1. Introduction
Metazoa first appeared in the fossil record during the Ediacaran Period. A variety of paleoenvironmental proxy records suggest a possible association of metazoan evolution with a perturbation in the redox structure of the atmosphere and ocean basins [Canfield et al. 2007; Fike et al. 2006; McFadden et al. 2008]. Specifically, the Shuram excursion, the largest negative carbonate carbon isotope excursion (δ13Ccarb) in the sedimentary record, is observed globally in strata of this age [e.g., Burns and Matter 1993; Grotzinger et al. 2011; Le Guerroue et al. 2006a]. The Shuram excursion has been suggested to represent a major perturbation in the marine carbon cycle and concomitant perturbation to the redox structure of the fluid Earth [e.g., Fike et al. 2006].
However, several aspects of the Shuram excursion (most notably, frequent covariation of δ13Ccarb and δ18Ocarb isotope ratios and the lack of a similar excursion in organic carbon isotopes (δ13Corg) in strata with low total organic carbon (TOC) abundance), have resulted in suggestions that this stratigraphic pattern instead resulted from post-depositional processes, ranging from interaction with meteoric fluids [Knauth and Kennedy 2009; Swart 2008] to late-stage burial diagenesis [Derry 2010a,b]. Each of these alteration hypotheses fails to explain the widespread occurrence of the Shuram excursion (and its correlatives) during mid-Ediacaran time, as these diagenetic processes are intrinsically local—and not temporally restricted to mid-Ediacaran age strata. A third mechanism—global diagenesis [Grotzinger et al. 2011]—combines aspects of each of these hypotheses. It differs, however, in that this hypothesis is fundamentally driven by a global and synchronous change in the redox composition of the sediments (toward a more oxidizing bulk composition) at the time of deposition, resulting in a sedimentary reactor that was preconditioned to produce a widespread δ13Ccarb excursion of similar magnitude in basins around the world [Calver 2000; Fike et al. 2006; Kaufman et al. 2007; McFadden et al. 2008].
In recent years, there is increasing evidence that the δ13C signature of the Shuram was acquired at the time of deposition. While, the general lack of covariation between δ13Ccarb and bulk δ13Corg is also seen in coeval high TOC sections [Lee et al. 2013], certain lipid biomarker classes record a smaller magnitude excursion in parallel with that observed in δ13Ccarb, supporting a primary origin for the Shuram excursion [Lee et al. 2015]. Further, detailed stratigraphic analysis from the equivalent sections in Australia [Husson et al. 2012] and Death Valley [Bergmann et al. 2011] also provide evidence that the δ13Ccarb excursion was acquired at the time of deposition, a conclusion further supported by subsequent Ca and Mg isotope analyses [Husson et al. 2015]. The mechanism to generate such low δ13Ccarb values, however, still remains enigmatic.
Iron isotope ratios provide a useful framework in which to further examine the Shuram excursion. Hydrothermal and weathering inputs are the main sources of Fe delivered to marine sediments and have δ56Fe values near 0‰ [Beard et al. 1999; Chever et al. 2015; Dauphas et al. 2017; Homoky et al. 2013]. Although many processes (e.g., reduction, oxidation, mineral precipitation) fractionate iron isotopes, it is the fractionations commonly observed between Fe(II) and Fe(III) that are thought to be dominant in sedimentary environments [Johnson et al. 2008]. The magnitude of this fractionation is approximately the same whether the transitions are biologically or abiotically mediated, by kinetic or equilibrium processes [Dauphas and Rouxel 2006]. The bacterial reduction of Fe(III) produces Fe(II) that is depleted in δ56Fe by ∼2‰ relative to the initial source of Fe [Beard et al. 1999]. Whereas Fe(III) is insoluble at typical pH values for marine waters and sediments, Fe(II) is highly soluble in anoxic waters. This 56Fe-depleted Fe(II) is mobile and its migration into or out of sediments can result in isotopic shifts to the sedimentary iron pool, driving it away from typical crustal values of ∼0.1‰.
