1 Introduction
The outbreak of a variant form of Creutzfeldt-Jakob disease (vCJD) in the United Kingdom as a result of infection with the agent of bovine spongiform encephalopathy (BSE) 〚1〛 has created an urgent need to understand the conditions surrounding BSE transmission to humans. Susceptibility to the infectious agent of BSE was recently described in some big-sized and long-lived non-human primate species. This appeared either spontaneously in macaque monkeys and Eulemurs living in zoological parks 〚2–4〛 or in cynomolgus monkey after experimental infection by intracerebral, intraperitonal or intravenous injections 〚5, 6〛. The need for an experimental model of the disease led us to choose the prosimian Microcebus murinus because of its close phylogenetic relation to humans, its small body size (height: 12 cm; weight: 70–100 g), and its comparatively short life expectancy (8 to 12 years in captivity, 3 to 4 years in its native Madagascar biotope). In addition, this species exhibits age-related clinical signs and cerebral lesions similar to those observed in aging humans, and 15 % of aged microcebes spontaneously develop an Alzheimer’s type pathology 〚7, 8〛. Finally, the evaluation of the social and cognitive impairments in this model could be achieved by tests similar to those used for humans 〚9〛.
2 Animals and methods
Eleven young adult (1–2 years old) microcebes were infected either orally or by inoculation with the BSE agent (table). Oral infection was accomplished with a single 0.5 g dose of brain homogenate mixed with 2 g of compote, and inoculations were performed as bilateral intracerebral (IC) injections into the parietal cortices of anaesthetized animals (Imalgène 500, 0.3 mL/100 g), with or without an accompanying intraperitoneal (IP) injection (0.2 mL of the same homogenate). All inocula consisted of 25 μL of a 10 % brain homogenate of a BSE-infected cow or macaque monkey obtained at the clinical stage of the disease. Four control microcebes were given a 10 % brain homogenate from an uninfected cow (3 were inoculated IC, one was inoculated IC and IP), and 5 other controls were fed uninfected cow brain tissue. None of the microcebes had ever been fed meat or bone meal of mammalian origin. Breeding of microcebes for this study conformed to recommendations of the Washington Convention on Endangered Species of Wild Fauna and Flora.
Summary of experimental protocols and results of attempts to infect microcebes with primary or macaque-passaged bovine spongiform encephalopathy.
| Animal | Inoculum/ | Survival | Behavior/ | Weight | Cause | PrP-IR | PrP-Western blot | ||
| number | route | (months) | cognition | loss | of death | Brain | / Spleen | Brain | / Spleen |
| 190 | BSE / Oral | 7 | Abnormal | No | Large open wound | NT | NT | NT | NT |
| 199 | BSE / Oral | 7.5 | Abnormal | Yes | Euthanasia | – | +++ | NT | NT |
| 197 | BSE / Oral | 13 | Abnormal | Yes | Neurological | + | ++ | – | – |
| 191 | BSE / Oral | >39 | Normal | No | |||||
| 162 | MBSE / Oral | >38 | Normal | No | |||||
| 183 | MBSE / Oral | >38 | Abnormal* | No | |||||
| 194 | MBSE / Oral | >38 | Normal | No | |||||
| 163 | BSE / IC+IP | 21 | Normal | No | Large open wound | – | – | – | – |
| 161 | MBSE / IC+IP | 17.5 | Abnormal | Yes | Neurological | +++ | – | ++ | ++ |
| 158 | MBSE / IC | 8 | Abnormal | Yes | Neurological | + | – | + | NT |
| 159 | MBSE / IC | 16 | Abnormal | Yes | Neurological | ++++ | +++ | NT | NT |
| 237 | Normal / Oral | 13 | Normal | No | Euthanasia | – | – | NT | – |
| 203 | Normal /Oral | 16 | Normal | No | Euthanasia | – | – | NT | – |
| 139 | Normal / Oral | 17 | Normal | No | Euthanasia | – | – | – | NT |
| 278 | Normal / Oral | 17 | Normal | No | Euthanasia | – | – | – | NT |
| 88 | Normal / Oral | 18 | Normal | No | Euthanasia | – | – | NT | – |
| 184 | Normal / IC+IP | >35 | Normal | No | |||||
| 240 | Normal / IC | 5 | Normal | No | Euthanasia | – | – | NT | NT |
| 238 | Normal/IC | >26 | Normal | No | |||||
| 241 | Normal/IC | >26 | Normal | No |
2.1 Behavioral and cognitive evaluations
After inoculation, the animals were kept in isolation for two weeks, following which they were housed as groups of three BSE-infected animals in large cages allowing freedom to climb, jump and run. They were kept under daily observation, and social behavior was assessed with respect to relations and time spent with cage-mates, grooming, vigilance, and aggressiveness or defensive reactions. Cognitive faculties, including attention, short-term memory, and learning, were evaluated every two weeks in tests of visual discrimination and analysis of time spent in the exploration of both familiar and new environments 〚8〛.
