1. Introduction
Pantellerites are typically identified as silica-oversaturated rhyolites with an alkali/alumina molar ratio [(Na2O + K2O)/Al2O3] (Peralkalinity Index, P. I.) higher than the unity, and they are rich in Na with an excess of Fe over Al [see the trend in MacDonald 1974; Le Maitre 2002; Jordan et al. 2021]. They are often associated with comendites, with slightly lower SiO2 and P.I. [e.g., Scaillet and MacDonald 2003]. Both occur mainly in interplate settings, including oceanic islands [Ascension Island; e.g., Jeffery Gertisser 2018] to continental rift zones, as for example the Sicily channel rift zone [Pantelleria Island; White et al. 2009], the Kenyan [e.g., Ren et al. 2006; MacDonald et al. 2011] and Ethiopian [e.g., Ronga et al. 2009; Iddon et al. 2018] Rift Valleys, and Mayor Island [NZ-Taupo Volcanic Zone; Barclay et al. 1996].
Peralkaline rhyolites have lower viscosity than calcalkaline rhyolites due to the high alkali content that strongly depolymerizes the melt structure [Stevenson et al. 1998; Mysen 2007; Mysen and Toplis 2007; Di Genova et al. 2013, 2017; Stabile et al. 2016, 2017, 2021], and they can shift between explosive and effusive eruptive behaviour, thus producing a wide variety of eruptive styles, ranging from lava flows and fountains to Strombolian to sub-Plinian and Plinian eruptions [e.g., Schmincke 1974; Mahood and Hildreth 1986; Duffield 1990; Lowestern and Mahood 1991; Houghton et al. 1992; Stevenson et al. 1993; Webster et al. 1993; Wilding et al. 1993; Barclay et al. 1996; Stevenson and Wilson 1997; Horn and Smincke 2000; Gottsmann and Dingwell 2002]. Although peralkaline rhyolites were once thought to be relatively H2O poor [Bailey and MacDonald 1987], more recent studies indicate magma water contents as high as 5–6 wt% H2O [e.g., Kovalenko et al. 1988; Webster et al. 1993; Wilding et al. 1993; Barclay et al. 1996; Gioncada and Landi 2010; Di Carlo et al. 2010; Lanzo et al. 2013; Romano et al. 2019].
Pre-eruptive magmatic volatile contents and pre- and syn-eruptive crystallization and degassing can strongly affect the rheology of magma in the chamber and during magma ascent, and in turn, they can influence the volcanic eruptive styles of magmas. Many variables, including melt composition, crystallinity, temperature (T), pressure (P), undercooling (𝛥T = Tliquidus − Tsubliqidus), time, melt water content, oxygen fugacity (fO2), and cooling and decompression rates, can influence magma crystallization at depth and during ascent to surface [e.g., Couch et al. 2003; Martel and Schmidt 2003; Hammer 2006; Brugger and Hammer 2010; Mollard et al. 2012; Martel 2012; Shea and Hammer 2013; Arzilli and Carroll 2013; Arzilli et al. 2016]. Crystallization has commonly been investigated in pantelleritic melts under equilibrium conditions [Scaillet and MacDonald 2001, 2003, 2006; Di Carlo et al. 2010; Romano et al. 2020], but disequilibrium crystallization kinetics deserve additional attention because of possible consequences for conduit flow processes and eruptive dynamics of peralkaline magmas [Arzilli et al. 2020].
In this review, we provide a comprehensive evaluation of the pre-eruptive conditions of volcanic activities at Pantelleria, which have implications for rheological and numerical eruption models that investigate magma ascent and fragmentation of peralkaline rhyolitic magmas. The aim of this contribution is to understand the eruptive dynamics of pantelleritic magmas by studying the phase abundances and chemical compositions of the main mineralogical phases (i.e., alkali feldspar (Afs) and clinopyroxene (Cpx)) present in natural products of the Pantelleria volcanic system. Here, we focus our attention on equilibrium and disequilibrium crystallization of alkali feldspar and clinopyroxene in different experimental and natural pantelleritic products, from Strombolian eruptions of Cuddia del Gallo/Randazzo and Fastuca pantellerite and the Green Tuff Plinian eruption, with the aim of constraining the pre- and syn-eruptive conditions of these eruptions. Specifically, we investigate how different parameters, such as pre-eruptive temperature and crystal fraction of the main mineralogical phases and (H2O)melt ( = concentration of H2O dissolved in melt) influence the eruptive style of pantelleritic magma, contributing to reach magma fragmentation and promoting explosive eruptive behaviour.
2. Pantelleria volcanic system
Pantelleria Island is located in the Mediterranean Sea south of Sicily (Italy) within the Sicily Channel Rift Zone [Rotolo et al. 2007; Civile et al. 2008]. The volcanic island of Pantelleria is composed of “La Vecchia” caldera (114 ka) and the “Cinque Denti” [45.7 ± 1.0 ka; Scaillet et al. 2013; Liszewska et al. 2018], which suggest the presence of magma beneath the central area of the island [Civetta et al. 1984; Mahood and Hildreth 1986; Rotolo et al. 2013, 2017]. Pantelleria has a bimodal magmatism association of transitional to alkali basalts, located mostly in the northwest sector of the island [Civetta et al. 1988; Rotolo et al. 2007], and trachytes-pantellerites which are more wide spread [e.g., Mahood and Hildreth 1986; Civetta et al. 1998; White et al. 2005, 2009; Liszewska et al. 2018; Scaillet et al. 2011, 2013; Williamsetal 2010; Jordan et al. 2018; Rotolo et al. 2021]. There is a clear compositional gap (Daly gap) between alkali basalt and peralkaline rhyolite end-members. Two main hypotheses have been proposed on the origin of the rhyolitic magmas: (i) low-degree partial melting of mafic cumulates in the lower crust to form trachyte, followed by crystal fractionation in shallow reservoirs to generate the most evolved pantellerites [Lowestern and Mahood 1991; Bohrson and Reid 1997; Avanzinelli 2004; MacDonald et al. 2008, 2011; Marshall et al. 2009]; (ii) fractional crystallization from an alkali basaltic parental magma [Civetta et al. 1998; White et al. 2005, 2009; Neave et al. 2012; Romano et al. 2019, 2020]. Rocks of intermediate compositions (such as mugearite and benmoreite) are rare and, in many cases, are thought to be the result of magma mixing based on textural observations [Romengo et al. 2012; Liszewska et al. 2018]; their rare eruption may represent a physical (density, viscosity) discrimination in the magma reservoirs [Civetta et al. 1988; White et al. 2009; Neave et al. 2012; Liszewska et al. 2018]. For instance, according to this last hypothesis, the Daly gap in Pantelleria compositions can be explained by the fact that the intermediate melts are not erupted because felsic magmas (trachytic to pantelleritic) within the magma chamber behave as a density filter for high-viscosity and crystal-rich intermediate magmas [Mungall and Martin 1995; Peccerillo et al. 2003]. Moreover, Prosperini et al. [2000] proposed a process of mixing plus fractional crystallization between the less evolved comenditic trachyte and the more evolved pantelleritic sample. This magmatic interaction process has been specifically considered the force triggering the magmatic events that produced the Khaggiar lava dome [6–8 ka; Speranza et al. 2010; Scaillet et al. 2011], which was followed by intense volcanic activity, characterized by explosive eruptions and lava flows emissions from different effusive centres [Civetta et al. 1998; Orsi et al. 1991; Scaillet et al. 2011; Neave 2020].
