances (EPS). |
| Capsule | A thick and relatively rigid gelatinous layer completely surrounding the cell wall of certain bacteria. |
| Causal role function | Contribution of a trait to the functioning of a biological system. |
| Chemotroph(ic)* | Refers to an organism whose energy is the result of endogenous, light-independent chemical reactions. |
| Clone, clonal | Refers to a group of genetically identical cells or organisms all descended from a single common ancestral cell or organism. |
| Convergent evolution | The development of apparently similar structures in unrelated organisms, usually because the organisms live in the same kind of environment. |
| Cytoplasm(ic) | Refers to the content of the cell, located within the plasma membrane. |
| EPS | Extracellular polymeric substances secreted by microorganisms. Sometimes used as a synonym for exopolysaccharides, a specific range of sugars produced either as insoluble capsules or a soluble slimes in the extracellular medium. |
| Exaptation | A character that provides a selective advantage under current conditions but had a different function at its origin. |
| Extracellular(ly) | Located or occurring outside the cell. |
| Extracytoplasmic | Located or occurring outside the cytoplasm. |
| Fitness | A measure of the contribution of an individual to the genetic composition of subsequent generations through its offspring. |
| Fitness contribution function | The contribution of a trait to the fitness of the organism. |
| Heterotroph(ic)* | Refers to an organism whose energy is derived from the intake and digestion of organic substances |
| Homeostasis, homeostatic | Refers to the regulation by an organism of the chemical composition of its internal environment. |
| Homologous | Referring to structures or processes in different organisms that show a fundamental similarity because of their having descended from a common ancestor. |
| Interspecific | Occurring between members of different species. |
| Intracellular(ly) | Located or occurring within cells. |
| Intraspecific | Occurring between members of the same species. |
| Isogenic | Genetically identical. |
| Knockout | A technique for inactivating a particular gene or genes within an organism or cell. |
| Lithotroph(ic)* | Refers to an organism that uses an inorganic substance as a substrate in its energy metabolism. |
| Magnetotaxis | The movement of a cell or microorganism in response to a magnetic field. |
| Maladaptive | Refers to a trait that does not lead to the highest relative fitness of the traits in the allowed set |
| Microaerophilic | Describes an aerobic environment with a lower partial pressure of dioxygen than that under normal atmospheric conditions. Alternatively, describes an organism whose maximal rate of growth occurs in such an environment. |
| Mixotroph(ic) | Refers to an organism that can alternatively or simultaneously adopt both autotrophic and heterotrophic growth modes. |
| Mucilage, mucilaginous | Refers to abundant, complex polysaccharides formed by organisms. Mucilages are typically slimy when wet. |
| Organelle | A minute structure within a cell that has a particular function. |
| Periplasm(ic) | Refers to the volume enclosed between the plasma membrane and the outer membrane in Gram-negative bacteria. |
| Phenotype | The observable characteristics of an organism. These are determined by its genes and by the interaction of the genes with the environment. |
| Phototroph(ic)* | Refers to an organism that uses light as the source of energy for metabolism and growth. |
| Pleiotropy, pleiotropic | Describes a situation in which a version of a gene has more than one effect in an organism. |
| Recombinant DNA | A composite DNA molecule created in vitro by joining a foreign DNA with a vector molecule. |
| Selected effect function | The effect of a trait which has been responsible for its selection upon evolution. |
| Symbiosis | A long-term intimate relationship between individuals of different species. The term is generally used to describe relationships in which both species benefit. |
| Syntrophy | A relationship where the metabolic product of a partner is used by the other partner as a metabolic substrate (and vice versa). A narrower definition is an obligately mutualistic metabolism. |
| Trait | A phenotypic characteristic of an organism that can be quantified. In particular, a quantitative trait shows continuous variation and can be measured quantitatively. |
| Transformation | A permanent heritable change in a cell that occurs as a result of its acquiring foreign DNA. |
| Transposon | A small, mobile DNA sequence that can be replicated and inserted at other sites in the genome. |
| Wild-type | The phenotype that is characteristic of most of the members of a species occurring naturally and contrasting with the phenotype of a mutant. |
2. Prokaryotic biomineralization processes and their functions: the classical framework
In this section, we present the conceptual framework originally laid out by Lowenstam [1981] and later developed by other authors to conceptualize biomineralization, and describe how they intersect with the question of the biological functions of prokaryotic biominerals (Figure 1).
The classical framework of microbial biomineralization. In biologically controlled mineralization (illustrated here by intracellular magnetite biomineralization), the bacteria exert a strict control on mineral nucleation, growth, and final location of the biominerals. The involvement of molecular machineries to direct mineral formation is associated with an important physiological cost, compensated by supposed fitness benefits for the organism. On the other hand, in biologically induced and biologically influenced mineralization (illustrated here by extracellular calcium carbonate precipitation in a biofilm), mineral precipitation respectively occurs as a result of local chemical changes caused by metabolic activity of the cells (here, a pH increase), or following passive nucleation on cellular or extracellular structures (here, favored by adsorption of Ca2+ ions on negatively charged EPS). Mineral properties are typically poorly controlled, and biomineral formation is thought to not necessarily provide fitness benefits (it may in some cases be detrimental to the cells). See Section 2 for more details.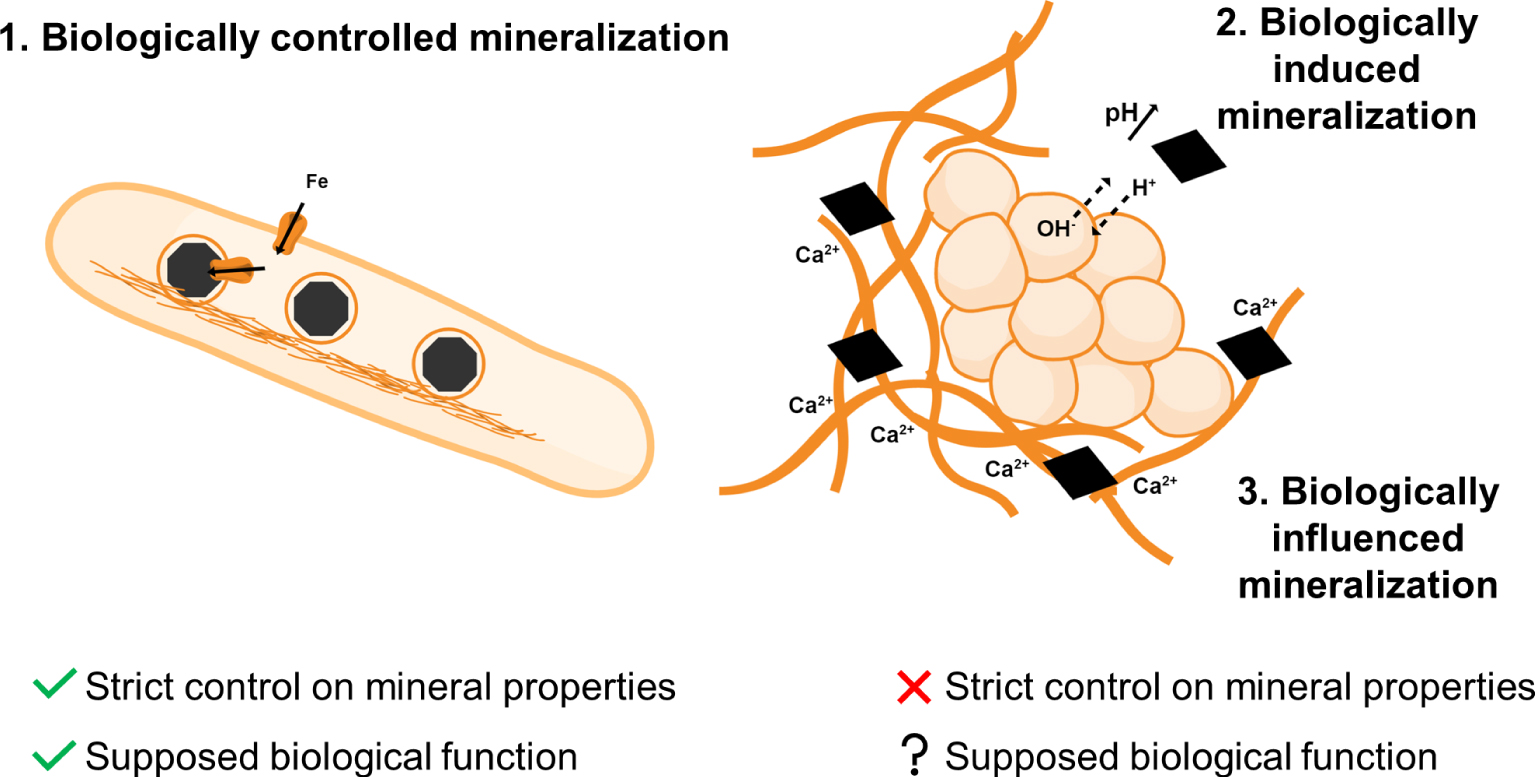
2.1. Controlled biomineralization
Biologically controlled mineralization was introduced by Mann [1983], generalizing the “organic matrix-mediated” notion coined by Lowenstam [1981]. As its name implies, in controlled biomineralization, the organism exerts strict genetic control to direct the nucleation, growth, and final location of the formed biominerals. Controlled mineralization can be intracellular* or extracellular* and/or start intracellularly with a final extracellular location of the biominerals. Many eukaryotic biominerals are formed by controlled mineralization and serve clearly identified biological functions (in both “causal role” and “selected effect” views), such as structure (e.g. apatite in bones), protection (e.g., aragonite or silica in shells), or food acquisition (e.g., apatite in teeth). The chemical, morphological and crystallographic properties of the biominerals are under tight genetic control, and they most often form within organic vesicles or matrices where mineralization is precisely directed [Veis 2003]. Such strict genetic control on biomineral formation and properties also exists in prokaryotic systems, but it is thought to be rare.
The best studied and most frequently cited example of biologically controlled (or directed) prokaryotic biomineralization is the formation of intracellular magnetic iron minerals by magnetotactic bacteria [Faivre and Schüler 2008] (Figure 1). These biominerals, either magnetite (Fe3O4) or greigite (Fe3S4), are located within a specialized bacterial organelle*, the magnetosome. The molecular-level machineries involved in iron acquisition and transport, iron redox chemistry, and mineral formation and positioning have now been described [Komeili 2007; Lower and Bazylinski 2013; UebeSchüler 2016]. Magnetic iron biominerals possess several defining properties that sets them apart from abiotic “equivalent” mineral phases, which makes them of particular interest for paleobiology and astrobiology [Benzerara et al. 2019; Li et al. 2020]: they usually have few crystal defects; their trace element content and isotopic composition are different from that of magnetites forming extracellularly in the same environment; they have restricted size and shape ranges so as to form stable single-magnetic domains, and they are aligned into a chain within the microbial cell, stabilizing and maximizing their magnetic dipole moment [Amor et al. 2020, 2016, 2015; Martel et al. 2012]. As a result, magnetotactic bacteria are equipped with the equivalent of an efficient single magnet, allowing them to align with and swim along Earth’s magnetic field lines. Combined with aerotaxis*, magnetotaxis* is thought to increase the bacteria’s efficiency to find and stay within their preferred oxygen conditions in natural environments [Frankel et al. 2007; Stephens 2006], corresponding to a “causal role” and a “fitness contribution” function. Originally, magnetosomes may have had another biological function (a “selected effect” function), such as harvesting reactive oxygen species [Lefèvre and Bazylinski 2013; Lin et al. 2020]. Interestingly, it has been shown that the navigation function of magnetite biominerals sometimes contribute to the onset of symbioses* between magnetic bacteria and unicellular eukaryotes, adding another potential function [Monteil et al. 2019]. An important idea associated with biologically controlled mineralization is that strict genetic control inevitably involves metabolic costs (to produce the enzymes and organic matrices directing biomineral formation). Such costs need to be compensated with beneficial effects for the organisms, and thus point toward the existence of important biological functions. In magnetotactic bacteria, this idea is further supported by the observation of frequent spontaneous losses of magnetotaxis under non-selective cultivation conditions due to deletions in the genomic “magnetosome island” [Ullrich et al. 2005].