2.2 Preparation of brain and spleen homogenates
Tissues from each animal were individually collected at the time of necropsy and stored at –80 °C. Samples were then homogenized to 10 % w/v in cold phosphate buffer saline (PBS) containing 5 % glucose, using Eppendorf disposable potters. Proteins were extracted by mixing the homogenate with an equal volume of 2' Triton/DOC lysis buffer (150 mM NaCl, 0.5 % Triton X-100, 0.5 % sodium deoxycholate, 50 mM Tris-HCl; pH 7.5). The protein concentration was adjusted to 3 mg/mL and the lysate digested with 100 μg/mL of proteinase K at 37 °C for 30 min. The samples were then mixed with an equal volume of 2' Laemmli buffer, boiled for 5 min, and analysed for the presence of proteinase-resistant protein by Western blotting. In some cases, the proteinase K-digested lysate was concentrated by centrifugation for 45 min at 20 000 g at 4 °C and the pellet directly resuspended in Laemmli buffer. Samples were electrophorezed in a 12 % acrylamide gel and electroblotted onto nitrocellulose membranes. Proteinase-resistant PrP (PrPres) was detected using the monoclonal antibody Pri-308 〚10〛 or a mix of three mAbs (SAF-60, SAF-69 and SAF-70) as previously described 〚11〛. Each lane was loaded with the equivalent of 12.5 μg of protein of the brain extract in the standard protocol and 190 μg in the centrifugation concentration protocol.
2.3 Immunohistochemistry
PrP was visualized by histological examination of treated 6-μm sections incubated with a panel of antibodies (3F4 〚12〛, 8G8 and 12F10 〚13, 14〛, SAF34 and SAF60 〚11〛) directed against the PrP, using formic acid and autoclaving pretreatments as previously reported 〚4〛. In some experiments, incubation with the antibodies was preceded by a treatment with proteinase K (25 μg/mL, 37 °C, 20 min). Various parts of the digestive tract, spleen, lymph nodes, peripheral nerves, and central nervous system were examined. Identical sections from microcebes inoculated with normal cow brain (negative controls), and from formalin-fixed brain of patients with Gertschmann–Sträussler–Scheinker disease and CJD (positive controls), were processed and stained simultaneously with those of the infected microcebes.
Neurodegenerative lesions other than PrP amyloid deposition were detected by staining with Harrisˈ hematoxylin (for intraneuronal vacuoles), anti-β42 amyloid (for β-amyloid deposits), anti-961S28T and anti-PHF 87S5 〚for abnormal Tau proteins in neurons: aggregated form and paired-helical filaments (PHF) respectively〛, and anti-gliofilament acidic protein (GFAP) Dako (for reactive astrocytes).
3 Results
3.1 In vivo observations
Beginning about three months after infection (whether by oral ingestion or intracerebral inoculation), and lasting until death, all animals that succumbed to neuropathologically and immunohistochemically confirmed BSE, had shown abnormalities of cognitive function and a progressive indifference to their environment before dying, becoming apathetic and immobile and usually staying in close contact with their mates in one corner of the cage or in the nesting box (table). Animals that did not develop a neurological disease (animal #163) or are still alive (191, 162, 183, and 194), and all nine animals given uninfected control brain remained normal throughout the period of observation.