There exist many debates on pantelleritic magma genesis and their evolution and eruptive behaviour [e.g., see Romano et al. 2018, 2020, and references therein]. Outcrops in Pantelleria show fall units (pumice fall, welded fall (splatter), etc.) and deposits of pantelleritic magmas, which were originated from lava fountains, with a continuous transition from explosive to more effusive style [e.g., Jordan et al. 2018]. An important question remains as to the mechanisms and processes triggering this shift in eruptive style for magmas with almost identical compositions [Schmincke 1974; Duffield 1990; Houghton et al. 1992; Stevenson et al. 1993; Webster et al. 1993; Wilding et al. 1993; Barclay et al. 1996; Stevenson and Wilson 1997; Horn and Smincke 2000; Gottsmann and Dingwell 2002; Hughes et al. 2017].
For instance, it is well-known [e.g., Sparks 1978; Papale and Polacci 1999] that the increase in magma viscosity due to volatile loss can produce the conditions necessary for magma fragmentation and explosive eruptions [Di Genova et al. 2013]. However, given the low viscosity of pantelleritic liquids [Neave et al. 2012], there should be other mechanisms, which trigger the most explosive style. Previous studies have demonstrated that these peralkaline rhyolites, at low temperatures, have lower viscosity than metaluminous rhyolites due to the effect of alkalis that strongly depolymerize the melt, decreasing their configurational entropy and the viscosity [Di Genova et al. 2013, 2017; Stabile et al. 2016, 2017, 2021].
Viscosity effects on the eruption of pantelleritic magmas are further modelled and discussed by Campagnola et al. [2016], who presented numerical simulations on the conduit dynamics of the highly explosive Green Tuff eruption, the most recent catastrophic eruption on Pantelleria Island [Mahood and Hildreth 1986; Williams 2010; Williamsetal 2010; Jordan et al. 2018, 2021; Rotolo et al. 2021]. The petrological data and the thermodynamic and numerical modelling indicate that pre-eruptive temperatures of the Pantelleria volcanic system for several explosive eruptions could range between 950 °C (high end-member associated with trachytic magmas) and 720–680 °C (associated with pantelleritic magmas) [White et al. 2005; Di Carlo et al. 2010; Campagnola et al. 2016; Liszewska et al. 2018; Romano et al. 2020].
In the following discussion, we attempt to provide a more refined picture of the most probable pre-eruptive conditions for the Pantelleria eruptions, with a particular focus on the resulting rheological implications and eruptive behaviour.
3. Review of equilibrium and disequilibrium experiments on peralkaline rhyolitic melts
3.1. H2O solubility in peralkaline rhyolite melts
Phase equilibrium studies can constrain the storage conditions of the specific magma system investigated, providing information on the magma evolution and magma chamber state prior to eruption [Rutherford et al. 1985; Geschwind and Rutherford 1992; Gardner et al. 1995; Rutherford and Devine 1996; Barclay et al. 1998; Cottrell et al. 1999; Di Carlo et al. 2010; Romano et al. 2018].
In this work, we consider studies on the phase relations as function of pressure, temperature, and water content in pantelleritic compositions (Table 1). In particular, water abundance in rhyolitic magmas can influence magma physical properties and crystallization behaviour [e.g., Hammer 2004; Gualda et al. 2012] and in turn, rheological properties and eruptive styles [e.g., Roggensack et al. 1997; Huppert and Woods 2002; Sparks 2003; Cashman 2004; Aiuppa et al. 2007; Edmonds et al. 2008; Stock et al. 2018; Stabile and Carroll 2020]. Much effort has been devoted to study water solubility in different silicate melt compositions, but only a few studies document water abundance in strongly peralkaline rhyolites and Fe-rich pantelleritic compositions [e.g., Scaillet and MacDonald 2001; Schmidt and Behrens 2008; Di Carlo et al. 2010; Stabile et al. 2018, 2020; Romano et al. 2021].
(a) Water content solubility calculations (at 750 °C) as function of pressure for pantelleritic compositions [Di Carlo et al. 2010; Arzilli et al. 2020] obtained by using Papale et al. [2006], Moore et al. [1998], and Ghiorso and Gualda [2015] solubility models. The purpose of the figure is to illustrate the H2O-saturated conditions for the selected magma compositions for a given P–T range. See Romano et al. [2021] for more detailed discussion on water solubility in pantelleritic melts. (b) Phase diagram for alkali feldspar (Afs) and clinopyroxene (Cpx) under water-saturated conditions at NNO + 0.8 and NNO − 1,2 in pantelleritic compositions from Arzilli et al. [2020] and Di Carlo et al. [2010], respectively.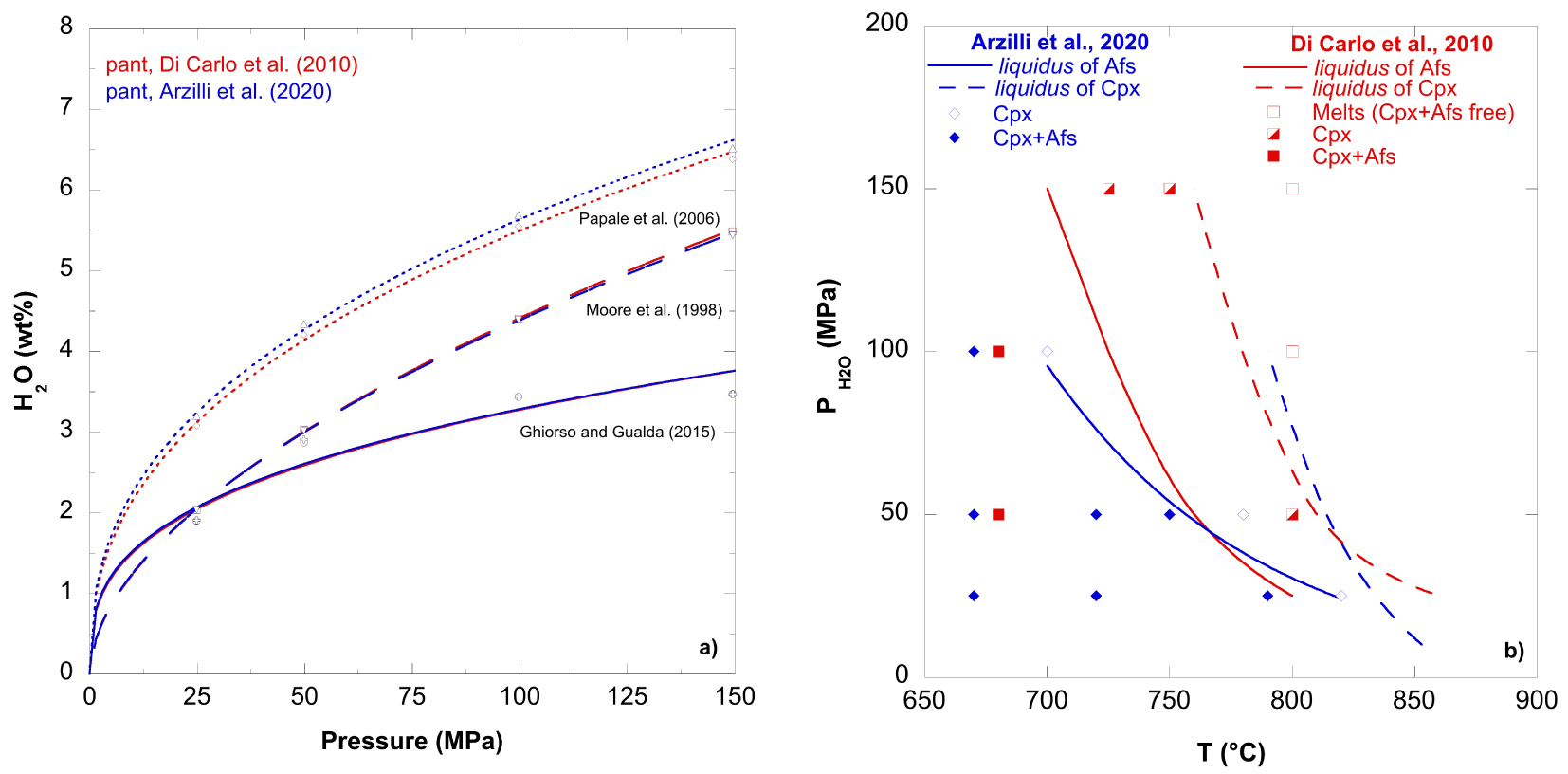
Summary of pantelleritic data used in this study
| Experimental data | Natural samples | |||||||
|---|---|---|---|---|---|---|---|---|
| (wt%) | Arzilli et al. [2020] Cuddia del Gallo | Di Carlo et al. [2010] Fastuca | Romano et al. [2020] Fastuca | Romano et al. [2020] Green Tuff | Rotolo et al. [2007] Fastuca (PAN0113) | Campagnola et al. [2016] Green Tuff | Gioncada and Landi [2010] Cuddia del Gallo (PANT15) | |
| SiO2 | 69.13 | 70.40 | 69.45 | 72.60 | 69.98 | 69.4 | 66.3 | |
| TiO2 | 0.54 | 0.48 | 0.48 | 0.52 | 0.47 | 0.50 | 0.39 | |
| Al2O3 | 10.46 | 10.30 | 10.15 | 9.00 | 9.75 | 8.40 | 10.28 | |
| Fe2O3 | n.a. | — | — | — | 8.52 | 8.60 | — | |
| FeO* | 8.06 | 7.52 | 7.87 | 6.24 | — | 8.41 | ||
| MnO | 0.30 | 0.26 | 0.21 | 0.24 | 0.27 | 0.30 | 0.29 | |
| MgO | 0.09 | 0.06 | 0.10 | 0.51 | 0.00 | 0.10 | 0.05 | |
| CaO | 0.56 | 0.52 | 0.53 | 0.46 | 0.55 | 0.40 | 0.43 | |
| Na2O | 6.30 | 5.67 | 6.71 | 7.29 | 7.02 | 6.30 | 6.10 | |
| K2O | 4.54 | 4.74 | 4.46 | 2.87 | 4.43 | 4.20 | 4.29 | |