2.2. Induced and influenced biomineralization
Two other mechanisms of prokaryotic biomineralization, which by contrast do not necessarily imply biological functions (in a “selected effect” view) or benefits to the cells, are often described [Benzerara et al. 2011; Dupraz et al. 2009; Lowenstam 1981]. As opposed to controlled biomineralization, which in bacteria is typically intracellular, these two modes of biomineralization often occur extracellularly (even at distance from the cells), or at least extracytoplasmically* within the cell wall. In a first process termed “biologically induced” or “indirect” biomineralization, minerals precipitate as a result of local chemical changes (e.g. redox transformations or shifts in pH) caused by the metabolic activity of the cells. In this case, the microorganisms play an active role in mineral precipitation by causing or increasing solution supersaturation, but there is no organic control on crystal nucleation or growth. Biologically induced mineralization thus requires metabolically active cells or enzymes. In the second mechanism, called “biologically influenced” or “passive” biomineralization, microbial cell walls or extracellular organic structures such as extracellular polymeric substances (EPS*) act as nucleation surfaces catalyzing mineral precipitation in supersaturated solutions. By allowing heterogeneous nucleation, these surfaces decrease the free energy barrier for mineral precipitation, which may become kinetically favored [e.g., Giuffre et al. 2013]. Biologically influenced biomineralization does not require cells to be alive or metabolically active. We note that some authors do not refer to this process as biomineralization but use the term organomineralization instead [Dupraz et al. 2009]. These two processes—induced and influenced biomineralization—can occur simultaneously. For example, in microbial mats, calcium carbonate mineral precipitation may occur as a result of (often photosynthetic) metabolism, which increases alkalinity and hence solution saturation with respect to calcium carbonate phases, while the EPS matrix acts as a template for mineral nucleation [Dupraz et al. 2009; Iniesto et al. 2021] (Figure 1). It is often assumed that biologically induced or influenced biominerals do not necessarily serve a function (other than “waste”), and their precipitation may even be described as purely accidental or “unintended” [Frankel and Bazylinski 2003], when not detrimental to the microorganisms (for instance if cells become completely entombed by the growing minerals). While this does not mean that there is no genetic basis to this kind of biomineralization (for instance, genes are needed to encode the production of EPS), it is meant that the effects of these biomineralization processes do not increase the fitness of the microorganisms and are therefore not adaptive, at least under the considered environmental conditions. We will see later that this view may be questioned based on recent results on the potential benefits of extracellular biomineralization in biofilms (Section 8.2).
3. Does prokaryotic biomineralization need to have a function?
The apparently inadvertent and detrimental nature of biologically induced or influenced mineralization has led several authors to focus on the question of how microorganisms can survive mineral precipitation and subsequent entombment (as reviewed for instance in Benzerara et al. [2011] and Konhauser et al. [2008]). However, the idea that non-controlled prokaryotic biomineralization is an accidental process is somewhat unsatisfying. Indeed, life has been co-evolving with the mineral world for nearly 4 billion years [Hazen et al. 2008; Williams and Rickaby 2012], and there is now experimental evidence that microbial communities are uniquely adapted to their mineral environment [e.g., Jones and Bennett 2014]. Several habitats of the early Earth, such as hydrothermal vents and stromatolite-supporting shallow waters, were particularly prone to mineral precipitation, and the geological record contains numerous and clear evidence of rapid encrustation of bacterial cells by minerals, a phenomenon which is at the origin of their fossilization and preservation in rocks [Li et al. 2013; Javaux and Lepot 2018]. Entombment within a mineral matrix is likely to greatly limit the transport of essential nutrients and energy sources to the bacteria [e.g., Miot et al. 2015], while also limiting the transport of waste away from the cells and preventing their motility. If overall detrimental, one can wonder why mineral encrustation has persisted through billions of years of microbial evolution. In cases where mineral precipitation is triggered by the bacteria themselves, we may ask ourselves whether biomineralization could provide benefits that outweigh its potential detrimental effects to the cells. We may thus be led to take into account concepts such as antagonistic pleiotropy*, in which the same trait (for instance here, the capability to biomineralize) is beneficial under certain environmental and/or physiological conditions [e.g., at a specific growth rate; Maharjan et al. 2013] and detrimental under other conditions. Alternatively, the capability to biomineralize may be one trait under the control of a pleiotropic gene, i.e. a gene controlling several traits at the same time. One of the other traits might be beneficial to the overall fitness of the microorganism and therefore adaptive, while the biomineralization trait might be indirectly selected [Lande and Arnold 1983]. We will further discuss this point below.
Phoenix and Konhauser [2008] proposed a list of potential benefits of prokaryotic biomineralization. While some proposed benefits were supported by data (such as screening from detrimental solar radiation, nutrient storage, or toxin immobilization), others were more speculative (such as physical protection from grazing or desiccation, and participation in biofilm strength and structural integrity). In a recent review, Mansor and Xu [2020] cited additional possible functions of prokaryotic biominerals, such as extracellular electron transfer, detoxification of harmful metabolic products, or the conservation of thermodynamic potentials for metabolic reactions. In the next sections, we will present several examples of microbial biomineralization systems with demonstrated or supposed biological functions, with no attempt to be exhaustive.
Significant advances have been recently achieved regarding “proximate” questions (“how?”) on microbial mineral formation, as we are learning about the genetic and enzymatic systems involved as well as fine details of crystal nucleation and growth mechanisms at the microbe–mineral interface. Here, we are deliberately setting these details aside to focus on studies where “ultimate” questions (“why?”) have been addressed, or at least raised.
4. Elemental sulfur biominerals: energy storage materials
Several biominerals are proposed to serve a storage function, either of nutrients or energy (i.e. electron donors or acceptors for energy metabolism). Here we will address elemental sulfur (S0) as an example of biomineral for which an energy storage function is well established, and explain what observations are supporting this proposed function (in a “causal role” and a “fitness contribution” view).
4.1. Elemental sulfur storage and utilization
Elemental sulfur, or zero-valent sulfur (S0), is an intermediate of the biogeochemical sulfur cycle that is found as a mineral in many natural environments such as marine sediments, water columns of lakes or oceans, cold or hot springs, hydrothermal environments, salt marshes and caves [Findlay et al. 2014; Hamilton et al. 2015; Jørgensen et al. 2019; Lau et al. 2017; Zerkle et al. 2010]. S0 is formed by abiological or biological oxidation of more reduced sulfur species, although in low-temperature environments biological S-oxidation rates are typically more than three orders of magnitude faster than abiotic ones [Luther et al. 2011]. A wide diversity of microorganisms can oxidize sulfide, polysulfides, or thiosulfate and precipitate S0, through phototrophic* (purple sulfur bacteria, green sulfur bacteria, cyanobacteria) and chemotrophic* (colorless sulfur bacteria) pathways [Dahl and Prange 2006; Kleinjan et al. 2003]. In turn, microbially formed S0 can be used as a source of energy for diverse S-oxidizers, S-reducers, and microorganisms that perform S0 disproportionation [Dahl 2020] (Figure 2A). Some, such as the thermoacidophile Acidianus, can grow from all three reactions [Amenabar and Boyd 2018].
Elemental sulfur biominerals as energy storage materials. (A) Simplified biogeochemical sulfur cycle, adapted from Zopfi et al. [2004]. Elemental sulfur (S0) occupies a central ecological role in the S cycle. It is produced by oxidation of more reduced S species, and can be consumed by S reduction, oxidation, or disproportionation. (B) Intracellular S0 storage in Thiothrix sp. (Image: C. Nims, University of Michigan). (C) Extracellular S0 formation by Chlorobaculum tepidum [Marnocha et al. 2016]. Image reproduced with permission from the Microbiology Society. (D) Extracellular S0 stores stabilized by EPS in a Sulfurovum-rich biofilm [Cron et al. 2021] (Image: B. Cron, Northwest Indian College). (E) Multiple C. tepidum cells degrading a S0 globule, illustrating “mutualistic” S0 utilization [Hanson et al. 2016]. Image reproduced with permission from John Wiley and Sons. (F) Thiobacillus ferrooxidans retaining S0 colloids within its organic capsule, illustrating “selfish” S0 utilization [Rojas et al. 1995]. Inset: after cell division, the S0 stores are shared with the daughter cell. Images reproduced with permission from Springer Nature.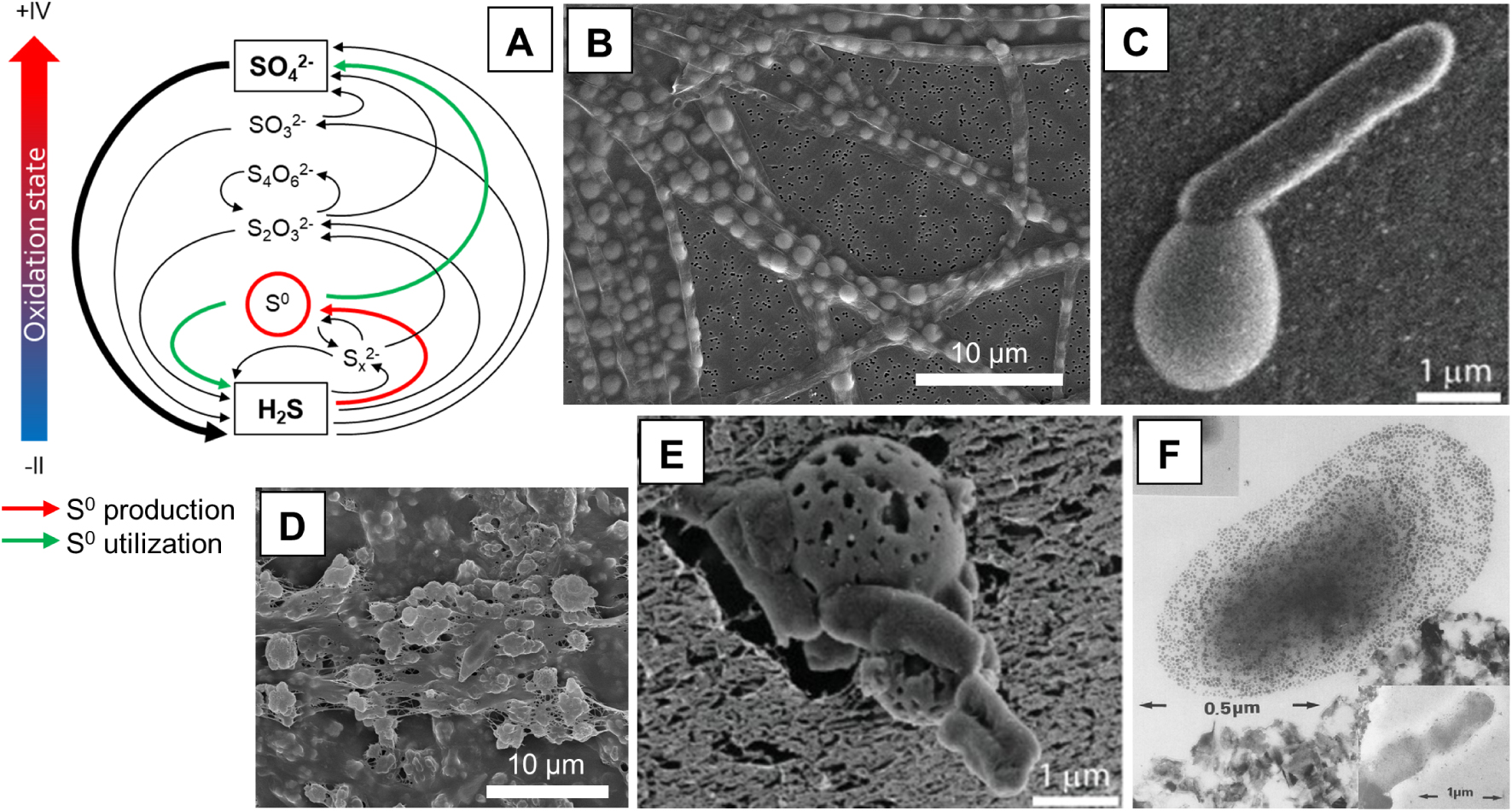
The enzymatic machinery involved in microbial S-oxidation has been described for many known S-oxidizers [Dahl et al. 2008; Friedrich et al. 2001; Ghosh and Dam 2009; Gregersen et al. 2011; Koch and Dahl 2018] and will not be detailed here. Elemental sulfur formed through S-oxidation can be found either intracellularly or extracellularly [Dahl and Prange 2006; Kleinjan et al. 2003; Maki 2013] (Figure 2B,C). In both cases, S0may serve an energy storage function. Indeed, S0 produced under favorable environmental conditions can then be used as an electron donor or an electron acceptor if conditions change. For instance, the S-oxidizer Thiobacillus denitrificans accumulates intracellular S0from the oxidation of thiosulfate, but the transiently stored S0 can later be further oxidized to sulfate for energy production when thiosulfate is depleted in the medium [SchedelTrüper 1980]. Beggiatoa oxidizes sulfide to intracellular S0 under high-sulfide conditions, while under low-sulfide conditions this stored S0 is oxidized to sulfate [Berg et al. 2014]. Purple sulfur bacteria such as Achromatium vinosum use intracellular S0 as a source of reducing power for anoxygenic photosynthesis in the absence of sulfide, releasing sulfate, while in the dark S0 is used as an electron acceptor, releasing sulfide [Mas and Gemerden 1995]. S0 reduction to sulfide was also observed in the anaerobic growth of Beggiatoa in the presence of an organic carbon source (acetate) [Nelson and Castenholz 1981]. Bacteria that precipitate S0 extracellularly may also utilize their own S0 biominerals for energy metabolism and growth in the absence of other reduced sulfur sources. For example, the purple sulfur bacterium Ectothiorhodospira halochloris precipitates extracellular S0 from sulfide oxidation. During growth in the absence of sulfide, they reduce S0 back to sulfide [Then and Trüper 1984]. More recently, the green sulfur bacterium Chlorobaculum tepidum was shown to form extracellular S0 globules during sulfide oxidation, and then uses this biogenic S0 as an electron donor for photosynthesis when sulfide is exhausted [Hanson et al. 2016; Marnocha et al. 2016] (Figure 1C,E).