Here, we present iron isotope ratio data from Nafun Group strata, Sultanate of Oman to better understand the depositional and diagenetic history of these sediments and the causative mechanism(s) for the Shuram excursion. Because the strata spanning the Shuram excursion are lithologically heterogeneous, we specifically measured the iron associated with the carbonate phase (Fecarb). Only Fe(II) is partitioned in any abundance into the carbonate mineral lattice [Reeder 1983] and since ferrous iron is soluble under anoxic conditions, analysis of δ56Fecarb provides insights into the redox conditions associated with the deposition and subsequent diagenesis of the carbonate fraction.
Replicated δ56Fecarb measurements of MQ1 3740 and MQ1 3852.
2. Materials and methods
We analyzed subsurface cuttings samples of Nafun Group strata from well MQR-1, which have been previously characterized in detail regarding their sedimentary geology, and carbon and sulfur isotopic composition [Burns and Matter 1993; Fike et al. 2006]. Cuttings are rock chips (typically 1–5 mm in size) produced during drilling that were collected by the well-site geologist every 2–5 m. Thus, cuttings represent a geochemical and lithologic average, which has the tendency to smooth high-frequency variations sometimes observed in stratigraphic time series data [Fike and Grotzinger 2008].
Fe isotopic data for Nafun Group strata
| Sample names | Depth | δ56Fe | δ57Fe |
|---|---|---|---|
| MQ1 3240 | 3240 | 0.00 | 0.04 |
| MQ1 3260 | 3260 | 0.07 | 0.16 |
| MQ1 3300 | 3300 | 0.11 | 0.04 |
| MQ1 3320 | 3320 | − 0.02 | − 0.05 |
| MQ1 3350 | 3350 | − 0.02 | − 0.13 |
| MQ1 3360 | 3360 | 0.09 | 0.20 |
| MQ1 3404 | 3404 | 0.07 | 0.19 |
| MQ1 3428 | 3428 | 0.00 | − 0.16 |
| MQ1 3510 | 3510 | − 0.11 | 0.02 |
| MQ1 3530 | 3530 | − 0.37 | − 0.67 |
| MQ1 3556 | 3556 | − 0.55 | − 0.71 |
| MQ1 3600 | 3600 | − 1.05 | − 1.55 |
| MQ1 3620 | 3620 | − 0.77 | − 1.22 |
| MQ1 3636 | 3636 | − 0.94 | − 1.35 |
| MQ1 3660 | 3660 | − 0.57 | − 1.00 |
| MQ1 3700 | 3700 | − 0.38 | − 0.41 |
| MQ1 3722 | 3722 | − 0.29 | − 0.48 |
| MQ1 3740a | 3740 | − 0.27 | − 0.62 |
| MQ1 3740b | 3740 | − 0.31 | − 0.36 |
| MQ1 3740c | 3740 | − 0.31 | − 0.57 |
| MQ1 3756 | 3756 | − 0.29 | − 0.16 |
| MQ1 3780 | 3780 | − 0.15 | − 0.09 |
| MQ1 3800 | 3800 | − 0.11 | − 0.12 |
| MQ1 3806 | 3806 | − 0.26 | − 0.27 |
| MQ1 3810 | 3810 | − 0.44 | − 0.66 |
| MQ1 3826 | 3826 | 0.10 | 0.16 |
| MQ1 3832 | 3832 | 0.05 | − 0.08 |
| MQ1 3848 | 3848 | 0.04 | 0.02 |
| MQ1 3852a | 3852 | 0.02 | − 0.16 |
| MQ1 3852b | 3852 | − 0.01 | 0.06 |
| MQ1 3860 | 3860 | 0.00 | − 0.03 |
| MQ1 3880 | 3880 | − 0.09 | − 0.23 |
| MQ1 3900 | 3900 | 0.06 | 0.00 |
| MQ1 3920 | 3920 | 0.11 | 0.16 |
| MQ1 3926 | 3926 | 0.04 | 0.11 |
| MQ1 3932 | 3932 | 0.05 | 0.12 |
| MQ1 3936 | 3936 | 0.06 | 0.00 |
| MQ1 3948 | 3948 | − 0.15 | − 0.40 |
| MQ1 3970 | 3970 | 0.08 | 0.04 |
| MQ1 3984 | 3984 | 0.10 | 0.12 |
| MQ1 4000 | 4000 | − 0.03 | − 0.11 |
Fe isotopic compositions (δ56Fe) of the carbonate fraction, reported in permil (‰) relative to the terrestrial standard IRMM014. Note: a, b and/or c indicate laboratory replicates (dissolution, chemical purification, and mass-spectrometry analysis) of a given sample. The precision of these independent replicates is in the same range as the reproducibility of the standard (2𝜎 = 0.05‰ for δ56Fe) and is much smaller than the range of isotopic fractionation (1‰) observed in this study.