| P2O5 | 0.01 | — | 0.04 | 0.06 | — | 0.03 | ||
| SO2 | n.a. | — | — | — | — | — | ||
| F | n.a. | — | — | 0.20 | — | — | ||
| Cl | n.a. | — | — | — | — | — | ||
| Total | 99.75 | 100.00 | 100.00 | 100.00 | 100.00 | 98.2 | 99.42 | |
| PI1 | 1.46 | 1.40 | 1.56 | 1.68 | 1.68 | 1.77 | 1.43 | |
Notes: PI1 (Peralkalinity Index) = molar (Na2O + K2O)/Al2O3. n.a. = not analysed.
Figure 1a shows the general state of knowledge concerning water solubility in pantelleritic magmas under water-saturated conditions. In the figure, we show solubility calculations as a function of pressure—at 750 °C—for two similar pantellerite compositions [Di Carlo et al. 2010; Arzilli et al. 2020] with all plotted results obtained from the thermodynamic models of Papale et al. [2006], Moore et al. [1998], and Ghiorso and Gualda [2015]. Generally, the data reflect the well-known strong pressure dependence of water solubility, although we are aware that the Papale et al. [2006] model tends to slightly overestimate water solubility in such melt compositions, while Moore et al. [1998] better reproduces the solubility data [see Romano et al. 2021]. Overall, the solubility data demonstrate that water loss from pantelleritic melts during ascent can alter the fluid-phase mass fraction produced, depending on initial magma water content and dynamics of degassing (bubble nucleation, growth, coalescence), and possible eventual fragmentation (for explosive eruptions).
3.2. Liquidus curves of alkali feldspar and clinopyroxene
Previous petrological studies have worked to better define the T–P range and the redox conditions of comenditic to pantelleritic magmas [Scaillet and MacDonald 2001, 2003, 2006; White et al. 2005, 2009; Di Carlo et al. 2010; Romano et al. 2020], as well as their pre-eruptive water contents [e.g., Gioncada and Landi 2010; Neave et al. 2012; Lanzo et al. 2013; Romano et al. 2019]. The best-estimate alkali feldspar and clinopyroxene liquidus curves for two pantelleritic melts are shown in Figure 1b at temperatures of 700–850 °C and P(H2O) of 25–150 MPa. The two melts differ slightly in composition, with slightly higher wt% concentrations of FeO and Na2O for the melt composition used by Arzilli et al. [2020], as well as a small difference in the P.I. of 1.46 vs. 1.40 for the Di Carlo et al. [2010] study (see Table 1).
Di Carlo et al. [2010] studied the role of different intensive parameters (P, T, H2O in the melt and fO2 on crystal–liquid equilibria) in a Pantelleria rhyolite belonging to the Fastuca pumice fall eruptive unit. Phase equilibria show that clinopyroxene is the first liquidus phase, followed by alkali feldspar and then quartz (which is here not reported) over the entire range of T–H2Omelt investigated by Di Carlo et al. [2010], with aenigmatite being stable at temperature ⩽700 °C, at pressures ⩽100 MPa. Slightly different results on the same composition have been found by Romano et al. [2020], where the mineralogical assemblage is dominated by alkali feldspar, with minor aenigmatite and clinopyroxene, but also fayalite, amphibole, and quartz occurring in minor amounts at lower temperatures. In particular, the crystallization of fayalite in peralkaline magmas depends on a combination of temperature, fO2, and melt peralkalinity (and SiO2 activity) and for Fastuca it is limited to T between 690–750 °C, for suitable peralkalinity of melt and fO2 [Romano et al. 2020]. These small differences between Romano et al. [2020] and Di Carlo et al. [2010] results, despite the use of nominally identical starting materials, are most likely due to the high sensitivity of the phase stabilities to small variation of intensive parameters, as for instance, the slightly lower redox conditions investigated in Romano et al. [2020] compared to Di Carlo et al. [2010]. However, in these evolved pantelleritic magmas, ferromagnesian phases are always limited to relatively small abundances and Afs, followed by Qz are the main phases to crystallize—these are all near-eutectic-type melts in which incompatible elements can vary widely in abundance, while major elements show only small variations.
In Figure 1b, we also report the liquidus temperatures of alkali feldspar obtained by Arzilli et al. [2020] and of clinopyroxene, using the composition of a peralkaline rhyolitic pumice (PANT15) from the eruptive fall unit of Cuddia del Gallo. These liquidii are consistent with those obtained from Di Carlo et al. [2010] at similar pressures, near water saturation. Although the oxygen fugacity of NNO +0.8 considered in Arzilli et al. [2020] is higher than those investigated (NNO − 1 to NNO − 2) by Di Carlo et al. [2010], the liquidii of the alkali feldspar are similar at pressures lower than 50 MPa (Figure 1b), which suggest that alkali feldspar is not strongly sensitive to fO2 (which mainly influences melt FeO contents, and indirectly, SiO2 activity). However, at pressures higher than 50 MPa, the alkali feldspar at NNO + 0.8 is stable at slightly lower temperatures compared with experiments at NNO − 1. This temperature difference at pressures higher than 50 MPa may be related to compositional difference between the two peralkaline rhyolitic melts (see Table 1). Moreover, reducing the redox conditions from NNO + 0.8 to NNO − 1.2 shifts the clinopyroxene liquidus at pressures higher than 50 MPa to lower temperatures (temperature difference of ∼30 °C). On the other hand, the clinopyroxene liquidus from Di Carlo et al. [2010] shows broadly the same pattern than the alkali feldspar liquidus curve, but appearing at higher T (>750 °C). Overall, the relative order of crystallization of the main mineralogical phases is the same and persists over the P–T range for both melt compositions used by Di Carlo et al. [2010] and Arzilli et al. [2020].