Three animals (190, 199, and 163) were sacrificed 7, 7.5, and 21 months after infection, without neurological signs (two of the three had large open wounds; those often occur in breeding, where impaired animals are attacked by healthy congeners). Four other animals (197, 158, 159, and 161) experienced a clinical phase manifested by a dramatic loss of weight accompanied by prostration and generalized tremors: two of them (158 and 159) had been infected 8 and 16 months earlier by IC inoculation of MBSE, one (161) had been inoculated 17.5 months earlier by both IC and IP routes with MBSE, and one (197) had been orally infected with BSE 13 months earlier (this animal also developed a large irritated patch of skin and became progressively blind over the last few weeks before death). Four animals infected with BSE or MBSE (162, 183, 191, 194) remain alive and well after an observation period of 38 months.
3.2 Post-mortem studies
3.2.1 Animals without neurological signs
One animal (190) with a large open wound was sacrificed 7 months after the ingestion of BSE brain without any immunohistological demonstrable PrP in either brain or peripheral organs (table). The second animal (199) was sacrificed 7.5 months after ingestion of BSE brain: PrP was detected in the spleen, mesenteric lymph nodes, and small intestine, being particularly notable in intra-epithelial lymphocytes, Paneth cells in the gland of Lieberkühn villous lymphoreticular elements, Peyerˈs patches, and the nerve plexus of Auerbach: the intramural intestine innervation located inside the smooth muscle (figure 1). PrP was not detected in the brain, even in areas with vacuolated nerve fiber tracts. The third animal (163), inoculated IC and IP 21 months earlier with BSE brain, had no immunohistologically detectable PrP in any of its organs, and no PrPres in brain or spleen on Western blot (figure 2); it had presumably not been infected by the inoculum.
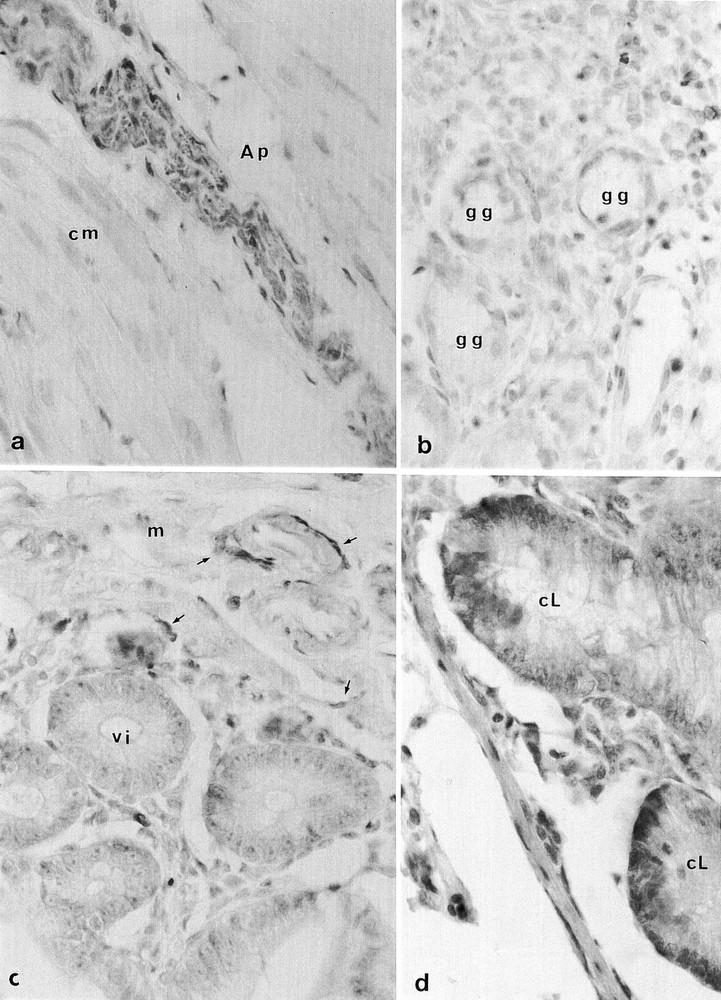
Microcebe 199 orally infected 7.5 months earlier with BSE a. PrP immunoreactivity (yellow-brown) of the myenteric Auerbachˈs plexus (Ap) in the circular layer of the muscularis externa (cm) (anti-PrP 8G8, 1/10000), × 350; b. PrP (yellow) in the lymphoreticular system lying around the gastric glands (gg) (anti-PrP 8G8, 1/1000); Microcebe 197 orally infected 13 months earlier with BSE, × 850; c. isolated lymphocytes and lymphatic submucous plexus (arrows) around the villi (vi) of the small intestine (vi: in transversal section) and in the mucosa (m) (anti-PrP 8G8, 1/1000), × 850; d. PrP immunoreactivity in the Paneth cells (brown) at the base of the crypt of Lieberkühn (cL) (cL:in longitudinal section) (anti-PrP SAF34, 1/1000), × 850.