It is not clear what determines whether S0 is produced intra- or extracellularly. From a functional perspective, the “choice” of external energy storage is problematic, since these stores are open to piracy by other cells within the same population. As further explained below, some S0-forming bacteria may have developed strategies to prevent such piracy. One possible advantage of the extracellular storage strategy is that an unlimited amount of S0 can be stored by an individual microorganism, since accumulation is not limited by the dimensions of the cell. Indeed, sulfur content is the main determinant of volume variation in cells of Allochromatium vinosum [Mas and Gemerden 1995]. Moreover, Beggiatoa filaments grown with a high-sulfide flux can accumulate so much intracellular S0 that they eventually burst [Berg et al. 2014], showing that cell dimensions are clearly limiting S0 storage capacities in microorganisms biomineralizing S0 intracellularly. Intracellular S0 accumulation furthermore increases cell density which may negatively impact the buoyancy of microorganisms with planktonic lifestyles [Mas and van Gemerden 1987].
4.2. The properties of biogenic S0: adaptation to the energy storage function?
It has been known for several decades that both intra- and extracellular biomineralized S0 possess properties that significantly differ from those of inorganically precipitated S0 minerals [Steudel 1989]. Microbial S0can be stored under different forms depending on the biological species, which suggests some level of genetic control on S0 chemical and mineralogical properties. For instance, some chemotrophic S-oxidizers such as Thiomargarita, Thioploca, Beggiatoa and Thiothrix store sulfur as intracellular globules of cyclo-octasulfur (S8) [Nims et al. 2019; Pasteris et al. 2001; Prange et al. 2002], possibly in combination with polysulfides (S) [Berg et al. 2014], while purple (phototrophic) S-oxidizers and some chemotrophic S-oxidizers from the Firmicutes phylum store S0 intracellularly as sulfur chains, possibly terminated by organic end groups (i.e., organic polysulfanes) [Lee et al. 2007; Prange et al. 2002]. The crystal structure of S0 in intracellular globules has not been systematically determined, but it is thought to be either liquid-like [Hageae et al. 1970], solid amorphous [Nims et al. 2019], or microcrystalline [Pasteris et al. 2001]. Extracellularly biomineralized S0 may also exist in different forms and structures. For instance, S0 globules produced by Acidithiobacillus ferroxidans are composed mostly of polythionates [Prange et al. 2002; Steudel et al. 1987], while extracellular S0 produced by the phototrophic green sulfur bacterium Chlorobium vibrioforme is composed mostly of sulfur chains terminated by organic residues [Prange et al. 2002]. Another green sulfur bacterium, C. tepidum, produces extracellular globules composed of amorphous and nanocrystalline S8, and they were found to be less stiff and more elastic than commercial S0 [Marnocha et al. 2019]. Sulfuricurvum kujiense, a chemoautotrophic* S-oxidizing bacterium, produces extracellular S0 under a metastable crystalline form, the monoclinic allotropes β- and γ-S8 [Cron et al. 2019]. Extracellular metastable monoclinic S8 allotropes were also found in the environment in Sulfurovum-dominated biofilms [Cron et al. 2021] (Figure 2D).
The stable form for S0 under ambient temperature and pressure conditions is crystalline orthorhombic α-S8 [Steudel and Eckert 2003], and it is likely that unstable S0 forms need to be stabilized by close interaction with organic structures. Internal S0 is typically periplasmic*, nested in an invagination of the cytoplasmic membrane [e.g., Larkin and Strohl 1983; Prange et al. 2004]. Protein membranes were identified around the intracellular S0 globules in diverse S-oxidizers including Beggiatoa, Thiothrix, Thiovulum and different purple sulfur bacteria [Maki 2013]. Three proteins forming an envelope around the S0 globules were identified (SgpA, SgpB, SgpC) in the purple sulfur bacterium Allochromatium vinosum, and their critical role in intracellular S0 formation and storage was demonstrated [Brune 1995; Prange et al. 2004]. Similarly, organic envelopes were observed at the surface of extracellular S0globules formed by Thiobacillus sp. W5 [Kleinjan et al. 2005], C. tepidum [Hanson et al. 2016; Marnocha et al. 2019], and S. kujiense [Cron et al. 2019], as well as in natural biofilms [Cron et al. 2021]. These organic envelopes contain polysaccharides and proteins [Cron et al. 2019; Marnocha et al. 2019], which are not homologous to the Sgp proteins forming the envelope around intracellular S0 globules in A. vinosum [Hanson et al. 2016]. The presence of this organic coating allows extracellular S0 to exist in a metastable form [Cosmidis et al. 2019; Cron et al. 2019, 2021] and slows down its crystallization to stable α-S8 [Marnocha et al. 2019; Steudel 2003]. Organically coated S0 globules may be formed through a self-assembly process, in which soluble organic molecules react with extracellular sulfide or polysulfides to form sulfurized polymers assembling into vesicles around S0 minerals [Cosmidis et al. 2019; Cron et al. 2019]. Alternatively, the mechanism of secretion of organically coated S0 globules could involve the production of outer membrane vesicles in some species [Li et al. 2020].
There is now ample evidence that S0storage under a metastable form (e.g., polymeric sulfur, or amorphous or monoclinic S8) favors its mobilization during consumption, showing an adaptation of the S0 biomineral properties to their biological function. For instance, when grown on S0 provided extracellularly, A. vinosum cells show a preference for polymeric sulfur over commercial crystalline S8, which they are unable to uptake [Franz et al. 2007]. Preference for polymeric sulfur utilization over S8 was also evidenced in natural mats of chemotrophic S-oxidizers [Engel et al. 2007]. Particle size, surface area, and S0 composition and structure affects S0 oxidation rate by Thiobacillus albertis [Laishley et al. 1986]. Incubation experiments of natural freshwater communities with different sulfur sources showed a preference for the utilization of a reactive form of colloidal S0—possibly polythionates—over S8 [Findlay and Kamyshny 2017]. The structure of the enzymes involved in sulfur utilization may provide a clue to the relationship between S0 form or structure and its utilization. For instance, sulfur oxygenase reductase (SOR) enzymes present in some archaea possess an elongated active site that accommodates linear polysulfides but not S8 [Li et al. 2008; Urich et al. 2006]. The presence of an organic envelope around biomineralized S0 may also be important for S0 utilization, by making S0 hydrophilic and thus allowing interaction with the cell surface. Indeed, it was recently shown that C. tepidum can grow on its own biogenic S0 globules but not on commercial S0, crystalline S0, or inorganically precipitated colloidal S0, which is mineralogically very similar to biogenic S0 but does not have an organic coating [Marnocha et al. 2019, 2016].
4.3. Selfish versus mutualist utilization of S0stores
As pointed out earlier, extracellular location of S0 resources exposes to the risk of piracy by “free riders” benefiting from their utilization without participating in their production. Different strategies have been developed to privatize S0 stores by physically excluding nonproducers. For instance, the green sulfur bacterium Chlorobium limicola, which forms extracellular S0 globules adjacent to the cell wall in the presence of sulfide, grows tubular surface appendages called spinae and surrounds itself with a mucilaginous* capsule* when subjected to sulfide starvation periods (i.e. under conditions where S0 may be used as an electron donor) [Pibernat and Abella 1996]. The spinae and capsule may favor the retention of the S0 globules near the cells and their attachment to the outer membrane, effectively excluding utilization by other bacteria [Mas and Gemerden 1995; vanGemerden 1986]. Similarly, transient extracellular colloidal S0 particles produced by Acidithiobacillus ferrooxidans are retained within a thick organic capsule which may play a similar role in the privatization of S0 resources [Rojas et al. 1995] (Figure 2F). In other cases, even if no such physical barriers exist, S0 consumption by rival cells may be limited by the fact that S0 utilization requires intimate physical contact with the S0 globules, as for instance with A. vinosum [Franz et al. 2007], Acidithiobacillus thiooxidans [Knickerbocker et al. 2000], or different strains of Acidithiobacillus and Acidiphilium spp. [Rohwerder and Sand 2003]. In biofilms where cell motility is limited, utilization of S0 would thus be limited to the producer and its immediate neighbors, which typically belong to the same clonal* cluster [Nadell et al. 2016].
Interestingly, for some species, a mutualistic utilization of S0 could be demonstrated. In the case of C. tepidum, both S0 formation and consumption are cooperative processes (Figure 2E). Indeed, multiple cells contribute to the formation of a single globule, and during the utilization phase, cells attached to the S0 globules and unattached cells have comparable growth rates, suggesting that attached cells could be solubilizing S0 as diffusing polysulfides, “feeding” the free cells [Marnocha et al. 2016]. A similar cooperative solubilization mechanism could exist for Acidithiobacillus ferrooxidans. Indeed, while adsorption of some cells to the surface of the minerals seems necessary for growth of S0, free cells also participate in sulfur oxidation and growth [Ceskova et al. 2002].
Ultimately, S0 can be traded in mutualistic relationships between members of different bacterial species. For instance, the S0-producing green sulfur bacterium Chlorobium virbrioforme can be grown in a syntrophic* co-culture with the S0-reducing Desulfuromonas acetoxidans [Warthmann et al. 1992]. In these cultures, S0 produced as a result of sulfide oxidation by Chlorobium is used as an electron acceptor by Desulfuromonas for acetate oxidation, leading to the regeneration of sulfide. Syntrophic interactions may be facilitated by the fact that S0 utilization by reduction and disproportionation does not always seem to require physical contact with the minerals [Amenabar and Boyd 2018; Blumentals et al. 1990; Boyd and Druschel 2013]. Potential roles of biomineralization in intra-* and interspecific* cooperation will be further developed later in this review.
5. Extracellular iron minerals: a biomineral type with multiple functions
Iron biominerals can be formed extracellularly by bacteria, as the end product of various iron-cycling metabolisms [Kappler et al. 2021]. Iron mostly exists in the environments under two redox states: Fe(II) (or ferrous iron) and Fe(III) (ferric iron). Ferric iron is relatively insoluble under circumneutral pH conditions, so iron oxidation may often result in the precipitation of Fe(III)-bearing phases such as Fe(III)-(oxyhydr)oxides and Fe(III)-phosphates, or mixed valence Fe(II)-Fe(III) phases such as magnetite or green rusts. Bacteria can oxidize ferrous to ferric iron via different metabolic pathways, namely: microaerophilic* Fe(II) oxidation (whereby O2 is used as the electron acceptor and Fe(II) the electron donor for lithoautotrophic* growth), phototrophic Fe(II) oxidation (whereby light and electrons from Fe(II) are used to fix inorganic carbon into organics), and nitrate-dependent Fe(II) oxidation (whereby Fe(II) oxidation is coupled with nitrate reduction, either through a chemolithoautotrophic* pathway leading to energy generation and inorganic carbon fixation, or, more often, through abiotic Fe(II) oxidation by reactive nitrogen species produced as a result of heterotrophic* denitrification; in the latter case, Fe(II) oxidation is not an energy-generating process) [Bryce et al. 2018; Emerson et al. 2010; Kappler et al. 2021].