3.3. Composition of alkali feldspar
The compositions of experimental alkali feldspars fall in the range of Or28–67 (Figure 2a). A broad negative correlation between Or (mol%) and T (°C) is evident when all the available data are plotted together (Figure 2a). For instance, for the pantellerites in Arzilli et al. [2020], the Or content of alkali feldspar crystals formed at 670 °C ranges from 47 to 67 mol% (different melt H2O), while the ones crystallized at temperatures ⩾720 °C are characterized by a lower Or content between 31 and 37 mol% (near the binary Alb-Or minimum). Hence, the alkali feldspar is more sodic at temperatures between 720 and 790 °C, independent of P and H2O dissolved in the melt [Arzilli et al. 2020]. The grey shaded band in Figure 2a indicates the range of Or contents (between 34 and 38 mol%) in natural alkali feldspar crystals obtained from Cuddia del Gallo (PANT15), Fastuca and Green Tuff eruption products [Di Carlo et al. 2010; Lanzo et al. 2013; Liszewska et al. 2018; Romano et al. 2020]. The variation in Or content with temperature is appreciable only at ⩽700 °C for experiments from Di Carlo et al. [2010] and Romano et al. [2020].
(a) Orthoclase (Or) mol% content of alkali feldspars as a function of experimental temperature (T °C) and (b) H2O dissolved in the melt in pantelleritic compositions [Di Carlo et al. 2010; Arzilli et al. 2020; Romano et al. 2020]; the total P range considered is between 50 and 150 MPa. GTP in the legend indicates Green Tuff pantellerite, while FP is Fastuca pantellerite [Romano et al. 2020]. The grey shaded band indicates the range of Or contents (34–38 mol%) in natural alkali feldspar phenocrysts [Lanzo et al. 2013].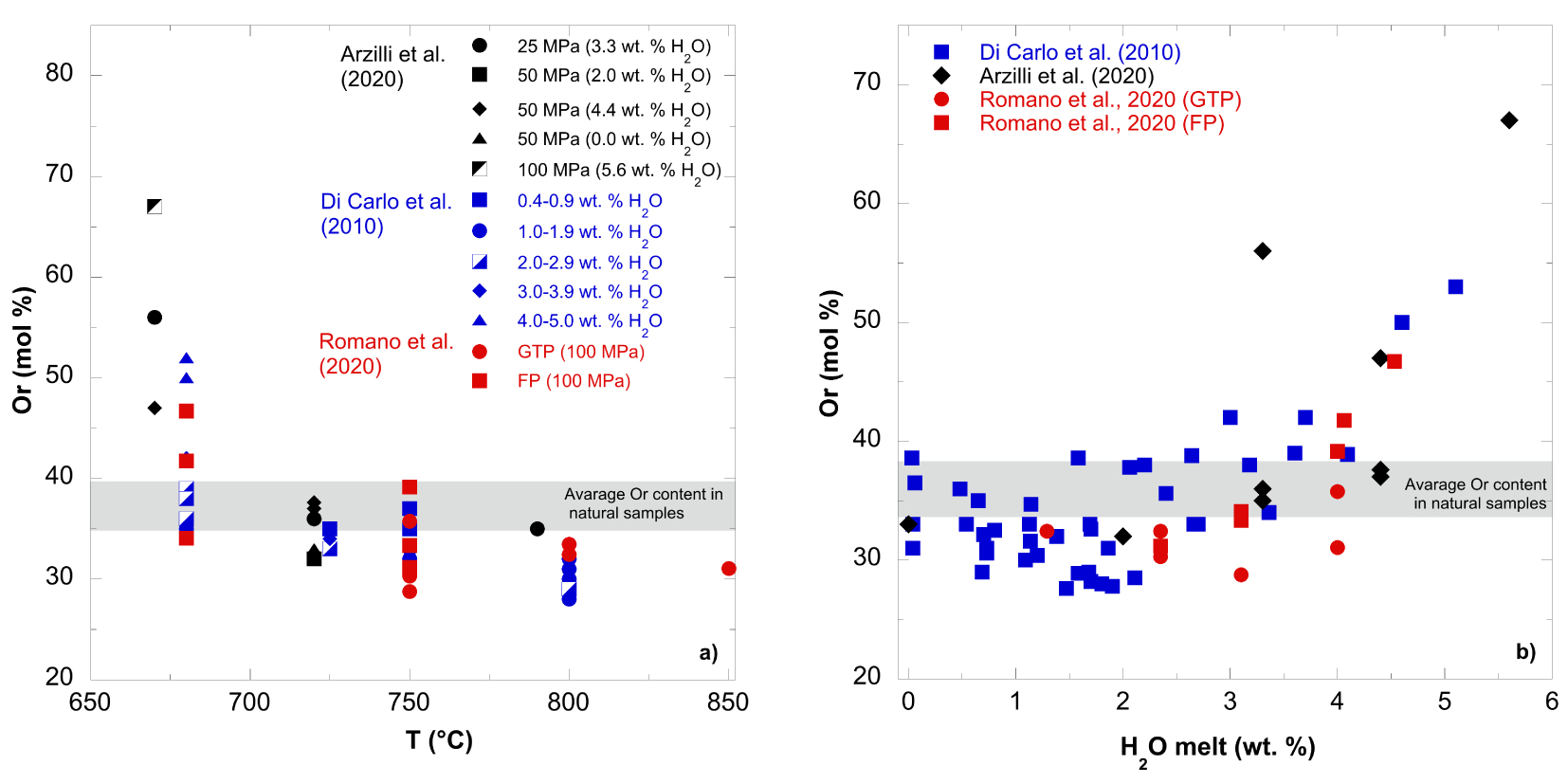
When we examine variations of Or content with H2Omelt (Figure 2b), the majority of data displays limited variation with H2Omelt except for the highest H2O charges which appear to show a general positive correlation between Or and H2O in the melt. However, when considering each subset of data relative to the melt compositions, which are reported from Di Carlo et al. [2010], Or defines a slight positive correlation with H2O only for H2O > 2.5 wt% at 100–150 MPa. Whereas an almost horizontal trend is obtained at lower H2O contents for compositions falling in the range Or28–39. Overall, the composition of natural alkali feldspar in pantellerites is well reproduced at 720–750 °C and (H2O)melt in the range 2.5–4 wt%. Higher or lower temperatures cannot reproduce the natural alkali feldspar composition (grey band in the figure).
3.4. Composition of clinopyroxene
Experimental clinopyroxenes have compositions in the range of XFe (=molar Fe/(Fe + Mg), with all Fe as Fe2+) between 0.54 and 0.97 for data reported on GTP (Green Tuff Pantellerite) and FP (Fastuca Pantellerite) from Romano et al. [2020], while it varies between 0.84 and 0.99 for data on FP of Di Carlo et al. [2010] (Figure 3a).
