1 Introduction
Flycatchers are generally easily distinguished by their broad flat bills, with bristles around the nostrils, and by their characteristic hunting behaviour. However, the similarity of these characteristics does not necessarily reflect their phylogenetic relationships 〚1〛. New World or sub-oscine flycatchers (Tyrannidae), for example, have been distinguished from others by their particular syringeal morphology, shared by many other endemic South-American bird families.
The Old World or oscine flycatchers have traditionally been grouped into one large family, which does not form a natural group. Among them, it is possible to distinguish the muscicapine flycatchers (Muscicapidae) by their spotted juvenile plumage 〚2–4〛 and the distinctive “turdine thumb” like pattern of their syrinx. These characters are also shared by the thrushes, Turdidae 〚5〛.
The non-muscicapine Old World flycatchers were split into two groups. The first one is the Platysteiridae (shrike-, black-and-white and puff-back flycatchers, wattle-eyes), endemic to Africa and supposedly related to the African bush-shrikes, Malaconotidae 〚1, 6〛. A second group of non-muscicapine flycatchers is composed mainly of Australo-Papuan species, which were defined by external morphology and behaviour 〚7–9〛. Most have been arranged into two main sub-families, Rhipidurinae (2 genera, 40 species) and Monarchinae (19 genera, 95 species), or tribes or families depending on the authors 〚2, 3, 8, 10–12〛. For convenience, we refer to these two groups respectively as rhipidurine and monarchine birds. Rhipidurine birds (fantails) are easily defined because of their characteristic tail shape and fanning or wagging movements. However, a rigorous definition of the monarchine group is impossible 〚9〛; they comprise rather varied genera (18 by 〚12〛; 19 by 〚11〛), some with numerous species like Monarcha and Myiagra, some with very few species like Arses (two species), Clytorhynchus (four species), Peltops (two species), or some that are monotypic like Chasiempis, Mayrornis or Neolalage. Several species bear glossy plumage and coloured steel blue-gray bills, some have peculiar crests, some have sexual dimorphism (Myiagra), and some have elongated tail streamers (Terpsiphone). The family name Monarchidae is an accepted synonym of Myiagridae, formed originally around the type genus Myiagra Vigors and Horsfield, 1827 〚13〛.
The taxonomic position of the four non-Australasian genera (Trochocercus, Hypothymis, Terpsiphone, Erythrocercus) has been long debated 〚1, 8, 14–17〛, but they are generally all believed to be monarchine birds 〚11, 12〛; this hypothesis has to be verified. Moreover, beside this problem of family limit, another question is to establish whether the genus Trochocercus is polyphyletic, as it has been reported by several authors 〚18–22〛. These questions will be addressed with the following taxonomic sampling. Ten members of these following putative monarchine genera from Africa and Asia are included in our study: four Trochocercus (crested flycatchers, five species in Africa), two Hypothymis (blue monarchs, three species in Asia), two Terpsiphone (paradise flycatchers, 14 species both in Africa and in Asia), one Elminia (blue flycatchers, two species in Africa) and one Erythrocercus (three species in Africa). In order to clarify the position of the target groups of this study, we sequenced DNA for 27 additional species from various passerine families (see § Material and Methods, Table 1), including some other monarchines and species from the related genera Rhipidura and Dicrurus 〚1〛, as well as two species of the Asian flycatcher genus Culicicapa, whose relationships are unclear.
List of taxa studied, geographic origin, number of the samples used and Genbank accession numbers.