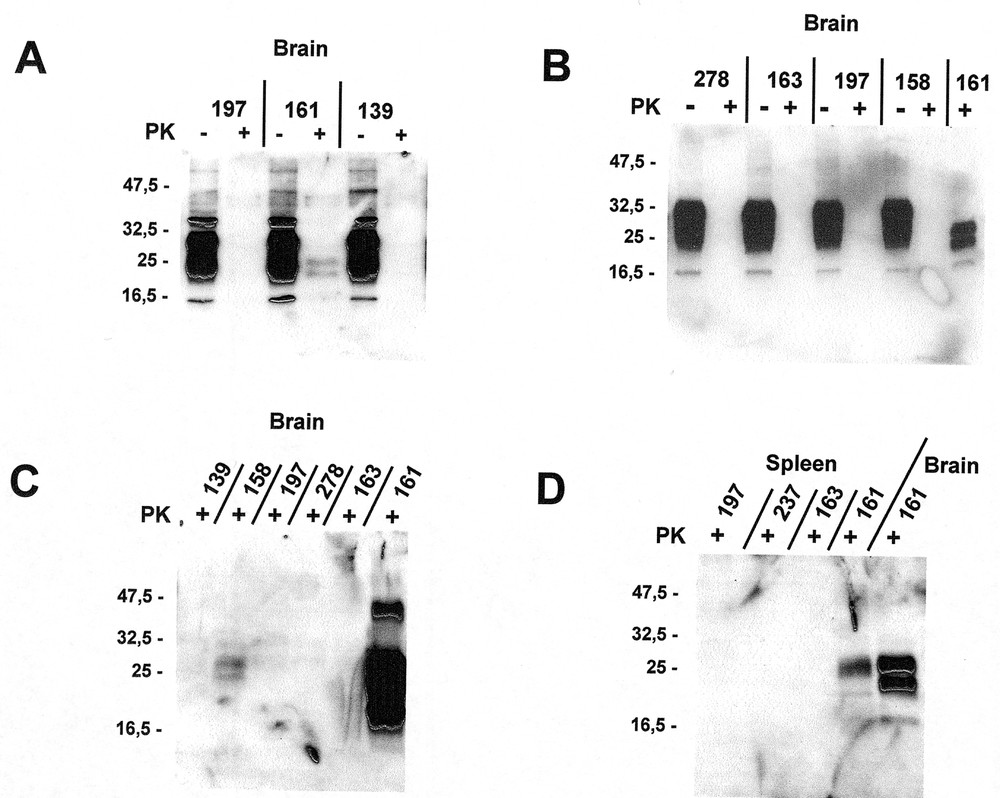
Western blots of brain (panels A, B, and C), and spleen (panel D). Specimens in panel A were prepared according to our standard protocol (12.5 μg/mL of proteinase K, and no concentration step), and reacted with an antibody mixture of SAF 60+69+70. Specimens in panel B and the last lane of panel D were also prepared according to our standard protocol, and reacted with Pri 308 antibody. Specimens in panel C and the first 4 lanes of panel D were prepared using a concentration step followed by incubation with 190 μg/mL of proteinase K and reacted with Pri 308 antibody. Note the difference produced by concentrating the brain tissue from animal # 161 (last lanes of panels B and C).