(a) Variation of XFe ( =FeO*/(FeO* + MgO) molar), where FeO* is total iron expressed as FeO of experimental Cpx, with Temperature (°C) at a range of pressures [Di Carlo et al. 2010; Romano et al. 2020]. The grey bands correspond to the natural compositions of Cpx (GTP = Green Tuff Pantellerite; FP = Fastuca Pantellerite). (b) Variation of CaO content (wt%) of experimental Cpx with melt water content (H2Omelt wt%) at a range of temperatures and pressures [Di Carlo et al. 2010; Romano et al. 2020]. The grey band corresponds to the average CaO content of Cpx phenocrysts in the starting rocks (Fastuca and Green Tuff Pantellerite).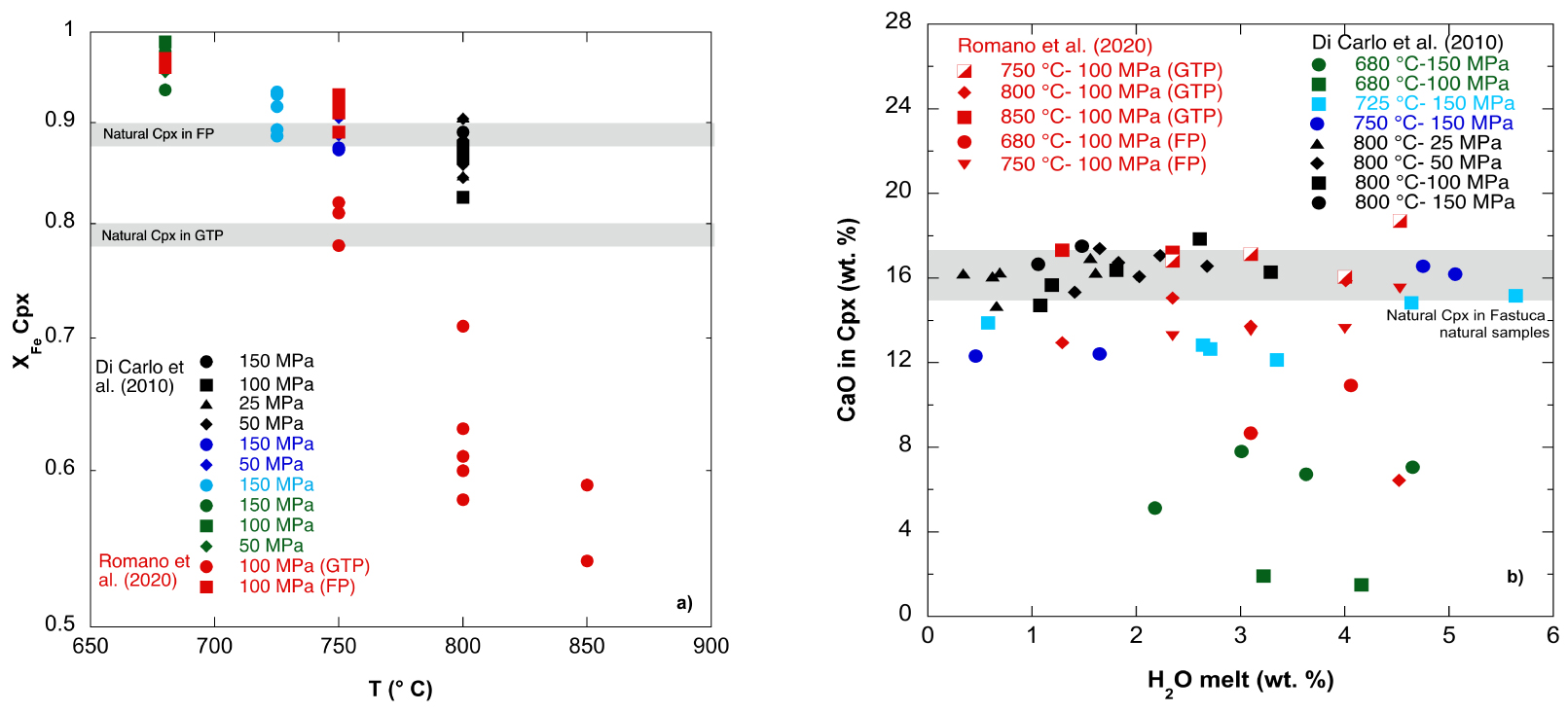
XFe shows a negative correlation with temperature in the different pantelleritic products. In particular, at constant H2Omelt, in GTP clinopyroxenes, a decrease in temperature of 50 °C (from 800 to 750 °C) increases XFe from 0.60 to 0.80, in FP, clinopyroxene only crystallizes at lower temperature ( <800 °C), and the XFe ranges from 0.89 to 0.97. The most Fe-rich clinopyroxene crystals are produced at 680 °C in FP compositions [Romano et al. 2020]. Similarly, experimental data from Di Carlo et al. [2010] display the highest XFe values (between 0.92 and 0.99) at the lowest temperature investigated of 680 °C. Clinopyroxene compositions synthesized at 750 °C reproduce the small range of natural clinopyroxene compositions [Romano et al. 2020], while clinopyroxenes obtained by Di Carlo et al. [2010] at temperatures between 720 and 800 °C better reproduce the natural clinopyroxene compositions of Fastuca products (upper grey band in Figure 3a).
3.5. Crystallization of alkali feldspar and clinopyroxene
Although the crystallization behaviour of peralkaline rhyolites is relatively well-understood at equilibrium, the prediction of the crystallization kinetics under non-equilibrium, time-dependent conditions is still difficult, even though kinetic data can potentially provide new insights about the timescales of the magmatic processes. Here, we summarize the current understanding of the crystallization kinetics of alkali feldspar and clinopyroxene under both equilibrium and disequilibrium conditions in pantelleritic magma compositions. Alkali feldspars are highly sensitive to variations of intensive variables in volcanic systems, which are recorded by variable textures and compositional zoning patterns, depending on the rate of change of intensive parameters promoting crystallization. Recently, several studies have investigated clinopyroxene crystallization in different alkaline magma because clinopyroxene crystallization can also rapidly change the magma rheology, and chemical zoning of clinopyroxene can provide information on the magma history [e.g., Ni et al. 2014; Polacci et al. 2018; Masotta et al. 2020; Pontesilli et al. 2019; Arzilli et al. 2019]. Here, we report the total crystal fraction (𝜙total), which represents the sum of both alkali feldspar and clinopyroxene crystal fraction, in experimental runs on pantellerites from Cuddia del Gallo, Fastuca, and Green Tuff [Di Carlo et al. 2010; Arzilli et al. 2020; Romano et al. 2020] as function of 𝛥TCpx (temperature below the clinopyroxene liquidus).
Results show 𝜙total between 0.08 and 0.29 for 𝛥TCpx between 10 and 140 °C and (H2O)melt in the range of 3.3–5.6 wt% for Arzilli et al. [2020] [consistent with melt inclusion water content estimations in phenocrysts; Gioncada and Landi 2010]. Considering both experimental data from Di Carlo et al. [2010] and Romano et al. [2020], 𝜙total (clinopyroxene + alkali feldspar phenocrysts) ranges between 0.01 and 0.49 for 𝛥TCpx up to ∼170 °C. The phase abundances along with the total crystal fraction (𝜙) for the investigated experimental conditions in Arzilli et al. [2020] are also reported in Table 2. The experimental durations vary between 24 and 288 h [Arzilli et al. 2020] and demonstrate long nucleation delay of alkali feldspar in pantelleritic melts (from several hours to several days). The nucleation delay of alkali feldspar under water-undersaturated conditions can be ∼230 h, and the nucleation delay time decreases with increasing melt H2O content at fixed P and/or 𝛥T. Particularly, under water-saturated conditions, the nucleation delay of alkali feldspar is <50 h, while the nucleation delay of clinopyroxene ranges from minutes to a few hours [Arzilli et al. 2020]. This indicates that clinopyroxene formation timescales can be significantly shorter than timescales for alkali feldspar formation upon changes of magmatic P–T conditions in pantelleritic magmas. Slow feldspar crystallization/recrystallization time scales are also observed in plagioclase-bearing basaltic magmas [Polacci et al. 2018; Masotta et al. 2020; Pontesilli et al. 2019]. Importantly, we observe that despite a wide range of 𝛥T, the variation of clinopyroxene crystal fraction is relatively small from 0.02 to 0.10 [Di Carlo et al. 2010; Romano et al. 2020]. This implies that peralkaline rhyolites may spend days in sub-liquidus conditions without experiencing significant changes in crystal fraction due to long nucleation delay times for alkali feldspar [discussed in more detail in Arzilli et al. [2020]].