Powdered samples of ∼150 mg were dissolved in 10 mL of 10% cold acetic acid in an ultrasonic bath for about two hours. The solution was centrifuged and the procedure repeated a second time on the solid residue. Fe was purified by anion-exchange chromatography using the procedure described by Wang et al. [2012]. Iron isotopic compositions were measured on a Thermo Scientific Neptune MC-ICP-MS as described by Wang et al. [2012]. Reproducibility of the full analytical protocol has been tested by independent analysis of several aliquots from the same powder for multiple samples (e.g., sample MQ1 3740 in triplicate and MQ1 3852 in replicate; Figure 1). For these samples, the δ56Fe of the replicates are in agreement within 0.05‰ (2𝜎, see Table 1), which is the same order of magnitude as the long-term reproducibility of known standard solutions. On the other hand, replicated measurements of δ57Fe return only a 2SD of 0.30‰. When using these errors all the samples fall on a mass-fractionation line with the expected slope 1.5 in a δ57Fe vs δ56Fe plot (Figure 2). Given the mass-dependency relationship and the better reproducibility of the δ56Fe, we will discuss all the data in terms of δ56Fe.
Triple isotope diagram (δ57Fe vs δ56Fe) for all the samples analyzed here. All the samples fall on a mass-fractionation line of slope 1.5.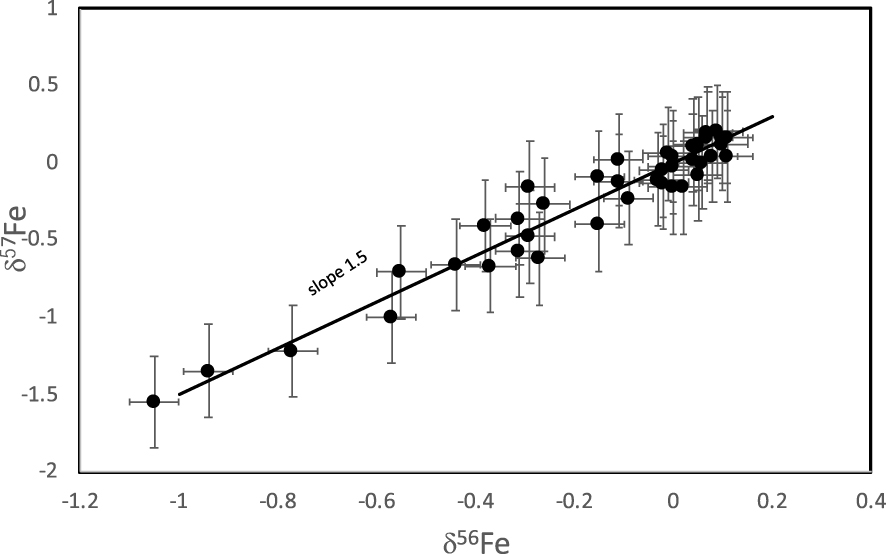
3. Results
Nafun Group sediments analyzed in the present study were deposited from 635–548 Ma in a regionally extensive sag basin under open, shallow marine conditions, and each formation can be traced laterally for several hundred km across Oman [Bowring et al. 2007; Grotzinger et al. 2002; Le Guerroue et al. 2006a; Mattes and Conway-Morris 1990; McCarron 2000]. The strata comprise two clastic-to-carbonate shallowing-upward successions (Masirah Bay Formation (Fm.) and Khufai Fm.; Shuram Fm. and Buah Fm.). The Shuram excursion spans several hundred meters of section from the uppermost Khufai Fm. through Shuram Fm. and into the mid-Buah Fm. [Burns and Matter 1993; Fike et al. 2006; Le Guerroue et al. 2006b]. The Shuram excursion has been identified in multiple sections, both from outcrops and the subsurface, and serves as an excellent stratigraphic marker for correlation across Oman [Burns and Matter 1993; Le Guerroue et al. 2006a; McCarron 2000].