3.2.2 Animals with neurological signs (158, 197, 159, and 161)
One animal (158) inoculated with MBSE brain 8 months earlier had no immunohistologically demonstrable PrP in spleen, while, in its brain, vacuoles associated to a faint PrPres immunoreaction were revealed both by microscopic immunolabelling and by Western blot (figure 2). A second animal (197), fed BSE brain 13 months earlier, exhibited a spongiosis that was particularly marked in the trigeminal nerve and solitary tract of the brain stem (some fibers of which were immunohistochemically positive for PrP). The two other animals (159 and 161), inoculated with MBSE 16 and 17.5 months earlier, showed a well-developed spongiosis, with numerous vacuolated neurons and granular PrP deposits in the brain stem, thalamus, septum, and periventricular grey substance (figures 3a, b). Western blots of brain and spleen from animal 161 revealed a moderate amount of PrPres (figure 2). Additionally, in the last 3 diseased animals (197, 159, 161) the optic nerve transversal sections showed some vacuoles. The ganglion cells of the retina were filled with small granules of PrP, which were especially prominent after proteinase K treatment (figure 3f).
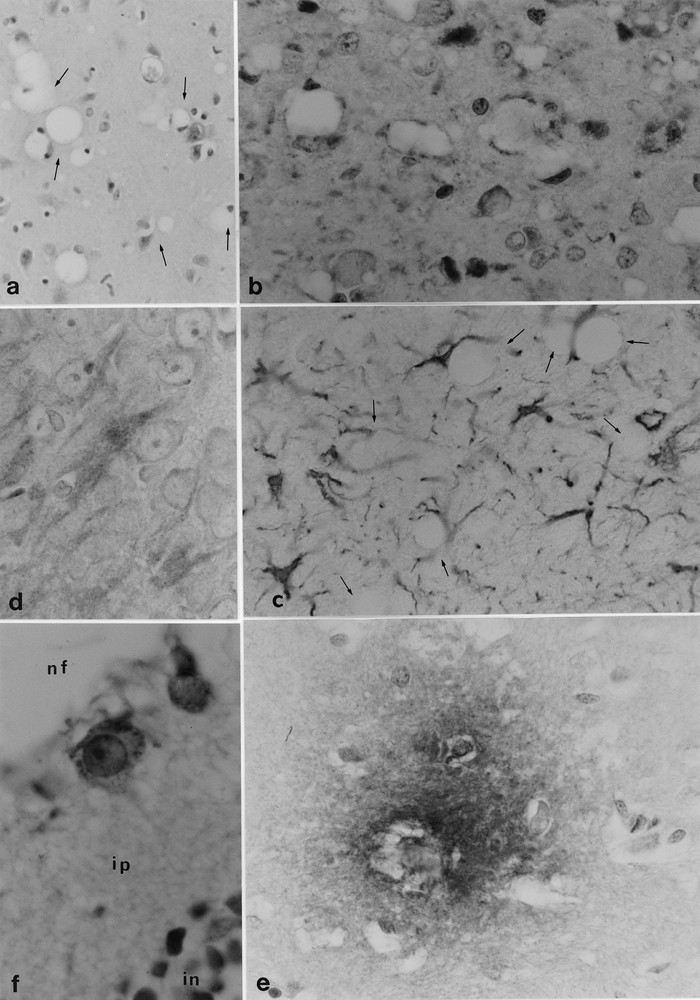
Microcebe 161 intracerebrally and peritoneally inoculated 17.5 months earlier with macaque-passaged BSE (MBSE). a. vacuolated neurons (arrows) in the periventricular grey substance (eosin-Harrisˈ hematoxylin), × 350; b. PrP deposits (brown) in the periventricular grey substance (anti-PrP SAF34, 1/10000), × 850; c. reactive astrocytes (brown) in the periventricular grey substance around the vacuolated neurons (arrows) (anti-GFAP Dako, 1/100), × 850; d. PHF-immunoreactivity (brown) in the hippocampal neurons (anti-PHF 87S5, 1/400), × 850; e. β42 amyloid diffuse plaque in the thalamus (anti-β42 amyloid FCA 3542, 1/1 000), × 850; f. PrP granular deposits in the cytoplasm of two ganglion cells of the retina: (nf: nerve fiber layer, ip: inner plexiform layer, in: inner nuclear layer) (anti-PrP 8G8, 1/1000), × 1 700.
It is also notable that infected animals showed conspicuous signs of neurodegeneration, which are never seen in normal microcebes less than five years old (and were not seen in the control animals). These signs were characterized by a large number of Tau protein aggregates in the pyramidal cortical and brain stem neurons. Also, in two diseased animals (159 and 161), some PHF-immunoreactive Tau proteins were detected in different cortical areas (figure 3d). β-amyloid deposits were seen in vessel walls, and diffuse plaques were found in the cortex, thalamus (figure 3e), and cerebellum. Astrocytic gliosis was also pronounced (figure 3c).