Biomineralized iron architectures: adaptation to life at a redox interface. (A) SEM image of a Mariprofundus ferrooxydans PV-1 cell and biomineralized twisted stalk [Chan et al. 2016b]. (B) Differential interference contrast image of M. ferrooxydans growing in the presence of an oxygen gradient in a microslide experiment. The corresponding radial plot shows directional orientation of the stalks in the oxygen gradient [Krepski et al. 2013]. (C) SEM of the biomineralized sheath of Leptothrix ochracea [Chan et al. 2016b]. (D) Confocal image of a mat of sheath-forming cells. The cells are shown in green, while the Fe-(oxyhydr)oxides of the sheaths are shown in red. Living cells occupy the edge of the mat. Cell filaments and sheaths are mostly parallel to the growth direction of the mat (white arrow) [Chan et al. 2016a]. (E) SEM image of dreadlock-like iron structures produced by the pelagic Zetaproteobacterium strain CP-8. The biomineralized structures are easily shed, which may help cells maintain suspension in the water column. The likely location of the missing cell is denoted as a yellow oval. (F) Gradient tube cultures inoculated by Zetaproteobacteria strains CP-5 and CP-8, displaying distinct orange growth bands (white arrows). The cells occupy a narrow zone of the tubes, corresponding to optimal oxygen conditions [Chiu et al. 2017]. Images (A), (B), and (C) are reproduced with permission from John Wiley and Sons. Images (D), (E), and (F) are under Creative Commons Attribution License (CC BY).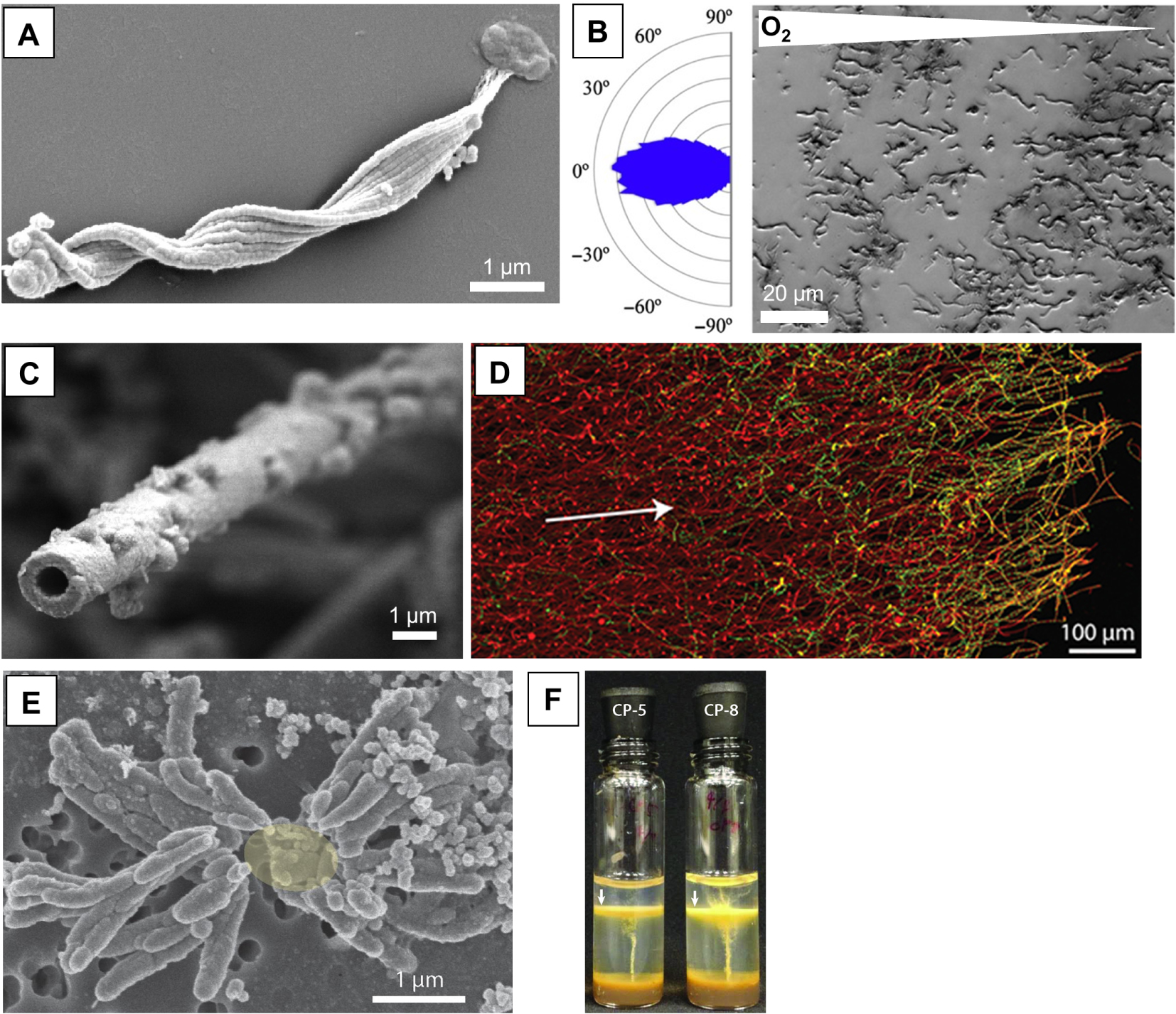
5.1. Extracellular biomineralized structures of microaerophilic iron oxidizers: adaptation to life at a redox interface?
Parallel to the diversity of metabolic pathways leading to Fe(II) oxidation, there is a variety of proposed functions of the Fe(III) biominerals formed as products of these microbial oxidation processes. The question of the biological functions of Fe biomineralization has often been raised in the case of microaerophilic iron oxidizers (Figure 3). Some of them direct Fe(III) precipitation onto organic extracellular structures, which may consist in tubular sheaths around the cells [e.g., the betaproteobacterium Leptothrix ochracea; Chan et al. 2009], twisted stalks [e.g., the freshwater betaproteobacterium Gallionella ferruginea or the marine zetaproteobacterium Mariprofundus ferrooxydans; Chan et al. 2011], or structures resembling “dreadlocks” [e.g., the Mariprofundus strains CP-5 and CP-8; Chiu et al. 2017] (Figure 3A,C,E). These extracellular structures are mostly composed of acidic polysaccharides and saturated aliphatic chains (probably lipids) [Chan et al. 2011, 2009; Laufer et al. 2017], and serve as templates for the nucleation of poorly crystalline Fe(III)-(oxyhydr)oxides. The organic structures direct Fe(III) precipitation away from the cells, preventing their encrustation by iron minerals [Chan et al. 2011], a function that is further aided by specific mineral-repelling properties of the cell surface such as low charge and hydrophilicity [SainiChan 2013]. In addition, sheaths and stalks appear to serve as attachment and support structures that help the microbes occupy niches where O2 and Fe(II) gradients overlap [Chan et al. 2016a], an important requirement for these microaerophilic iron oxidizers [Emerson et al. 2010; Maisch et al. 2019] (Figure 3D). Indeed, when Mariprofundus ferrooxydans is grown in opposing O2 and Fe(II) concentration gradients, their stalks, forming rigid holdfasts anchoring them to surfaces, grow directionally toward higher oxygen concentrations, allowing the bacteria (living at the end of stalks) to orient themselves within the redox gradient and colonize a narrow band with well-defined O2 and Fe(II) conditions [Krepski et al. 2013] (Figure 3B). On the other hand, the marine Zetaproteobacteria Mariprofundus strains CP-5 and CP-8 are pelagic, occupying the oxic–anoxic transition zone of a stratified estuary [Chiu et al. 2017], and thus do not appear to need anchoring or support structures. However, their iron “dreadlocks” can easily be shed (Figure 3E), which may help the cells maintain suspension and adjust buoyancy in the water column. The correlation between the morphology of biomineralized iron structures (sheaths and stalks versus “dreadlocks”) and microbial lifestyle (benthic vs. planktonic) reinforces the hypothesis that biomineralized structures of microaerophilic iron oxidizers are used by the cells to colonize environmental niches with adequate O2 and Fe(II) conditions [McAllister et al. 2019] (Figure 3F). Interestingly, we note here a convergence of functions with extracellular sulfur filaments produced by Candidatus Arcobacter sulfidicus, a microaerophilic sulfur oxidizer affiliated to Epsilonproteobacteria. These microorganisms thrive in deep-sea hydrothermal vents [Taylor and Wirsen 1997] and form mat-like biomineralized architectures at interfaces between oxygen and sulfide gradients, permitting retainment within their specialized niche [Sievert et al. 2007].
Another potential function of mineralized extracellular structures of microaerophilic iron oxidizers was proposed by Hallbeck and Pedersen [1995]. Using competition experiments between stalk-forming Galionella ferruginea and a spontaneous non-stalk forming mutant, they showed that the capacity of stalk formers to direct iron oxidation onto the stalks and away from the cells conferred them competitive advantage in iron-rich environments, by protecting them from toxic oxygen species (e.g., hydroxyl radicals) produced by the chemical reduction of oxygen by ferrous iron.
5.2. Other potential functions of extracellular iron biominerals
Potential functions of Fe(III) biominerals in other types of iron-oxidizing bacteria have more rarely been discussed. It has been proposed that biogenic ferrihydrite minerals forming at the surface of anoxygenic phototrophic Fe(II) oxidizers Rhodopseudomonas palustris and Rhodobacter ferrooxidans may act like “sunscreen”, protecting them from harmful UV radiation and DNA damage [Gauger et al. 2015]. Note that shielding from UV-induced DNA damage by minerals has also been suggested for silicifying cyanobacteria [Phoenix et al. 2006, 2001], although it remains to be formally proven that this protection is not coincidental but rather results from an adaptation to high-UV environments.
Although somewhat speculative, a possible benefit of Fe(III) biomineralization in general may be the establishment of an energy-generating proton motive force in the vicinity of the cells [Mansor and Xu 2020]. Indeed, as shown by Equations 1 and 2, Fe(II) oxidation and Fe(III)-oxyhydroxide precipitation release protons. Fe(III) biomineralization, if occurring close to the cells, may thus result in the formation of a proton gradient across the cell wall, which may be harnessed for ATP production.
| (1) |
| (2) |
While it has been proposed by Chan et al. [2004] for microaerophilic iron oxidizers, this mechanism may apply to any iron-oxidizing bacteria locating Fe(III) precipitation near the cell wall. However, it remains to be experimentally demonstrated that bacteria indeed harvest this proton motive force for energy generation.
Finally, an interesting, and probably underexplored function of Fe(III) biomineralization may be the establishment of syntrophic relationships between Fe-oxidizers and Fe-reducers. In a broad sense of the term, syntrophy can be used as a synonym for mutual co-feeding, i.e. a relationship where the metabolic product of a partner is used by the other partner as a metabolic substrate (and vice versa) [Morris et al. 2013]. Several studies have shown a coupling between Fe-oxidation and Fe-reduction in the environment, i.e. situations in which biogenic Fe(III) minerals produced by Fe-oxidizing bacteria are used as substrates for Fe-reducers [Blöthe and Roden 2009; Emerson et al. 2010]. Interestingly, experiments have shown that biogenic Fe(III) minerals are more easily colonized and reduced than abiogenic ones [e.g., Glodowska et al. 2021], suggesting that Fe-reducers may rely on Fe-oxidizers to supply bioavailable Fe(III) substrates in some environments. Such cooperative metabolic relationships may be particularly relevant to environments where Fe-reduction and Fe-oxidation processes spatially overlap [e.g., Laufer et al. 2016]. In some cases, Fe redox cycling may even occur within mineral particles containing both Fe(II) and Fe(III) [e.g., magnetite; Byrne et al. 2015], effectively acting as mineral “biogeobatteries” sustaining Fe-cycling bacterial communities [Peiffer et al. 2021]. It would be interesting to test whether metabolic relationships based on the formation and consumption of biogenic Fe(III) minerals fall within a stricter definition of syntrophy, i.e. an “obligately mutualistic metabolism” in which otherwise endergonic reactions become exergonic through the removal of reaction products by the other partner [Morris et al. 2013]. Biogenic magnetite has been shown to facilitate the (stricto sensu) syntrophic relationship between Fe-reducing Geobacter and methanogenic Methanosarcina, by promoting extracellular electron transfer between the two partners [Tang et al. 2016]. Other examples for the role of (potentially biogenic) Fe minerals in the mediation of electron transfers supporting metabolic networks are provided in the recent review by Mansor and Xu [2020].