Experimental run conditions and phase abundances for the data reported in Arzilli et al. [2020]
| Sample | Pressure (MPa) | Temperature (°C) | H2O (wt%) | t (h) | Phase abundances (wt%) | Total 𝛷 |
|---|---|---|---|---|---|---|
| C136 | 100 | 670 | 5.6 | 192 | Gl(83), Afs(7), Cpx(10), Ox( <1) | 0.17 |
| C144 | 50 | 750 | 4.4 | 288 | Gl(92), Afs(6), Cpx(2) | 0.08 |
| C155 | 50 | 720 | 4.4 | 96 | Gl(89), Afs(7), Cpx(3), Ox(1) | 0.11 |
| C148 | 50 | 720 | 4.4 | 175 | Gl(85), Afs(8), Cpx(6), Ox(1) | 0.15 |
| C149 | 50 | 720 | 4.4 | 195 | Gl(83), Afs(10), Cpx(6), Ox(1) | 0.17 |
| C141 | 50 | 670 | 4.4 | 72 | Gl(71), Afs(20), Cpx(9) | 0.29 |
| C146 | 25 | 790 | 3.3 | 288 | Gl(83), Afs(12), Cpx(3), Ox(2) | 0.17 |
| C151 | 25 | 720 | 3.3 | 130 | Gl(75), Afs(19), Cpx(5), Ox(1) | 0.25 |
| C138 | 25 | 670 | 3.3 | 24 | Gl(90), Afs(2), Cpx(8), Ox(<1) | 0.10 |
Gl = glass; Afs = Alkali feldspar; Cpx = Clinopyroxene; Ox = oxides (Magnetite–Ülvospinel solid solution, Fe–Ti oxides). Phase abundances calculated by multiple linear regression using known starting composition and crystal compositions analysed by microprobe; total 𝛷 means total crystal fraction (% crystals/100).
4. Discussion
4.1. Pre-eruptive conditions of the Pantelleria volcanic system
4.1.1. Strombolian eruptions
The strombolian pantelleritic products of Fastuca, Cuddia Randazzo, and Cuddia del Gallo have a mineral assemblage that consists of alkali feldspar, clinopyroxene, and minor amounts of fayalite, aenigmatite, amphibole, and quartz [Di Carlo et al. 2010; Gioncada and Landi 2010; Lanzo et al. 2013; Landi and Rotolo 2015; Romano et al. 2020]. Alkali feldspar is the dominant crystal phase, and together with clinopyroxenes, occurs as both phenocrysts (between 500 μm to mm sizes) and microlites (from a few microns to 100–200 μm) [Di Carlo et al. 2010; Gioncada and Landi 2010; Romano et al. 2020]. The abundance of phenocrysts is similar among the strombolian products of Fastuca, Cuddia del Gallo, and Cuddia Randazzo. The phenocrysts crystal fraction is ∼0.15 (alkali feldspar + clinopyroxene). Fastuca samples also contain alkali feldspar phenocrysts, and alkali feldspar and clinopyroxene microlites [Romano et al. 2020]. Similarly, alkali feldspar microlites are present within the groundmass of the Cuddia Randazzo products. For most of the samples, the abundance of alkali feldspar microlites ranges between 0.56 and 0.66 of total crystals, while mafic mineral crystal fractions are ∼0.05–0.11 [Landi and Rotolo 2015]. Experimental temperatures between 720 and 800 °C and pressures of 25–100 MPa produce crystal fractions (∼0.15; considering alkali feldspar + clinopyroxene) similar to the phenocryst abundances observed in the strombolian products of Fastuca, Cuddia Randazzo, and Cuddia del Gallo. Experiments also indicate that a crystal fraction of ∼0.50 can be produced at temperatures between 680 and 750 °C (Figure 4). Therefore, abundant microlites may be produced within this range of temperature in the strombolian eruptions.
Regarding the Fastuca strombolian eruption, equilibrium experiments indicate that the composition of natural clinopyroxene phenocrysts (XFe = 0.88–0.90) can be reproduced at temperatures between 725 and 800 °C and pressures between 25 and 150 MPa (Figure 3a). Instead, the compositions of natural alkali feldspar phenocrysts are reproduced at temperature between 680 and 750 °C, pressures between 50 and 100 MPa (Figure 2a), and water contents between 2 and 3.5 wt%. These results indicate that there is a narrow temperature window, between 725 and 750 °C, in which clinopyroxene and alkali feldspar can crystallize at the same conditions. This implies that either pre-eruptive temperatures of Fastuca eruption were between 725 and 750 °C or that magma was cooled down in a magma reservoir or during magma ascent from 800 °C to temperatures at which alkali feldspar can crystallize (680–750 °C). Furthermore, the rare occurrence of amphibole in Pantelleria rhyolites [Jordan et al. 2018; Rotolo et al. 2007; White et al. 2009] suggests that this mineral crystallizes from a wetter and cooler magma storage region [Di Carlo et al. 2010]. In this way, amphibole and alkali feldspar would coexist at T > 680 °C for crystal contents comparable with those observed in natural pantellerite [Romano et al. 2020].
Eruptive temperatures ⩾800 °C, as we have demonstrated in Figures 2, 3 and 4 are not consistent with observations on crystal compositions and abundances of alkali feldspar and clinopyroxene for the different strombolian products considered here (Fastuca, Cuddia Randazzo and Cuddia del Gallo). This is also supported by the presence of aenigmatite, which is only stable at temperature of 750 °C and 50 MPa for dry conditions and 750 °C, 100 MPa, and wet conditions, as reported from Di Carlo et al. [2010] and Romano et al. [2020], respectively.
Considering the total crystal abundance of alkali feldspar and clinopyroxene (phenocrysts and microlites) in the strombolian products and the compositions of alkali feldspar and clinopyroxene phenocrysts observed in Fastuca eruptive products, we propose that the more likely pre-eruption conditions are 680–750 °C, 25–100 MPa under water-saturated (or near-saturated) conditions.
Total crystal fraction (𝛷) of Afs + Cpx as function of 𝛥TCpx (°C below estimated Cpx liquidus temperature). The diagram shows data for peralkaline rhyolites from Di Carlo et al. [2010], Arzilli et al. [2020], and Romano et al. [2020] and data of total 𝛷 of main eruptive pantellerite products [i.e., Fastuca, Cuddia del Gallo, Green Tuff; Gioncada and Landi 2010; Lanzo et al. 2013; Landi and Rotolo 2015]. See text for discussion of 𝛥TCpx.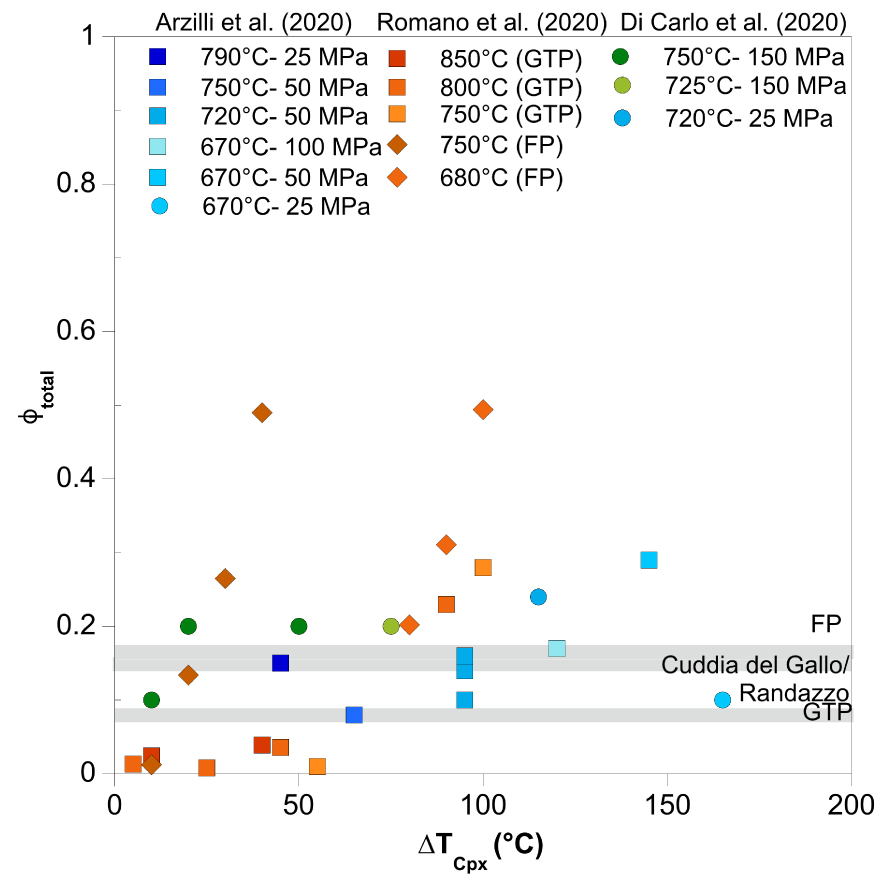
4.1.2. Plinian eruption
The Green Tuff Plinian eruption produced an ignimbritic deposit that is compositionally zoned, from pantelleritic at the base to comenditic trachyte at the top of the deposit. This suggests that the magma reservoir of the Green Tuff was also compositionally zoned before the eruption: pantelleritic magma at the top and comenditic trachyte at the bottom of the reservoir [Liszewska et al. 2018].