Nafun Group strata have δ56Fecarb values that range between − 1.05‰ to + 0.24‰ and preserve a record of stratigraphically coherent variations with small scatter between successive sample data points (Figure 3). Masirah Bay and Khufai strata possess δ56Fecarb isotopic compositions typical of bulk crustal materials (∼0.1‰). However, a progressive drop in δ56Fecarb values, down to − 1.05‰ characterizes the stratigraphic interval of the Shuram excursion. This δ56Fecarb minimum is stratigraphically offset from the nadir of the δ13Ccarb excursion. As the δ13Ccarb excursion ends, there is a gradual return to “typical” terrestrial δ56Fecarb values (∼0.1 −−0.2‰), which are retained in the overlying Buah strata.
Isotopic and geochemical data from well MQR-1 through the Nafun Group strata, plotted alongside a stratigraphic column. Data shown are δ13Ccarb, δ34SSO4 (carbonate-associated sulfate), TOC (wt%), δ56Fecarb, FeP∕FeT (iron in pyrite relative to total iron), and total iron and titanium (at 20 × abundance), both in wt%. The red dashed lines indicate bulk terrestrial δ56Fe (0.1‰). Age dates come from correlation based on δ13Ccarb chemostratigraphy [Bowring et al. 2007; Fike et al. 2006]. Carbon and sulfur isotope and TOC data are from Fike et al. [2006].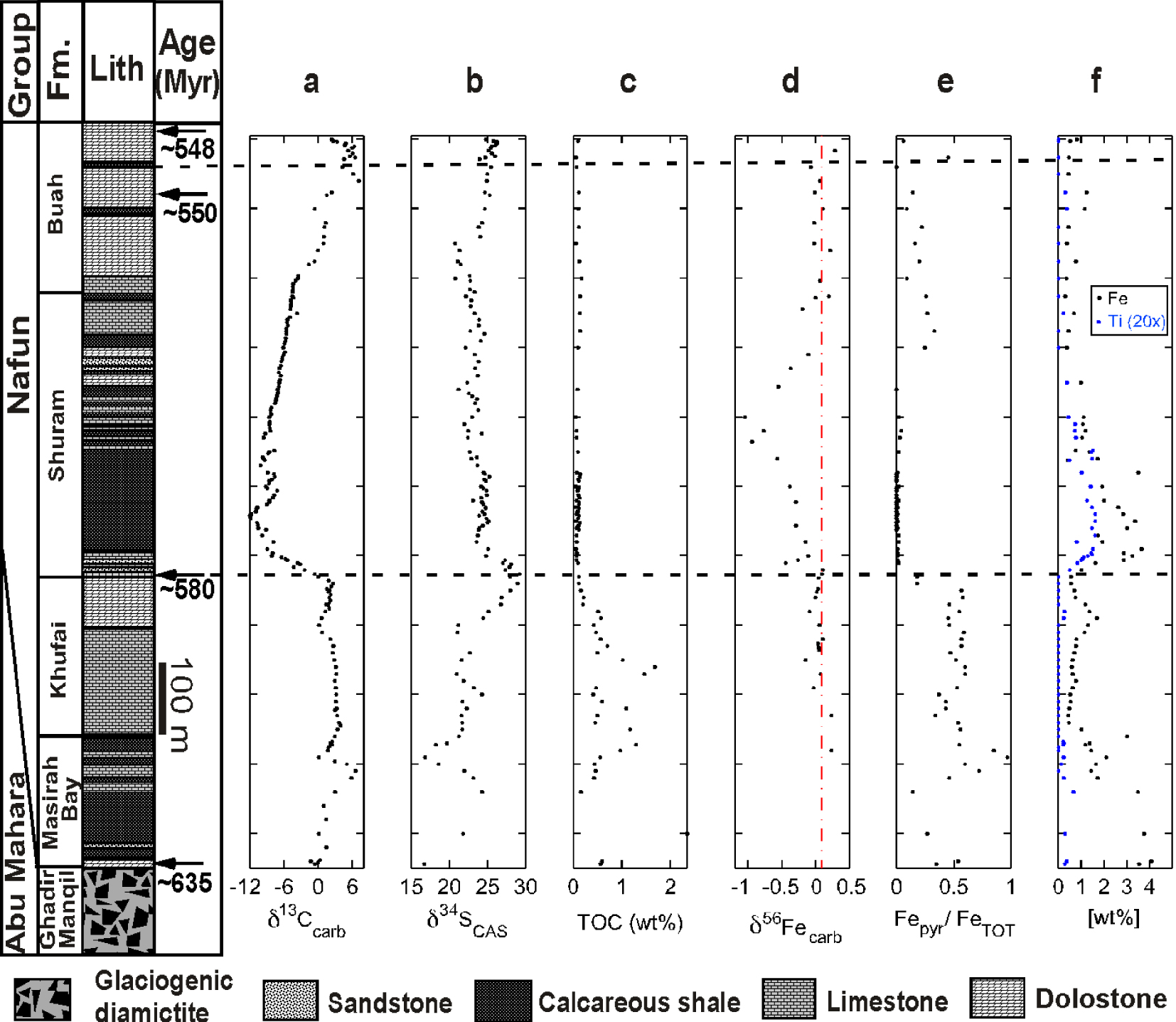
Complementary data from iron speciation and TOC abundance are also presented. Iron speciation data (Figure 3) show high ratios of pyrite to total iron in the Masirah Bay and Khufai formations. At the onset of the Shuram excursion, there is a substantial drop in pyrite to total iron ratios, in parallel with a large increase in total iron. This increase is coincident with increased siliciclastic input (based on parallel increases in Ti abundance). In the upper Buah Fm., following the recovery of the Shuram excursion, there