5.3. Adaptation to mineralizing environments: avoiding iron encrustation
It is possible that in some cases, iron biominerals are just accumulated as metabolic waste products of ferrous iron oxidation and do not serve any other biological function. However, an absence of function (other than “waste”) does not necessarily imply a complete lack of control on mineral formation, since some iron oxidizers may have evolved strategies to locate mineral precipitation away from the cells, avoiding formation of Fe(III) minerals intracellularly or on the cell surface, which would be damaging to the bacteria. This control on mineral location is achieved through different strategies, evidenced in several phototrophic iron oxidizers [e.g., Schädler et al. 2009]. For instance, some bacteria produce extracellular polymeric fibers nucleating Fe(III) minerals [e.g., Rhodobacter sp.; Miot et al. 2009a], while others lower the pH around the cells, which decreases the rate of Fe(III) absorption to the cell surface and increases its solubility [e.g., Thiodictyon sp.; Hegler et al. 2010], or secrete Fe(III)-complexing EPS [e.g., Rhodovulum iodosum; Swanner et al. 2015; Wu et al. 2014]. On the one hand, encrustation of the cells by precipitation of Fe(III) minerals at the cell surface and/or within the periplasm* was frequently observed in the case of iron oxidizers performing heterotrophic or mixotrophic* nitrate reduction [e.g., Acidovorax sp. strain BoFeN1; Miot et al. 2009b; Schädler et al. 2009]. On the other hand, in chemolithoautotrophic nitrate-reducing Fe-oxidizers [e.g., the enrichment culture KS; Blöthe and Roden 2009], no Fe(III) encrustation is observed, possibly evidencing the existence of strategies to avoid cell entombment [Nordhoff et al. 2017]. There thus appears to be a divide between chemolithoautotrophic nitrate oxidizers, for which Fe(II) oxidation is an enzymatically catalyzed, energy-generating process, and which may have evolved adaptations to avoid the damaging effects of Fe(III) encrustation, and heterotrophic or mixotrophic nitrate oxidizers, for which Fe(II) oxidation does not generate energy, and for which such adaptations may not exist. However, Nordhoff et al. [2017] note that even heterotrophic nitrate oxidizers do not become encrusted under environmentally relevant, relatively low Fe(II) concentrations, or when these organisms are present in co-culture with autotrophic nitrate reducers, suggesting that Fe(III) entombment may not be a problem for nitrate-reducing bacteria in nature regardless of the mechanism.
6. Intracellular carbonates: a controlled biomineralization process with unidentified functions
Bacterial calcium carbonate precipitation is often considered a non-controlled, extracellular process. However, an increasing diversity of bacteria has been shown to biomineralize amorphous calcium carbonates (ACC) intracellularly [Segovia-Campos et al. 2021] (Figure 4). The giant uncultured sulfur-oxidizing Achromatium, affiliated to the Gammaproteobacteria, was the first bacterium identified among them [Schewiakoff 1893]. More recently, biomineralization of intracellular ACC was also discovered in several species of Cyanobacteria (Figure 4A–C) [Benzerara et al. 2014; Couradeau et al. 2012] as well as several undescribed members of the Alphaproteobacteria [Monteil et al. 2021; Liu et al. 2021]. Interestingly, the latter are magnetotactic bacteria, controlling the intracellular mineralization of magnetite crystals (Figure 4D). Some of these intracellularly calcifying bacteria had been studied before but their biomineralization capability had been overlooked, suggesting that intracellular ACC biomineralization may be much more widespread than presently acknowledged [Li et al. 2016a]. The capability to biomineralize intracellular carbonates is found in certain taxa only, and is sometimes shared by all the members of a clade, showing that it is a heritable trait [Benzerara et al. 2014].
Diversity and function of intracellular ACC biomineralization. (A) SEM image in the secondary electron (SE) mode of the cyanobacterium Neosynechococcus sphagnicola forming numerous ACC inclusions (brightest spots) together with polyphosphate granules (bright grey). (B) Overlay of Ca (red)and P (green) chemical maps obtained by energy dispersive X-ray spectrometry over the SEM image shown in (A). (C) SEM image of the cyanobacterium Synechococcus sp. PCC 6717 forming intracellular ACC mostly located at the poles of the cells. (D) SEM image in the SE mode of a magnetotactic alphaproteobacterium forming intracellular ACC. Two large ACC granules are observed as well as a small one close to the middle of the cell. Bright spots appearing in the bottom part of the cell correspond to a magnetite chain. (E) Cryo-transmission electron microscopy image of a cryo-ultramicrotomy section in a magnetotactic cell forming intracellular ACC. The ACC granule appears in dark and is surrounded by a thin feature which corresponds to a lipid bilayer. (F) Light microscopy image of an Achromatium oxaliferum cell. The refringent granules correspond to ACC. (G) SEM image of an Achromatium cell showing the numerous ACC granules paving the whole volume of the cell. (H) Diversity of the functions postulated for intracellular ACC in bacteria. One function (Fct 1) relates to buffering of intracellular pH upon carbon fixation by e.g., oxygenic photosynthesis and/or the use of inorganic C stored in ACC for C fixation. A second function (Fct 2) relates to intracellular pH buffering upon S-oxidation (e.g., in Achromatium and/or magnetotactic alphaproteobacteria). Oxidation of sulfides to elemental sulfur is associated with ACC formation. Oxidation of elemental sulfur to sulfates or reduction of elemental sulfur to sulfides are associated with ACC dissolution. Last, a third function (Fct 3) is related to cell buoyancy modification, allowed by the relatively high density of ACC compared to the rest of the cells. (Images: KB).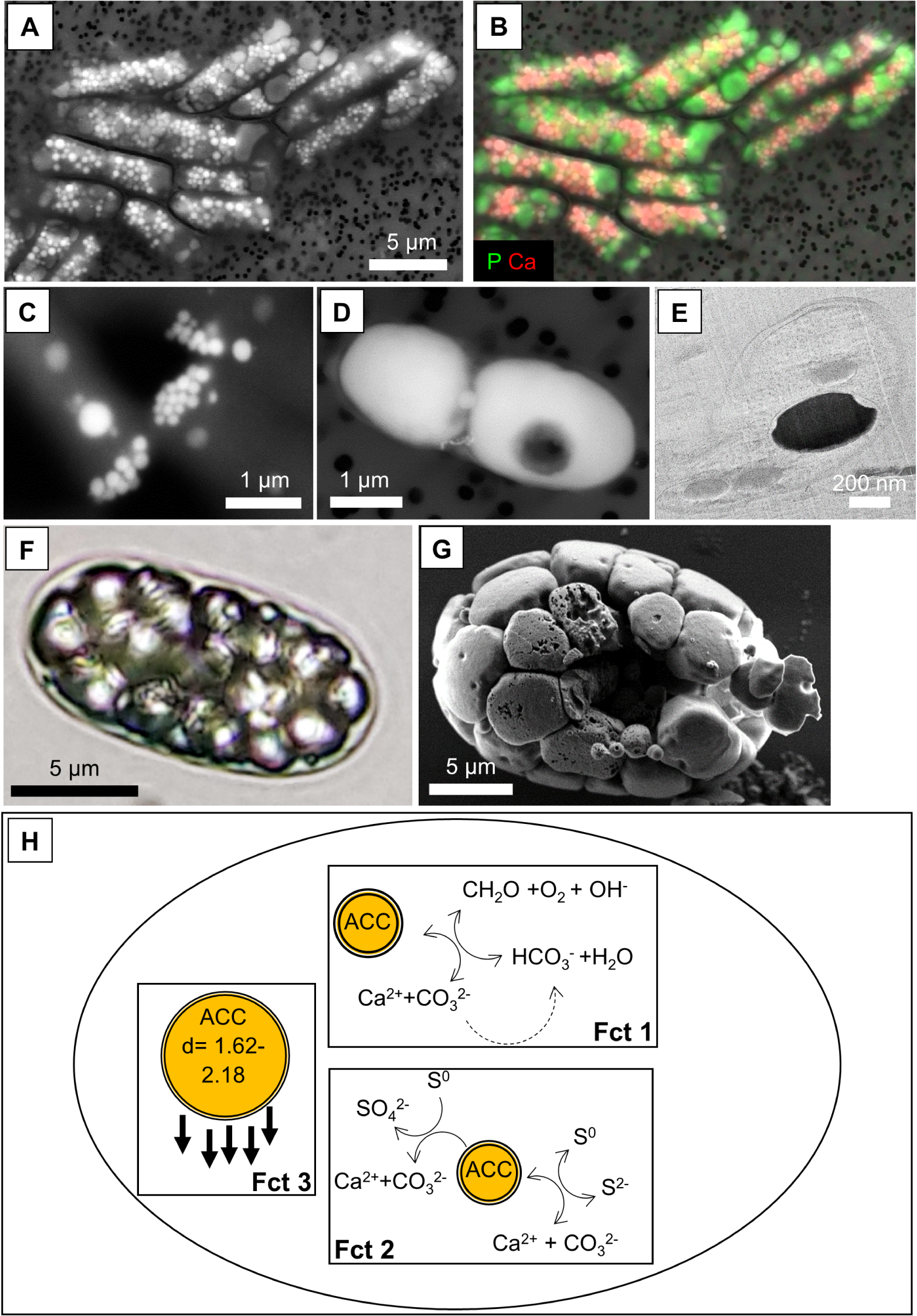
Cells may contain significant volumes or masses of intracellular ACC (Figure 4F,G), accounting for up to ∼2/3 of the total cell volume in Alphaproteobacteria and leading to a 12% cell density increase in the cyanobacterium Gloeomargarita lithophora. It has been argued that whatever the function of these biominerals is, intracellular ACC formation must impact cell physiology and/or ecology [Couradeau et al. 2012; Monteil et al. 2021].
Bacterial intracellular biomineralization of ACC can occur in undersaturated extracellular solutions, where carbonate precipitation would not occur otherwise [Cam et al. 2018; Head et al. 2000]. Biomineralization thus involves some energy expense (hence, it is an active process), possibly in relation with the transport of alkaline earth elements and/or C, increasing the saturation of the intracellular solution in contact with the nascent precipitates. It has been pointed out that intracellular ACC formation was not consistent with the chemical composition of a bacterial cytoplasm which is typically inferred as undersaturated with respect to calcium carbonate phases [Cam et al. 2015]. However, this apparent paradox is solved by the existence of dedicated intracellular compartments within the bacteria, where chemical conditions are appropriate for mineral precipitation without being detrimental to the functioning of the rest of the cells. The nature of the envelope delimiting these compartments differ in nature between phyla: an envelope composed of a protein shell or a lipid monolayer in Cyanobacteria [Blondeau et al. 2018b] vs. a lipid bilayer in Proteobacteria [Gray 2006; Monteil et al. 2021] (Figure 4E). This suggests that at least part of the process of intracellular ACC biomineralization may have evolved convergently* in different bacterial groups.
Apart from these facts, the molecular mechanisms of intracellular ACC formation remain currently unknown. Several of these bacteria contain RuBisCO or a homologous* gene and can fix inorganic carbon. Consistently, fixation of CO2 favors carbonate precipitation. However, this may not be a requirement since some Achromatium populations do not contain any of these genes, while being able to biomineralize ACC [Gray 2006]. Moreover, calcium transporters were targeted in the genomes of intracellular-ACC-forming bacteria as possible important actors but no specificity was particularly evidenced compared with bacteria not biomineralizing ACC [Wever et al. 2019]. The mechanism of the stabilization of ACC as a reactive amorphous solid remains to be understood. It may be due to confinement within intracellular compartments [Cavanaugh et al. 2019; Zeng et al. 2018]. The genes involved in the formation of the compartments remain to be identified in all these bacteria.