The mineral assemblage of the pantelleritic magma consists mainly of alkali feldspar, with minor clinopyroxene and aenigmatite, and traces of fayalite and quartz. Alkali feldspar represents the main crystal phase ( >85 vol.%) of the phenocryst assemblage in every sample [White et al. 2009]. Microlites are not present within the Green Tuff pantelleritic products [Campagnola et al. 2016]. The textures of pantelleritic pumices erupted from Green Tuff Plinian eruption are mainly vitrophyric and crystal fraction of phenocrysts ranges between 0.05 and 0.25 [White et al. 2009; Lanzo et al. 2013; Campagnola et al. 2016; Liszewska et al. 2018; Romano et al. 2019]. Although the observed crystal fraction covers a broad range, observations on numerous natural samples, suggest an average crystal fraction of 0.08 is representative for the pantelleritic member, with the later trachytic part of the eruption being more crystal-rich compared with the earlier pantelleritic part [Campagnola et al. 2016]. The mineral assemblage of the trachytic member of the Green Tuff eruption consists of alkali feldspar and clinopyroxene, with minor olivine, ilmenite, and apatite [Campagnola et al. 2016; Liszewska et al. 2018; Romano et al. 2020]. Both phenocrysts and microlites are present in the trachytic magmas. Alkali feldspar is the main phase (phenocrysts = 0.18 (%vol); microlites = 0.05), whilst, clinopyroxene is the second main phase in terms of crystal abundance (phenocrysts = 0.04; microlites = 0.06, according to Campagnola et al. [2016]).
Previous studies indicate that a thermal gradient was present within the magma reservoir at pre-eruptive conditions: the pantelleritic magma at the top was colder (700–750 °C) than the trachytic member (900–950 °C) in the lower part of the reservoir [Campagnola et al. 2016; Liszewska et al. 2018]. Water contents range from 1 wt% in the trachytes to 4 wt% in the pantellerites [Liszewska et al. 2018]. Previous studies also estimated ∼100 MPa as pre-eruptive pressure, which correspond to depths of 3–4 km [Campagnola et al. 2016; Liszewska et al. 2018]. This depth is consistent with geophysical studies [e.g., Mattia et al. 2007], which place the top of the magma reservoir at 4 km beneath the caldera. This implies that at ∼100 MPa, and with 4 wt% of H2O the pantelleritic magma was likely near water-saturated conditions (see Figure 1a) prior to eruption, particularly if we consider that the Papale et al. [2006] model may slightly overestimate H2O solubility in pantelleritic magmas (as suggested by Romano et al. [2021]).
The composition of natural clinopyroxene phenocrysts (XFe = 0.78–0.80) of Green Tuff pantelleritic products can be reproduced experimentally at temperatures between 750 and 850 °C at ∼100 MPa (Figure 4a,b), whereas the compositions of natural alkali feldspar are reproduced at temperature of 750 °C, 100 MPa, and 3–4 wt% of H2O. Therefore, crystallization of the pantelleritic magma may occur near water-saturated condition before the Plinian eruption.
As we observed for the Fastuca products, clinopyroxene can crystallize at higher temperatures (750–850 °C) than alkali feldspar. However, the pre-eruptive temperatures of the Green Tuff pantelleritic products under water-saturated conditions cannot be higher than 750 °C, as the alkali feldspar is the main phase in terms of abundance. This is also confirmed by the experimental results of Romano et al. [2020], which show alkali feldspar is present only at temperatures ⩽750 °C at 100 MPa and 4 wt% of H2O, whereas at temperatures ⩾800 °C alkali feldspar is not able to crystallize. This implies that either pre-eruptive temperature of the pantelleritic magma was at ∼750 °C or that the magma cooled down in a magma reservoir from 850 °C to ∼750 °C forming clinopyroxene first at higher temperatures and then alkali feldspar at lower temperatures, before the Plinian eruption was triggered; these scenarios should be further investigated, as they have implications on the triggering of the eruption and the eruption styles.
Experimental results suggest that at 𝛥TCpx between 3 and 170 °C and experimental duration between 24 and ∼500 h, pantelleritic melts can produce crystal fractions (considering alkali feldspar and clinopyroxene) between 0.02 and ∼0.50. Figure 5 shows calculated magma viscosities and crystal fractions for different melt water contents, at temperatures ranging from 700–850 °C (see figure legend for details). In general, low 𝛥TCpx (<75 °C) can promote a total crystal fraction similar to the natural pantelleritic products of Green Tuff Plinian eruption which are shown with green arrow in Figure 5 [Lanzo et al. 2013; Campagnola et al. 2016; Romano et al. 2020]. For 𝛥TCpx higher than 75 °C, experimental crystal fractions are higher than those observed in the Green Tuff pantelleritic products. One condition in Figure 5 corresponding to 𝛥TCpx = 170 °C (25 MPa, 670 °C) shows a 𝜙total of 0.10, which is similar to that of the Green Tuff pantelleritic products, but the ratio of clinopyroxene to alkali feldspar is too high and not representative of the natural samples. This is due to the combination of low temperatures (670 °C) the high 𝛥TCpx, which are not likely pre-eruptive conditions of Green Tuff Plinian eruption. Based on the chemical compositions of clinopyroxene and alkali feldspar, the likely pre-eruptive temperatures of the pantelleritic magma is ∼750 °C (Figure 5b), which implies relatively small 𝛥TCpx (<75 °C). Small 𝛥TCpx may promote a nucleation delay of alkali feldspar crystals. Magma stagnation at small 𝛥TCpx for a few days prior to the triggering of the eruption may only produce a limited amount of crystallization. This indicates that although the pantelleritic member spends days rather than hours at sub-liquidus conditions, it may not produce drastic changes in (alkali feldspar) crystal fraction [Arzilli et al. 2020]. Instead, the trachytic magma at the bottom of the reservoir is more crystallized than the pantelleritic member, as trachytic magmas can reach faster the equilibrium crystal fraction [Arzilli et al. 2018, 2020]. The injection of hotter mafic-intermediate magma into the cooler reservoir destabilized the system, heating the trachytic magma (whose phenocrysts are partially resorbed but with no evidence of physical and chemical mixing) and triggering the Green Tuff eruption [Landi and Rotolo 2015; Romano et al. 2018; Liszewska et al. 2018; Neave 2020]. Thus, we propose that the Green Tuff pantelleritc magma was stored for days at pre-eruptive temperature of ∼750 °C, pressure 100 MPa, and under near water-saturation conditions and was erupted suddenly after the injection of hotter magma into the reservoir without having time for significant changes in crystal volume fraction.