4 Discussion
Although studies of BSE and vCJD in mice have provided some insights into host resistance, efficiency of different routes of infection, and distribution of tissue infectivity 〚15, 16〛, it is clearly desirable to verify and extend such information in a species more closely related to humans, i.e., in a primate model. We have shown that the prosimian primate species Microcebus murinus is susceptible both to oral and IC infection with BSE, and that incubation periods are considerably shorter than occur in infected macaque by IC and intravenous routes in BSE- and MBSE-infected macaques 〚6〛.
Animals sacrificed during the incubation period demonstrated an abnormal accumulation of PrP in various intestinal and lymphoreticular tissues and associated innervation as early as seven months after infection, and in the central nervous system beginning about 13 months after infection. These results are consistent with earlier immunohistological studies of primates fed with meat and bone meal nutritional supplements in French zoos 〚2–4〛. Our finding of PrPres deposits in the intestinal Paneth cells of the orally infected animal warrants further study in view of the role of these cells in protecting the small intestine crypts 〚17〛.
One particularly interesting observation, in view of the frequency with which emotional abnormalities occur in human patients with vCJD, is that many of our infected microcebes showed early and persistent behavioral changes.
The constellation of neurological illness, spongiform and degenerative neuropathology, progression of PrP tissue accumulation, and identification of PrPres, leaves no doubt about intracerebral transmission of macaque-passaged BSE to microcebes. The same ensemble of features, but without demonstrable PrPres in tissue extracts, provides highly suggestive but not conclusive evidence of oral transmission of primary (unpassaged) BSE. This could be explained by the presence of abnormal PrP that is sensitive to PK digestion, as previously reported in other experimental conditions 〚18〛. Intracerebral transmission of primary BSE and oral transmission of macaque-passaged BSE was not successful (primate-passaged BSE might occur without detectable PrPSc, as reported in mice 〚19〛).
Whether the reason for these irregularities lies in the small number of animals used in these experiments, or is the result of interspecies susceptibility factors, cannot at present be answered. It is worth noting in this regard that numerous primers reacting with human and simian PRNP genes have failed to induce PCR amplification of the microcebe PRNP gene, which has so far resisted all attempts at complete sequencing 〚20 and L. Cervenakovà, unpublished data〛. Thus, it is possible that heterogeneity between the microcebe and other primate PRNP genes may be responsible both for irregular transmission results and the difficulty of detecting PrPres with antibodies raised against non-microcebe PrP species.
PrP deposits in the ganglion cells of the retina accompanied by a vision deficit were noted in all diseased animals. This feature has been previously reported in scrapie-infected hamsters 〚21, 22〛, and may relate to the occurrence of visual disturbances in patients with CJD.
In the brain of naturally-infected macaque from the Montpellier zoo we previously reported prominent PHF-immunoreactive Tau proteins with numerous β-amyloid plaques and the presence of few β-amyloid diffuse plaques showing an acetyl-and butyryl-cholinesterase activities, accompanied by large numbers of PrP-positive plaques 〚23〛. PHF-immunoreactive Tau proteins are one of the hallmarks of an Alzheimer’s type pathology observed in some aged microcebes 〚7, 24〛, and in the present study using young animals, the occurrence of a few β-amyloid diffuse plaques, aggregated Tau proteins, PHF, and astriogliosis are consistent with this premature aging process.
Our results encourage the notion that this primate could be a useful experimental tool for pathogenetic investigations and elucidation of some parameters that govern transmissibility in primates with respect to BSE and vCJD. We have already undertaken experiments to evaluate the oral dose-response curve, define the distribution of infectivity in different organs and blood components, and study the possibility of vertical transmission of disease.
Acknowledgements
We thank C. Cohen-Solal, E. Huetter, B. Lasserre and S. Rouland for the quality of their technical assistance, F. Checler who provided the antibody anti-ß42 amyloid, and the Region Languedoc-Roussillon, Ministry of National Education, Research and Technology, and Naturalia et Biologia for their financial support.