is a slight increase in pyrite to total iron ratios and a return to baseline total iron concentrations, corresponding to a decrease in siliciclastic input (Figure 3). TOC contents vary strongly in upsection (Figure 3): Masirah Bay and Khufai Nafun strata are characterized by relatively high TOC (>∼1‰), whereas the Shuram and lowermost Buah strata coincident with low δ56Fe values have minimal TOC (<0.1%), and TOC levels increase slightly in the upper Buah (although remaining below 1%).
4. Discussion
The δ56Fecarb data provide new constraints on redox conditions prior to, during, and in the aftermath of the Shuram excursion. Without knowing the isotopic composition and relative abundance of all iron-bearing phases at the time of deposition, it is not possible to rigorously interpret a given δ56Fecarb value for a unique set of processes. We can, however, draw several end-member expectations for iron isotope ratios as a function of the redox state and processes in the sedimentary environment.
Strata in the Masirah Bay and Khufai have δ56Fecarb values of ∼0.1‰ and are characterized by high TOC, abundant pyrite, and enrichments in redox-sensitive trace elements. In organic-rich sediments, such as these, all reactive ferric iron is reduced to Fe(II) and thus liable to be incorporated into Fecarb. For reasons of isotope mass balance, the reduction of the readily biologically available iron oxides (hereafter referred to as quantitative iron reduction) results in a δ56Fecarb signature of ∼0.1‰ for a sample with composition similar to typical crustal materials. Depositional conditions for these strata were previously inferred to be anoxic to euxinic [Fike and Grotzinger 2008; Fike et al. 2006; Wu et al. 2015] and the δ56Fecarb values presented here are consistent with complete reduction of available iron oxides.