Intracellular carbonate phases formed by bacteria display several features that differ from those of abiotically precipitated carbonates. First, they are invariably amorphous and therefore unstable due to their high solubility relative to crystalline forms such as calcite or aragonite [Addadi et al. 2003]. Diverging conclusions were reached over time about the identity of the formed CaCO3 phase in Achromatium but the early identifications as an amorphous calcium carbonate phase were recently confirmed by Raman spectroscopy analyzes [Benzerara et al. 2021]. ACC is widespread among eukaryotic biominerals and it has been suggested that their high reactivity facilitates the homeostatic* function that was attributed to them [Addadi et al. 2003; Gower 2008]. Second, in some cases, intracellular carbonates formed by bacteria contain relatively high proportions of heavy alkaline earth elements such as Sr or Ba, indicating the existence of a fractionation mechanism favoring the uptake of these elements over Ca in the biominerals [Blondeau et al. 2018a; Cam et al. 2016; Mehta et al. 2019]. Part of the enrichment of ACC in trace elements might be related to some particle-size effects. However, overall, the different characteristics observed for intracellular ACC, in addition to an intracellular localization and the presence of an envelope around the carbonate inclusions, point toward a biologically controlled process in the classical definition of the term (see Section 2.1).
Several functions have been speculated for intracellular bacterial carbonates (Figure 4H). We note that these different potential functions are not exclusive and intracellular ACC biominerals may have varying functions in different microorganisms: (i) they may serve as a storage reservoir for inorganic C for C-fixating organisms. Some authors have also proposed that ACC may serve as an electron acceptor [Gray 2006]. As mentioned, some of these autotrophic organisms need large amounts of inorganic C for their metabolism. The large reactivity of ACC inclusions conferred by their non-crystalline structure, allowing a rapid remobilization, could be consistent with such a function. (ii) They may buffer intracellular pH variations, which are expected to be important in these bacteria due to their metabolisms. Achromatium is a S-oxidizing bacterium and can oxidize sulfide to S0 or S0 to sulfate. The first oxidation process consumes protons, while the second one produces protons. On the other hand, the formation of ACC produces protons, whereas its dissolution consumes protons. Consistently with a pH buffer role, ACC inclusions precipitate when cells oxidize sulfide to S0 and dissolve when cells are exposed to O2 and S0 is oxidized to sulfate [Yang et al. 2019]. Similarly, photosynthesizing bacteria tend to consume protons when fixing CO2 by RuBisCO and formation of ACC may contribute to pH buffering in addition to other molecular pH-buffering systems such as proton pumps [Görgen et al. 2021]. (iii) Last, it has been suggested that ACC inclusions, owing to their density (>2 g⋅cm−3), serve as ballasts for cells, and thus represent an adaptation to a benthic lifestyle [Couradeau et al. 2012; Gray and Head 2014; Monteil et al. 2021]. However, this has been questioned for some microorganisms based on ecological considerations showing an inverse relationship between the quantity of ACC and the depth within the sediments [Head et al. 2000].
Overall, although the genetic basis of intracellular calcium carbonate biomineralization is not known, it is likely that this is a controlled process which impacts cell fitness and has been selected by evolution. Yet, this example illustrates the difficulty to infer the genuine function of biominerals. The next section will describe some approaches and strategies that may be employed to make progress on this fundamental question.
7. A roadmap for functional studies of microbial biominerals
A deeper understanding of the functions of microbial biomineralization in a teleological view requires to shift our focus from descriptive and mechanistic (“How?”) to evolutionary and adaptive (“Why?”) questions. Importantly, it is crucial to discriminate biominerals which are adaptive traits, i.e. phenotypes* with genetically encoded (heritable) variation, which evolved through natural selection for a specific function, from non-adaptive ones, which are only the “unintended and uncontrolled consequence of metabolic activities” [Frankel and Bazylinski 2003]. Non-adaptive minerals may precipitate “accidentally” because of geochemical changes in the local environment, as a result of metabolism, or following heterogeneous nucleation on microbial surfaces or EPS, and do not serve any function that is beneficial to the cell. As we will explain below, in order to demonstrate that the formation of a biomineral is an adaptive trait, the first necessary (but not sufficient) step is to demonstrate that variation of this trait is genetically encoded (and therefore heritable). Secondly, it must be established that the biomineralization phenotype is beneficial to the organism, which means that it is associated with increased fitness (or reproductive success). Finally, hypotheses on the adaptive functions of the biominerals may be formulated and tested in the laboratory. We give further details on the different steps of this roadmap below (Figure 5).
A roadmap for functional studies of microbial biomineralization. The figure illustrates some of the experimental approaches described in the respective sub-sections of Section 7 (please refer to the main text for more details). Some drawings are adapted from Kobras et al. [2021] and McDonald [2019].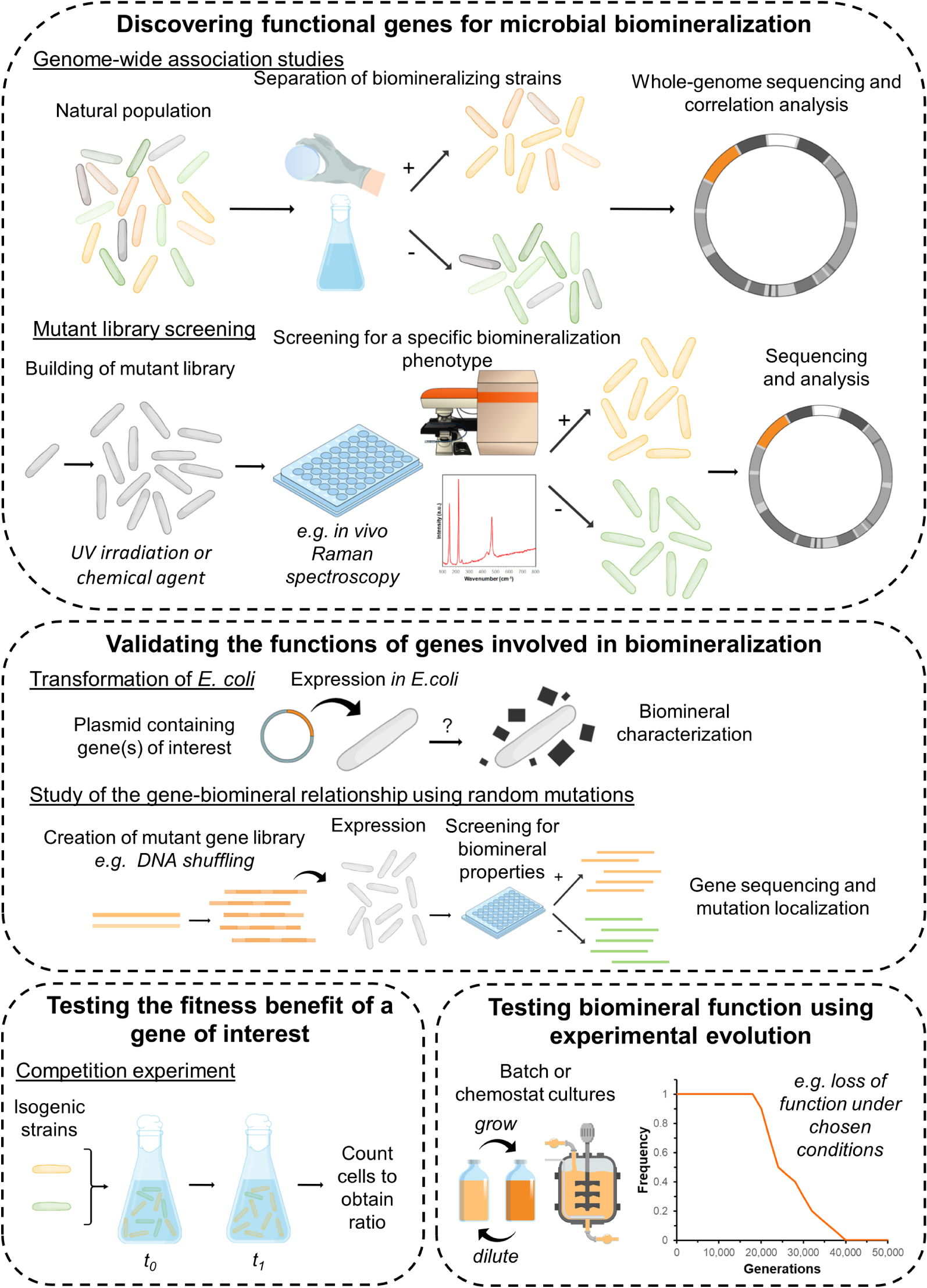
7.1. Discovering functional genes in microbial biomineralization systems
In recent decades, DNA sequencing techniques have allowed important progress in our understanding of the genetic mechanisms of microbial biomineralization. We have learned about specific genes involved in the formation of different biominerals [e.g., Keffer et al. 2021], discovered how they are distributed in the environment [e.g., Macalady et al. 2013], and how they may interact in complex networks in ecosystems [e.g., Hamilton et al. 2015]. However, there has been a clear bias toward the study of metabolic genes, involved for instance in energy-generating oxidation–reduction processes, which are often the primary drivers of microbial mineral formation. A functional understanding of biomineralization now requires turning our attention to genes that are involved in controlling biomineral properties such as morphology, structure, texture, chemical composition, or localization. Indeed, in the same way that the structures of proteins determine their functions, properties of adaptive biominerals are intimately linked with their biological functions (see for instance Section 2.1 for our description of the properties of intracellular biominerals in magnetotactic bacteria and their role in magnetotaxis). On the other hand, it is unlikely that cellular resources are spent to control mineral properties in the case of non-adaptive biominerals, i.e. minerals that are only formed as metabolic by-products or as a result of “accidental” precipitation.
Biomineralization processes typically involve a great number of genes acting together to control mineral nucleation and growth. These genetic pathways remain to be discovered for most microbial biomineralization systems. Microbiology approaches based on high-throughput sequencing techniques now allow to improve our understanding of the genetic basis of phenotypic variations, i.e. to determine how mutations (i.e. changes to encoding DNA sequences) can alter gene functions and phenotypes [Kobras et al. 2021]. These methods may now be applied to discover new genes involved in controlling the “mineral phenotype”. Genome-wide association studies (Figure 5) have been used to identify genes associated with traits of interest in natural microbial populations [Mallawaarachchi et al. 2021]. In these methods, genomes from hundreds of isolates from different environments are sequenced and compared to identify genomic elements that are statistically associated with a given phenotype [Chen and Shapiro 2015; ReadMassey 2014]. This comparative genomic approach can be used to understand the genetic basis of the production of some biominerals with specific properties. For instance, comparisons of whole genomes of stalk-forming and stalk-less microaerophilic iron oxidizers allowed for the identification of genes clusters involved in the formation of these organic-mineral structures in Betaproteobacteria and Zetaproteobacteria [Kato et al. 2015; Koeksoy et al. 2021]. As noted by Kobras et al. [2021], in the case of complex traits (such as biomineralization), genome-wide association studies may return thousands of genetic elements associated with the phenotype of interest, and it may be difficult to identify the role of individual genes and understand their interactions. Another important limitation of this approach is the need to assemble large genome databases comprehending both biomineralizing, poorly biomineralizing or non-biomineralizing bacteria, which often relies on culture-dependent approaches to test mineralization capabilities. Increasing use of single-cell genomic sequencing of environmental samples may facilitate this endeavor [e.g., Mansor et al. 2015]. Application of genome-wide association studies also requires that the genes of interest are relatively well conserved. Last but not least, this strategy relies on the assumption that the biomineralization capability is encoded by a same set of genes shared by the different microorganisms and does not result from convergent evolution, i.e. phylogenetically unrelated molecular systems.
It may be necessary to study the importance and functions of specific genes involved in biomineralization under more carefully controlled conditions. Other approaches may be employed to observe how small genetic differences in otherwise genetically identical (i.e., isogenic*) strains influence microbial phenotypes. Random chemical or physical (e.g., UV) mutagenesis can be used to generate mutant libraries of a specific microbial strain [e.g., Achal et al. 2009]. These libraries can then be screened to identify mutants with altered mineral phenotypes, and their whole genomes sequenced to locate the mutations and identify affected genes [e.g., Faivre and Baumgartner 2015; Rahn-Lee et al. 2015] (Figure 5). In the last decade, new methods based on transposon* insertion mutagenesis have been developed [Cain et al. 2020; vanOpijnen and Camilli 2013]. These methods require the construction of libraries of mutants for which most non-essential genes contain transposon insertions. After a screening process aimed at selecting mutants displaying the phenotype of interest, sequencing of the transposon–genome junctions allows for the determination of the insertion location of each transposon. Regions of the genome where transposon insertions are statistically underrepresented in selected mutants are likely to contain genes that are essential for the phenotype of interest. Whole-genome mutant libraries can also now be obtained using technologies based on CRISPR-Cas systems [e.g., Vo et al. 2021]. In the future, the more widespread use of these new technologies is likely to bring important progress in our understanding of the genotype–phenotype relationship in biomineralization systems.