Effect of crystal content on liquid (magma) viscosity as function of water content (from dry conditions to 6 wt% H2O) at different magmatic temperatures of (a) 700 °C, (b) −750 °C, (c) −800 °C, and (d) −850 °C. The arrows in the figures, starting from the natural phenocryst content (average) of the main eruptive products (Green Tuff or GTP, Cuddia del Gallo/Randazzo, Fastuca eruptions or FP/CUDDIA), indicate the different paths of rising magmas. The grey dashed line indicates the fragmentation level corresponding to a viscosity value of 106 Pa⋅s. The viscosities of crystal-bearing and vesicle-free suspensions have been estimated by using the Mader et al. [2013] equations as function of crystal fraction, of a strain rate of 𝛾 = 1 s−1, and a mean crystal aspect ratio of rp = 8. See text for discussion.
In the following, we present and discuss the different effects of the initial temperature, H2Omelt, crystal fraction, and crystal aspect ratio of alkali feldspar and clinopyroxene on the rheology and dynamics of the pantelleritic magma of the Pantelleria volcanic system.
4.2. Syn-eruptive conditions and rheological implications
Viscosity (𝜂) calculations on dry and hydrous pantelleritic magma compositions (Cuddia del Gallo, Fastuca, and Green Tuff) can help understand better the interactions between magma rheology and eruptive styles. Brittle magma fragmentation occurs when a critical viscosity-dependent strain rate is exceeded [Papale 1999]. Melt composition, crystal fraction, and vesicularity all influence the bulk magma viscosity [e.g. Giordano et al. 2008; Vona et al. 2011; Mader et al. 2013]. The bulk magma viscosity increases as the magma crystallinity increases and during degassing as magma water content decreases [e.g., Giordano et al. 2008; Vona et al. 2011]. This favours approaching brittle magma fragmentation conditions. Regarding peralkaline rhyolitic explosive eruptions, magma fragmentation still remains an unclear process [Di Genova et al. 2013; Campagnola et al. 2016; Hughes et al. 2017].
For viscosity calculations, we consider the pre-eruptive temperatures of the Pantelleria volcanic system, the melt water content, the crystal fractions (𝜙), and the crystal aspect ratio (rp). We use the model of Di Genova et al. [2013] for the prediction of the initial viscosity of peralkaline silicate melts as a function of temperature and water content. Assuming a pre- to syn-eruptive temperature of 700–850 °C, H2Omelt between 0 and 6 wt%, and pressure of 25–100 MPa, we calculate the viscosities of pantelleritic liquids at this range conditions (Figure 5).
To investigate how the presence of crystals can influence the rheology of peralkaline rhyolitic magmas, the viscosities of crystal-bearing and vesicle-free suspensions have been estimated by using the Mader et al. [2013] equations using a strain rate of 𝛾 = 1 s−1 and a mean crystal aspect ratio of rp = 8. For simplicity here, a strain rate of 1 s-1 has been considered assuming that the crystallization occurs at near-equilibrium conditions, while the crystal aspect ratio represents the average value between alkali feldspar and clinopyroxene obtained from crystallization experiments performed by Arzilli et al. [2020] under a wide range of 𝛥TCpx (3–170 °C). Viscosities are reported in Figure 5(a–d) as a function of the crystal fraction (alkali feldspar and clinopyroxene phenocrysts) for a given eruptive temperature (700–750–800–850 °C, Figure 5a–d) and H2Omelt varying from 0 (dry conditions) up to 6 wt%. We consider 0.3 wt% of H2O the residual water content at the exit of the vent, following the modelling of Green Tuff eruption [Campagnola et al. 2016].
Overall, the viscosity increase is not linear with crystal fraction and it becomes steep for 𝜙 values >0.35, approaching infinite values for further 𝜙 increases [a consequence of the form of viscosity-crystal fraction relationship used by Mader et al. [2013]]. At a given temperature, the decrease of H2Omelt during magma ascent can change the viscosity by up to 6 log units (Figure 5). At viscosities higher than 106 Pa⋅s (grey dashed line corresponding to the fragmentation level in Figure 5), brittle fragmentation may be invoked in agreement with modelling results obtained by Campagnola et al. [2016] and the rheological calculations reported by Hughes et al. [2017].
The pre-eruptive conditions of the strombolian cases (Fastuca, Cuddia Randazzo, and Cuddia del Gallo—red paths in Figure 5) can promote crystallization of phenocrysts and microlites of alkali feldspar and clinopyroxene at temperatures of 680–750 °C. The high total crystal fraction (phenocrysts and microlites) observed in the strombolian products may be produced during magma stagnation and slow ascent prior to the triggering of the eruption. As shown in Figure 5 large undercoolings and several days at pre-eruptive conditions are needed to produce high crystal fractions of alkali feldspar [Arzilli et al. 2020]. The pre-eruptive conditions proposed for the strombolian eruptions and crystal abundance observed in the natural samples can increase viscosity during magma ascent (Figure 5a,b) at 𝜂 > 106 Pa⋅s, which is sufficient to lead to brittle fragmentation.
Regarding the Green Tuff Plinian eruption (green paths in Figure 5), the pre-eruptive conditions of the crystal-poor pantelleritic member are likely a temperature of ⩽750 °C, a pressure ∼100 MPa, and water-saturated conditions (in agreement with Campagnola et al. [2016] and Liszewska et al. [2018]). Despite the crystal content of the pantelleritic products being relatively low (on average 0.08), magma viscosity can reach values higher than 106 Pa⋅s close to the surface (0.3 wt% of H2O) at temperatures ⩽750 °C (Figure 5a,b), which implies that brittle fragmentation may be promoted. At a temperature of 800 °C, magma viscosity can reach a value of 106 Pa⋅s only at the surface with a crystallinity of 0.08 (Figure 5c). At temperature of 850 °C this viscosity threshold is not reached (Figure 5d); therefore, it is unlikely that brittle fragmentation and explosive eruptions are promoted at temperatures ⩾800 °C. This result is in agreement with previous studies [Campagnola et al. 2016; Hughes et al. 2017], however, our simple model considers only the effect of crystals, temperature, and magma water content at the equilibrium conditions, whilst, the effect of decompression rate, adiabatic cooling ascent rate, strain rate, vesicularity, and outgassing during magma ascent should be considered [La Spina et al. 2021]. For example, Hughes et al. [2017] suggest that bubble overpressure driven by rapid decompression and strain localization around crystals may also promote brittle magma fragmentation.
In conclusion, we note that crystal abundance does not play a fundamental role in changing the viscosity of the pantelleritic magma for the Green Tuff Plinian eruption, in agreement with Campagnola et al. [2016] and Hughes et al. [2017]. This contrasts with basaltic compositions, where fast syn-eruptive crystallization has been proposed as a driving mechanism triggering fragmentation and highly explosive eruptions [e.g., Sable et al. 2006, 2009; Arzilli et al. 2019; Bamber et al. 2020]. The pantelleritic magmas may favour highly explosive eruptions reaching viscosity of 106 Pa⋅s even with a low crystallinity at ⩽750 °C (Figure 5). Therefore, the tendency of such magmas to fragment brittle or to flow effusively may be strongly controlled by temperature (Figure 5) and/or rapid decompression and strain localization, as suggested by Hughes et al. [2017]. Another process that should be considered is the crystallization of nanolites during fast perturbation of undercooling [Mujin and Nakamura 2014; Di Genova et al. 2020]. Nanolites may form during fast magma ascent in iron-rich peralkaline rhyolitic magmas, and their formation could change the magma viscosity by several orders of magnitude [Di Genova et al. 2020]. All these hypotheses indicate that the complexity of pantellerite eruptive phenomena should be further investigated with more experimental and modelling studies to better understand the fragmentation process and the highly explosive eruptions in peralkaline rhyolitic systems.
Acknowledgements
This research has been supported by PRIN2017 (2017J277S9-MC). We thank John C. White and the other anonymous reviewer for their contribution to the review of this work and useful comments that helped us to improve this manuscript.




 CC-BY 4.0
CC-BY 4.0