Strata in the upper Buah Fm. are also characterized by δ56Fecarb values of ∼0.1‰. However, iron speciation, 𝛥33S data, and the distribution of redox-sensitive trace elements suggest that depositional conditions in these strata were oxic [Fike and Grotzinger 2008; Fike et al. 2006; Wu et al. 2015]. Both TOC and pyrite are found in very low levels in these strata. While organic carbon is not overly abundant (<1%), it was sufficient to reduce the much smaller reservoir of iron oxides. Consistent with these observations, the δ56Fecarb again approximates bulk silicate values (∼0.1‰), supporting complete reduction of available iron oxides.
In contrast, the strata containing the Shuram δ13Ccarb excursion are characterized by exceptionally low δ56Fecarb values (down to − 1.05‰). These strata are also characterized by very low (<0.1%) residual TOC and low pyrite abundances; nearly complete consumption of available organics may explain the relatively enriched δ13Corg values from this core and their high stratigraphic scatter [Fike et al. 2006], making them unreliable indicators of ambient carbon cycling [Dehler et al. 2005; Johnston et al. 2012]. In addition, both iron speciation and the distribution of redox-sensitive trace elements point to oxidizing conditions during deposition.
During deposition of these strata, TOC abundance was sufficient to reduce just a fraction of the available ferric iron. As such, the isotopic signature of the resulting ferrous iron is expected to express a large part of the fractionation (∼2‰) associated with iron reduction [Beard et al. 1999]. Specifically, carbonates forming in these environments of incomplete iron reduction would be expected to have lower δ56Fecarb values relative to available ferric iron, which is the signal observed during the Shuram excursion (Figure 3). The low δ56Fe values in these strata preclude reduction of the majority of the available pool of ferric oxides. This partial reduction of ferric oxides resulted in the generation of a local 56Fe-depleted Fe(II) reservoir incorporated into the carbonates precipitating at the sediment–water interface and the underlying sediments (i.e., during deposition and early diagenesis).
While the migration of Fe(II) is limited in strictly oxic or euxinic environments by rapid and nearly quantitative conversion to iron oxyhydroxides or iron sulfides, respectively, the mobilization and net migration of 56Fe-depleted Fe(II) can occur under ferruginous (anoxic but not sulfidic) conditions. For example, net migration of dissolved iron occurs in the modern Black Sea, where Fe(II) diffuses out of sediments beneath anoxic waters following in situ reduction and can subsequently be transported along the chemocline [Anderson and Raiswell 2004; Wijsman et al. 2001]. As such, the presence of a ferruginous iron source during Shuram time could explain the low δ56Fecarb values observed [Severmann et al. 2006]. However, the smooth stratigraphic profile observed in our δ56Fecarb data would also require a gradual onset and cessation for the advection of ferruginous waters, a situation that we view as unlikely given the high-energy depositional environment of these strata (e.g., as evidenced by hummocky cross-stratified intraclast–ooid grainstones) [Bergmann 2013; Grotzinger et al. 2011; Le Guerroue et al. 2006a]. Moreover, the minimum in δ56Fecarb is observed to coincide with the minimum in TOC abundance, stratigraphically above the nadir in δ13Ccarb (Figure 3), a correlation not readily explained by a marine ferruginous iron source.
Thus, the stratigraphic expression of the δ56Fe signal observed during the Shuram excursion is more likely to reflect local redox conditions within the sediments during and following deposition. This is inferred to be mediated primarily by the abundance of organic carbon relative to that of oxidized iron-bearing phases delivered to the sediments [Bergmann 2013]. Specifically, the δ56Fe data show that there was never more organic matter present than reactive iron during the formation of the carbonates recording the Shuram excursion (be it in the water column, during sedimentary lithification, or during late diagenesis). This observation effectively excludes late-stage burial diagenesis [Derry 2010b] as a potential source of the Shuram excursion. The Fecarb isotopic composition during the Shuram excursion thus was controlled by the relative abundance of organic C and oxidized Fe delivery to sediments [Bergmann 2013; Bergmann et al. 2013], while the carbon isotopic signature is controlled by the residence time of carbon in the ocean/atmosphere. A decoupling between these two timescales could produce the observed stratigraphic decoupling in the isotopic composition of these systems.