We see that the methods described above rely both on high-throughput sequencing methods, allowing for the obtention and processing of large DNA sequence datasets, and high-throughput screening techniques, allowing for the selection of microbial strains displaying phenotypes of interest among environmental populations or mutant libraries. While falling costs have massively democratized sequencing approaches, screening may remain a major bottleneck for the implementation of next-generation microbiology methods to biomineralization studies. Indeed, analytical tools for the identification and characterization of biominerals classically include crystallography approaches such as X-ray diffraction, or microscopy techniques such as electron microscopy or X-ray (micro)spectroscopy [Cosmidis and Benzerara 2014; Miot et al. 2014], which require time-intensive sample preparation and cannot be used to process hundreds of samples at a time. There is thus a need for the development of high-throughput characterization methods for the characterization of biominerals, that could be implemented for instance directly in vivo, with minimal sample preparation, on microplates where different mutants or environmental strains have been separated. These methods would not only need to determine whether or not biominerals are present in each well of the microplate, but also to characterize the functional properties of these biominerals, such as morphology or crystal structure. Raman spectromicroscopy may be a very powerful tool in this context, since it allows for in vivo identification of biominerals and characterization of their structures at a micron of sub-micron scale [e.g., Nims et al. 2019]. Coupling spectroscopic analyzes with confocal microscopy imaging, data on mineral morphology (e.g., size distribution, aspect ratios) could be acquired simultaneously with mineralogical and crystallographic information in a single step. For intracellular biominerals, it may be possible to screen cells using flow sorting techniques based on the light-scattering or fluorescent properties of the biominerals [e.g., Bawazer et al. 2012]. Screening techniques based on more specific properties such as mineral magnetism may also be implemented [e.g., Liu et al. 2016].
7.2. Validating the functions of genes involved in biomineralization
Once genes involved in biomineralization have been identified, their specific role in controlling mineral formation and properties must be confirmed and refined. “Knockout”* studies consist in deleting or inactivating a gene of interest and investigating the effect of the gene loss by comparing the resulting mutant phenotypes with that of a “wild-type”* strain. This approach was for instance used to demonstrate that filaments formed by the actin-like protein mamK are crucial to align and position intracellular iron biominerals in magnetotactic bacteria. Indeed, while these biominerals are organized into a chain in the wild-type strain, they were randomly ordered in a mamK deletion mutant [Komeili et al. 2006; Scheffel et al. 2006]. However, many bacterial strains are currently not amenable to genetic manipulation [e.g., Chenebault et al. 2020; Corts et al. 2019]. Another possible approach consists in transforming* the model bacterium Escherichia coli, in order to test whether the expression of a gene (e.g. coding for an enzyme of interest) induces biomineralization [e.g., Bachmeier et al. 2002], considering that the rest of the necessary cellular machinery is available in this bacterium (Figure 5). The genetically engineered E. coli strains can then be used to study how altering different factors such as the activity of the recombinant enzyme [Heveran et al. 2019] or its cellular sublocation [Cosmidis et al. 2015] affects biomineralization. In some cases, it may be relevant to determine the intra- or extracellular localizations of the proteins of interest, and determine whether they coincide with biomineral localization. Separation of different cell fractions (e.g., cytoplasm, cell wall, membranes) followed by western blotting can be used to localize proteins at the sub-cellular level [e.g., Schüler 2004]. Protein localization can also be achieved using fluorescence microscopy after fusion with a fluorescent reporter, an approach that was for instance used to localize proteins involved in silica biomineralization in diatoms [Scheffel et al. 2011].
Fine details of the genotype–mineral phenotype relationship may be revealed through mutation and screening in synthetic biomineralization systems. Different methods such as error-prone PCR or DNA shuffling (i.e., random recombination* with a similar gene, or “molecular sex”) can be used to induce random mutations in the sequence of the gene of interest and obtain a mutant library. These mutant sequences may be expressed in genetically engineered microbial cells [Liu et al. 2016] or biomimetic vesicles [Bawazer et al. 2012] and screened for specific biomineral properties. Mutant sequences resulting in biominerals with altered properties can then be sequenced to localize the mutations and determine the relative importance of specific gene regions or amino acids in the biomineralization process (Figure 5).
7.3. How to determine whether a biomineral is beneficial?
The discovery of genes controlling the formation and properties of a biomineral is a useful step toward demonstrating that the biomineralization process is a functional, adaptive trait. However, it is not sufficient, since even the formation of non-adaptive minerals is encoded in pleiotropic genes. For instance, genetically encoded changes in EPS composition or abundance can alter the morphology or structure of (non-adaptive) minerals forming under the influence of these substances [Tourney and Ngwenya 2009; Yin et al. 2020]. In fact, a trait can only be considered to be adaptive if both its variation is genetically encoded (heritable trait), and if it helps an organism to maximize its fitness. In microbial experiments, fitness is often measured as a growth rate [Kussell 2013]. It is particularly useful to perform competition experiments, in which a bacterial strain expressing the trait of interest (here, certain property or a biomineral) is grown in co-culture (in a 50:50 mixture) with an isogenic strain for which this trait was suppressed or has a different value (e.g., for which the biomineral property of interest is altered). Measuring the relative growth rates of these two strains gives a quantitative measure of the benefit of the trait (the mineral property) with respect to the fitness of the bacteria (Figure 5). Competition experiments require both strains to be labeled, using for instance, fluorescent protein expression constructs, so that they can both be counted on plates or using flow cytometry. Obviously, determining the fitness benefits of biomineralization is complicated in the case where mineral formation is correlated with important metabolic functions (under the control of pleiotropic gene systems). For this reason, we stress on the fact that the trait we are proposing to test in these competition experiments is not mineral formation itself, but a specific property of the biomineral (for instance, a certain morphology of structure). It may also be useful to measure the relationship between fitness and certain quantitative values of the correlated traits among variable populations in order to disentangle their respective selection effects [Lande and Arnold 1983].
7.4. Testing the functions of microbial biominerals
Once it has been established that the formation of a biomineral with specific properties is an adaptation, we may formulate and test hypotheses regarding its biological function. This final step of our roadmap is difficult to describe in general terms, since the details are likely to vary significantly from one biomineral function to another. However, as a general rule, hypotheses on biomineral functions should preferably be formulated from field observations [e.g., Chan et al. 2016a], and tested under controlled conditions in the laboratory [e.g., Krepski et al. 2013]. Ideally, experiments should be conducted using adequate controls, such as isogenic strains that do not produce the biominerals [e.g., Gauger et al. 2015], or that produce biominerals with different properties or at different rates.
Microbial evolution experiments may be particularly useful to demonstrate biomineral functions, although, to our best knowledge, these approaches have yet to be used in this context. Microbial evolution experiments are designed to study how microbial populations adapt to different environmental pressures. These experiments consist in propagating microbial populations over a large number of generations (typically thousands or tens of thousands) under controlled conditions, allowing for numerous “rounds of evolution”, using for instance serial dilutions in batch cultures or continuous cultures in chemostats [Kussell 2013; Lenski et al. 1991; McDonald 2019] (Figure 5). The evolved strains can be sampled at different times throughout the experiments (which may be continued over many months or years) and compared with the founder strains, to identify new phenotypes and associated mutations through DNA sequencing. Microbial evolution approaches are thus also powerful tools for the discovery of new gene functions. Once a hypothesis on biomineralization function has been formulated, i.e. once it has been proposed that a biomineral with specific properties is an adaptation to a particular niche or lifestyle, then it should be possible to make predictions on how biomineralization should evolve when environmental conditions are altered. For instance, if the extracellular accumulation of bioavailable S0 is an adaptation to life under fluctuating redox conditions (Section 4.1), then it can be predicted that S0 biomineralization may be lost, or that S0 properties are altered in a way that reduces bioavailability, in a cell lineage evolving with a constant supply of reduced sulfur [through the accumulation of loss-of-function mutations; Hottes et al. 2013; McDonald 2019].
8. New roles for biominerals
Here we describe new conceptual frameworks for future functional studies of biomineralization. We consider prokaryotic biominerals as potential “public goods”, and interrogate their role in building biofilm architectures. While more speculative, this section describes how future research may be conducted within these frameworks to shine light on potential roles of biomineralization in microbial social interactions in natural biofilm habitats.
8.1. Are extracellular biominerals “public goods”?
When they are extracellular, biominerals are in principle available to microorganisms other than their producers, and their potential benefits are thus shared with neighboring cells. Cooperative behaviors in which a good produced by one individual is available to neighbors may be problematic when the production of this good comes with a physiological cost. Indeed, “cheating” nonproducers may emerge and rapidly outcompete producers, as they do not pay the fitness cost associated with production. Thus, it has to be explained how such individually costly cooperative behavior can be selected and sustained in a population, a puzzle often termed the “public good problem” in evolutionary biology [Smith and Schuster 2019]. Different types of extracellular substances produced by bacteria have been described as public goods, including siderophores [Kramer et al. 2020], digestive enzymes [Drescher et al. 2014], and EPS structuring biofilms [Nadell and Bassler 2011]. It may be interesting to figure out whether in certain systems, extracellular biomineralization may be considered as a public good (i.e., an exploitable and costly cooperative behavior), and, if so, how this trait can be maintained in microbial populations (i.e., how exploitation can be averted).
We do not expect all extracellular biominerals to be public goods. However, this concept may be useful for instance in the case of extracellular S0 biominerals, the formation of which seems to require the (costly) production of S0-stabilizing organics [e.g., Cron et al. 2019], and which may be used as energy resources by neighboring cells (Section 4). To fully demonstrate this, it would be necessary to show that biomineral production (or the control of certain biomineral properties, such as a metastable structure increasing bioavailability) carries a fitness cost, which can be achieved through competition experiments with isogenic nonproducers. Then, it would be interesting to determine how bacterial populations overcome the public good problem, i.e. how exploitation of the public goods is averted.
In order to remain evolutionarily stable against exploitation, the benefits of a cooperative behavior must be directed toward other cooperating individuals (as compared to toward “cheaters”). Bacteria may adopt different strategies to solve public good problems, for instance by privatizing the goods through uptake strategies that exclude competitive strains [e.g., Niehus et al. 2017], or through negative frequency-dependent selection mechanisms making cheating beneficial only when rare but not when common [Ross-Gillespie et al. 2007]. An important mechanism to avert exploitation of public goods consists in increasing the spatial structure of the population by producing biofilm-building EPS, which limits both the motility of cells and the diffusion of their goods. As a result, the distance over which production by one individual benefits its neighbors is reduced, and thus the benefits of the public goods are preferentially provided to nearby clonemates, who are more likely to be cooperative producers than cheaters. This mechanism has been described for bacterial public goods such as extracellular enzymes [Drescher et al. 2014] and siderophores [Julou et al. 2013], and is likely to be relevant in the case of extracellular biominerals which are poorly diffusible, and thus for which spatial segregation between producers and nonproducers is likely to have major effects on availability.
The study of biominerals as public goods, and more generally all “social” aspects of microbial biomineralization, are topics that remain largely unexplored. Given the importance of population spatial structure for the evolution of cooperation and competition in natural communities, it is unfortunate that microbial biomineralization experiments are in the immense majority of cases performed in liquid cultures, where bacterial populations are uniformly mixed, and thus where structure is nonexistent. Future studies on the adaptive functions of microbial biominerals may thus benefit from working with colonies growing on agar plates or on mineral surfaces. Aside from any consideration of social behaviors, biofilms are a major form of bacterial habitat [Hall-Stoodley et al. 2004; Nadell et al. 2016], where many biomineralization processes actually occur in nature [e.g., Cron et al. 2021]. In the next section, we will discuss the idea that some functions of biominerals may be specific to microbial populations living in biofilms.