The strata recording the Shuram excursion are associated with increased detrital contribution of wind-blown silt [Bergmann 2013], likely replete with iron oxides (as is seen Saharan dust today). Interestingly, increased detrital input and enhanced iron delivery are also seen associated with the Shuram excursion in the coeval Johnnie Fm. (Death Valley, CA) [Bergmann et al. 2013]. Together, these suggest that both enhanced oxidative weathering on the continents and the subsequent delivery of detrital iron oxides to marine sediments played a role in the origin and timing of the Shuram excursion. This enhanced flux of iron oxides led to an oxidative pulse in marine sediments and a partial reduction of these oxides is the most parsimonious explanation of the stratigraphically coherent variations observed in δ56Fecarb during the Shuram excursion. The associated oxidation of organic matter can help explain both the characteristic depletion in δ13Ccarb that constitutes the Shuram excursion as well as the abnormally low net organic carbon burial observed globally during this interval [Bergmann et al. 2011; Calver 2000; Fike and Grotzinger 2007; Kaufman et al. 2007; McFadden et al. 2008].
These data add to the growing body of work that suggests the Shuram δ13Ccarb excursion was formed at the time of deposition, either reflecting a primary perturbation to the marine carbon cycle, or, if diagenetic in origin, mediated by a novel, global means to “precondition” sediments for subsequent chemical modification during deposition or shortly thereafter [e.g., Grotzinger et al. 2011]. Our results suggest further that this preconditioning could be mediated by enhanced transport of terrestrial iron oxides to the sediments. Through microbial iron reduction (and associated abiotic reactions), these iron oxide phases would have consumed existing reducing pools within the sediments and overlying water column (including e.g., hypothesized reservoirs of dissolved organic carbon; [Rothman et al. 2003]). The associated oxidation of organic carbon during Shuram time gave rise to C isotope patterns that were essentially global in scope and timing. As such, be it a primary signature of the water column or a unique example of “global sedimentary diagenesis”, these data point to the mid-Ediacaran as a time of increasingly oxidized conditions that set the stage for the appearance and subsequent evolutionary radiation of metazoa.
The redox composition of recently deposited sediments serves as an important buffer on atmospheric oxygen concentrations. The oxygen demand by chemical weathering is directly related to the abundance of electron-rich substrates (e.g., organic matter, pyrite, ferrous silicates, and carbonates) in sedimentary rocks. All else being equal, a more reducing reservoir translates to more oxygen demand and lower atmospheric oxygen concentrations [Berner 2006]. We suggest that the Shuram excursion represents the terminal loss of a long-lived reducing reservoir, one titrated out by enhanced delivery of detrital iron oxides. The oxidation of these surficial sediments would consume a much smaller oxidant pool than that needed to ventilate an anoxic deep ocean [e.g., Fike et al. 2006], in line with modeled constraints on oxidant budgets [Bristow and Kennedy 2008; Le Guerroue et al. 2006b]. Furthermore, this transition spanning the Shuram excursion was accomplished over a protracted interval [Bowring et al. 2007; Rooney et al. 2020] characterized by very low net organic carbon burial in marine sediments.
In a strict budgetary sense, these processes represent a transient drop in oxygen levels on the fluid Earth associated with the oxidation of these reduced sediments (i.e., generating the Shuram δ13C excursion). However, once this buffer was removed, reorganization of the major sinks on oxygen may have subsequently allowed pO2 levels to rise to higher levels (e.g., 10% or greater) typical of the Phanerozoic. Our observations are consistent with previous reports of a transition in the redox composition of the sediments toward more oxidizing conditions displayed in other Ediacaran-age strata ([e.g., Canfield et al. 2007], but see Sperling et al. [2015]), and directly connect the Shuram excursion to this transition. By removing a long-lived reducing sedimentary buffer, this event set the stage for atmospheric oxygen to rise, promoting the diversification of the metazoa.



 CC-BY 4.0
CC-BY 4.0