8.2. Biominerals and biofilm architecture
As explained in the previous section, it is important that questions around the roles of biominerals in cooperation and competition behaviors are investigated using experiments with microbial biofilm communities rather than liquid cultures, given the importance of surface adhesion and population structure in such interactions [Kees et al. 2021; Kim et al. 2014; Nadell et al. 2016; Schluter et al. 2015]. Moreover, as speculated by Phoenix and Konhauser [2008], some possible benefits of biominerals may mirror those afforded by EPS in biofilms, such as screening from detrimental radiation, physical protection from grazing or desiccation, and participation in biofilm strength and structural integrity. However, an advantage of biominerals over EPS would be that they can be produced at a smaller energetic cost [Phoenix and Konhauser 2008].
Roles of extracellular CaCO3 biomineralization in biofilms. (A) Competition experiment in a dual-species biofilm between non-biomineralizing P. aeruginosa (fluorescently stained in green) and biomineralizing P. mirabilis (fluorescently stained in red), expressing the urease. The confocal fluorescence micrographs show the distribution of CaCO3 minerals (blue) as well as of both types of cellsduring the experiments in the presence of a non-buffered medium, where CaCO3 biomineralization can occur (left), or in a buffered medium where CaCO3 precipitation is inhibited (right). When it can occur, biomineralization is responsible for the fitness advantage of P. mirabilis in the biofilm [Li et al. 2016a]. Figure reproduced with permission from Oxford University Press. (B) B. subtilis colonies and cross-sections of colonies after application of a fluorescein dye. The colonies were grown either with (+Ca) or without (−Ca) calcium (left and middle panels). The colonies on the right panel were grown in the presence of acetohydroxamic acid (AHA), a known inhibitor of the urease enzyme. Precipitation of CaCO3 minerals, mediated by urease activity, is crucial to form the complex structure of the biofilm and creates a diffusion barrier sheltering the inner cell mass of the colony. Scale bars: 0.5 mm [Keren-Paz et al. 2018]. Figure under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Extracellular precipitation of calcium carbonates in microbial mats has been extensively studied by geobiologists, in part due to its role in the formation of microbialites and stromatolites [e.g., Couradeau et al. 2013; Ferrer et al. 2021], which are important archives of microbial life on early Earth [Bosak et al. 2013]. The primary driver for calcification in biofilms is the local increase in calcium carbonate supersaturation, which may result from specific microbial metabolisms, for instance, photosynthesis, ureolysis, or sulfate reduction [e.g., Saghaï et al. 2016; White et al. 2016]. This active, biologically induced CaCO3 mineralization process occurs concurrently with passive binding of Ca2+ ions on the EPS matrix of the biofilm, which may favor CaCO3 heterogeneous nucleation, and influence mineral properties such as morphology, size, or structure [Dupraz et al. 2009; Görgen et al. 2021; Lyu et al. 2020]. Interestingly, it is now recognized that microbially induced CaCO3 precipitation may not be limited to heavily calcifying, mat-building environmental communities such as those forming stromatolites, but can also occur in biofilms of bacteria previously studied as pathogens in a medical context [e.g., Pseudomonas aeruginosa or Proteus mirabilis; Li et al. 2016a, 2015]. A variety of both gram-negative and gram-positive bacteria can induce calcium carbonate precipitation due to their ureolytic activity [the enzymatically catalyzed hydrolysis of urea, which locally increases pH and favors CaCO3 supersaturation; Castro-Alonso et al. 2019; Görgen et al. 2021]. A growing body of literature now suggests that CaCO3 minerals may be a previously overlooked component of the extracellular matrix of microbial biofilms containing ureolytic bacteria [reviewed in Keren-Paz and Kolodkin-Gal 2020] (Figure 6). Different lines of evidence suggest that this mechanism confers important fitness benefits to the bacteria, as shown for instance by competition experiments using dual-species biofilms [Li et al. 2016b] (Figure 6A), or studies using defective mutants [Oppenheimer-Shaanan et al. 2016]. The extracellular CaCO3 minerals are indeed thought to form a framework structuring and strengthening the biofilm, and protecting the bacteria from antibiotics and other harmful environmental substances [Dade-Robertson et al. 2017; Keren-Paz et al. 2018] (Figure 6B). CaCO3 precipitation may also be used as a detoxification mechanism preventing the accumulation of CO2 in non-photosynthetic biofilms [Oppenheimer-Shaanan et al. 2016]. It thus appears that it may now be time to reconsider extracellular calcification not as an “unintended” consequence of microbial metabolisms and passive Ca2+ adsorption onto EPS, but as an adaptive process and important feature of the extracellular matrix of biofilms, playing a crucial role in bacterial fitness and interspecies competition [Keren-Paz and Kolodkin-Gal 2020].
9. Conclusions
We have reviewed functional aspects of several prokaryotic biomineralization systems (see a summary in Table 2), and described approaches that may be used to discover or test new functions of biominerals. Many “ultimate” questions around microbial biomineralization remain to be understood. Fundamentally, it is still not clear in many systems whether biomineralization increases an organism’s fitness, and whether producing biominerals with specific properties contributes to adaptation to certain environmental conditions. Moreover, we note that the term “function” should be taken with caution as it encompasses diverse meanings. As we have seen here, these questions can be addressed through experimentation, but the use of these experimental approaches often requires prior understanding of the genetic pathways controlling biomineral formation and properties. It should be noted that genes controlling biomineralization may not always be found, especially in microbial species that are not amenable to culture or genetic manipulation. Moreover, the idea of specific genes for biomineralization should also be viewed as too restrictive or misleading, especially in cases when these genes are pleiotropic. Despite these difficulties, a deeper knowledge of these genetic controls is crucial to allow us to reconstruct the phylogenetic and evolutionary history of microbial biomineralization, which, in conjunction with a better understanding of the geological record, will assist efforts to decipher the history of life co-evolution with its mineral environment.
Summary of biomineral functions described in this review
Function | Biomineral example | Reference |
|---|---|---|
Protection: | ||
From UV radiation | Silica | |
Fe(III)-(oxyhydr)oxides | Gauger et al. [2015] | |
From grazing | ? (hypothetical) | Phoenix and Konhauser [2008] |
From dehydration | ? (hypothetical) | Phoenix and Konhauser [2008] |
From encrustation | Fe(III)-(oxyhydr)oxides | |
From antibiotics | Extracellular carbonates | |
Detoxification: | ||
Of reactive oxygen species | Magnetite, greigite | |
Fe(III)-(oxyhydr)oxides | Hallbeck and Pedersen [1995] | |
Of organic toxins | Clays | |
Of toxic metals | Metal nanoparticles | Hulkoti and Taranath [2014] |
Of CO2 | Extracellular carbonates | Oppenheimer-Shaanan et al. [2016] |
Storage: | ||
Of nutrients | Iron phosphates | Konhauser et al. [1994] |
Clays | ||
Of energy (electron donors or acceptors) | Elemental sulfur | Dahl [2020]; numerous references in the present review (Section 4) |
Of inorganic carbon | Intracellular carbonates | This review (Section 6) |
Structure: | ||
Biofilm strength and integrity | ? (hypothetical) | Phoenix and Konhauser [2008] |
Attachment and support | Fe(III)-(oxyhydr)oxides | Chan et al. [2016a] |
Magnetotaxis | magnetite, greigite | |
Positioning in redox gradients | Fe(III)-(oxyhydr)oxides | |
Elemental sulfur | Sievert et al. [2007] | |
Buoyancy adjustment | Fe(III)-(oxyhydr)oxides | Chiu et al. [2017] |
Intracellular carbonates | Couradeau et al. [2012], Gray and Head [2014], Monteil et al. [2021] | |
Interspecific interactions: | ||
Symbiosis | magnetite | Monteil et al. [2019] |
Syntrophy | Elemental sulfur | Warthmann et al. [1992] |
Magnetite | Tang et al. [2016] | |
Fe(III)-(oxyhydr)oxides | ||
Public goods | ? (hypothetical) | This review (Section 8.1) |
Competition in biofilms | Extracellular carbonates | Li et al. [2016a] |
Intraspecific interactions: | ||
Cooperative use of resources | Elemental sulfur | |
Extracellular electron transfer | Conductive or semi-conductive iron nanoparticles | |
Establishment of an energy-generating proton motive force | Fe(III)-(oxyhydr)oxides | |
pH homeostasis | Intracellular carbonates | This review (Section 6) |
Among emerging questions around the functions of microbial biomineralization, it will be particularly interesting to address the roles of biominerals in “social” interactions between prokaryotes, at both intra- and interspecific levels, in particular in biofilm habitats. Indeed, cooperative formation and utilization of biominerals may be important to sustain microbial communities in the environment. From a practical point of view, new knowledge on adaptive aspects of microbial biomineralization may be used in the future to develop and improve microbial bioremediation strategies [Gadd 2010], identify therapeutic targets to fight against pathogens [e.g., Keren-Paz et al. 2018], or design new biomaterials through directed evolution approaches [e.g., Liu et al. 2016].
As we shift our focus from “proximate” to “ultimate” questions, we may consider revisiting the classical framework used to categorize biomineralization processes (Section 2). An issue with the “controlled”/“induced”/“influenced” categories is that they combine mechanistic and adaptive aspects of biomineralization. Indeed, while “controlled” biomineralization is sometimes used as a synonym for adaptive biomineralization (i.e., a trait with variation under genetic control, which serves a specific function that increases fitness), “induced” and “influenced” biomineralization processes refer to the mechanisms leading to mineral precipitation (respectively: increased saturation due to metabolic activity, or mineral nucleation on organic surfaces). The variation of the latter two processes may or may not be under genetic control (and therefore, be heritable traits), and they may or may not be adaptive and increase fitness, leading to blurry boundaries between the three classical categories. For instance, until recently, extracellular CaCO3 precipitation as a result of the activity of the urease enzyme may have been viewed as a typical example of “induced” biomineralization. However, ureolytic CaCO3 biomineralization is clearly a heritable trait under genetic control [consistently, an E. coli strain transformed with the urease genes of an ureolytic Bacillus pasteurii strain acquires strong biomineralization capabilities; Bachmeier et al. 2002]. Moreover, with the discovery of important fitness benefits of extracellular CaCO3 biominerals due to their roles in interspecies competition, biofilm structure, and limitation of antibiotics diffusion [Keren-Paz and Kolodkin-Gal 2020; Li et al. 2016a; Oppenheimer-Shaanan et al. 2016] (Figure 6), ureolytic calcium carbonate biomineralization can be described as an adaptation to certain biofilm habitats. Often, the use of “controlled” biomineralization conveys the idea that the properties of the biominerals are tightly regulated by genes, so that the biominerals differ (e.g., in morphology, structure, or composition) from equivalent minerals precipitated through abiotic processes under similar conditions [Frankel and Bazylinski 2003]. However, it may be argued that this is also likely to be the case of any mineral precipitating in the presence of organic substances [e.g., Cosmidis et al. 2019]. Unusual mineral properties thus do not say anything about whether or not biomineralization is adaptive. Furthermore, when tight control on biomineral properties indeed exists, it operates through microbially induced changes in mineral saturation and/or under the templating effect of organic matrices, further blurring the lines between “controlled”, “induced”, and “influenced” classical biomineralization categories.
We propose that microbial biomineralization processes should be described either according to their adaptive value (e.g., adaptive or maladaptive*, based on whether or not they increase the organism’s fitness), or according to their mechanism (induced and/or influenced biomineralization, both of which may occur concurrently, and in both adaptive and maladaptive processes). Actually, in cases where biomineralization results from the activity of a pleiotropic gene (such as the gene coding for the urease, which is involved in both C and N metabolism and CaCO3 precipitation), biomineralization may be described as an exaptation*, i.e. a feature that now increases fitness but was not built by natural selection for its current goal [Gould and Vrba 1982]. Further complicating this categorization problem, the adaptive value of some biominerals may depend on the environment (i.e., biomineralization may increase or decrease fitness based on external conditions).
Overall, we hope that this review, rather than providing definitive answers to the fundamental question “why do microbes make minerals?”, participates in renewing curiosity for this interesting issue, and provides some foundation for future studies focussing on this topic.
Conflicts of interest
Authors have no conflict of interest to declare.
Acknowledgments
Karim Benzerara thanks the French National Research Agency (HARLEY: ANR-19-CE44-0017) for financial support. Both authors thank Emmanuelle Porcher for helpful comments and suggestions on the manuscript.




 CC-BY 4.0
CC-BY 4.0