| Family or tribe S&M1 | Family H&M2 | Species | Origin | Number and Collection6 | Genbank accession numbers7 Cytb 16S | ||
| Monarchini | Monarchidae | Terpsiphone | viridis | Cameroon | MNHN, no 2-23 | AF094616 | AF094646 |
| ‘’ | ‘’ | ‘’ | paradisi | Laos | MNHN, no 5-53 | AF096466 | AF096497 |
| ‘’ | ‘’ | Hypothymis | azurea | Thailand | MNHN, no 4-10B | AF096467 | AF096496 |
| ‘’ | ‘’ | ‘’ | helenae | Philippines | ZMC, no 03728 | AF096468 | AF096495 |
| ‘’ | ‘’ | Trochocercus 3 | cyanomelas | Tanzania | ZMC, no 03874 | AF096469 | AF096494 |
| ‘’ | ‘’ | ‘’3 | nitens | Cameroon | MNHN, no 3-28 | AF096470 | AF096493 |
| ‘’ | ‘’ | ‘’4 | nigromitratus | Kenya | MNHN, CG 1976-989 | AF096472 | AF096492 |
| ‘’ | ‘’ | ‘’4 | albonotatus | Tanzania | ZMC, no 02939 | AF096471 | AF096491 |
| ‘’ | ‘’ | Elminia | longicauda | Cameroon | MNHN, no 1-03 | AF096474 | AF096490 |
| ‘’ | ‘’ | Erythrocercus | mccallii | Cameroon | MNHN, no 3-25 | AF096465 | AF096489 |
| ‘’ | ‘’ | Myiagra | caledonica | Loyalty Isl. | MNHN, CG 1979-922 | AF096463 | AF096488 |
| ‘’ | ‘’ | ‘’ | cyanoleuca | Australia | AMNH, no FB1048 | AF096464 | AF096487 |
| ‘’ | ‘’ | Pomarea | iphis | Marquises | MNHN no D41 | AF135053 | AF135054 |
| Rhipidurini | ‘’ | Rhipidura | albicollis | Laos | MNHN, no 5-48 | AF096462 | AF096486 |
| ‘’ | ‘’ | ‘’ | cyaniceps | Philippines | ZMC, no 01876 | AF096461 | AF096485 |
| Dicrurini | Dicruridae | Dicrurus | paradiseus | Laos | MNHN, no 5-57 | AF096473 | AF096475 |
| Malaconotini | Platysteiridae | Platysteira | cyanea | Cameroon | MNHN, no 2-22 | AF096452 | AF096483 |
| Malaconotini | Laniidae | Telophorus | sulfureopectus | Malawi | MNHN, no 29 | AF096456 | AF096476 |
| Corvini | Corvidae | Corvus | corone | France | MNHN, no 13-16 | AF094613 | AF094643 |
| Oriolini | Oriolidae | Oriolus | xanthornus | Thailand | MNHN, no4-10D | AF094615 | AF094645 |
| Laniidae | Laniidae | Lanius | collaris | Thailand | MNHN, no 2-26 | AF094614 | AF094644 |
| Eopsaltriidae5 | Eopsaltriidae5 | Eopsaltria | australis | Australia | AMNH, no FB1550 | AF096455 | AF096484 |
| Sittidae | Sittidae | Sitta | europaea | France | MNHN, no 9-15 | AF135049 | AF135055 |
| Muscicapidae | Turdidae | Phoenicurus | phoenicurus | France | MNHN, no 22-43 | AF135050 | AF135057 |
| ‘’ | Muscicapidae | Muscicapa | striata | France | MNHN, no 13-1A | AF096458 | AF096480 |
| ‘’ | ‘’ | Culicicapa | ceylonensis | Thailand | MNHN, no 4-9G | AF096453 | AF096482 |
| ‘’ | ‘’ | ‘’ | helianthea | Philippines | ZMC, no 0783 | AF096454 | AF096481 |
| Pycnonotidae | Pycnonotidae | Andropadus | latirostris | Cameroon | MNHN, no 2-52 | AF096457 | AF096477 |
| Cisticolidae | Sylviidae | Camaroptera | brevicaudata | Cameroon | MNHN, no 2-15 | AF094626 | AF094654 |
| Sylviidae | ‘’ | Orthotomus | sutorius | Thailand | MNHN, no 4-8E | AF094622 | AF094652 |
| ‘’ | ‘’ | Acrocephalus | aedon | Thailand | MNHN, no 4-8D | AF094623 | AF094653 |
| ‘’ | ‘’ | Sylvia | melanocephala | France | MNHN, no S4 | AF135052 | AF135056 |
| ‘’ | Timaliidae | Garrulax | leucolophus | Thailand | MNHN, no 4-6E | AF094627 | AF094655 |
| ‘’ | ‘’ | Pellorneum | ruficeps | Thailand | MNHN, no 4-6F | AF094632 | AF094660 |
| Passeridae | Motacillidae | Anthus | pratensis | France | MNHN, no 13-5A | AF096460 | AF096479 |
| Passeridae | Ploceidae | Passer | domesticus | France | MNHN, no 13-5C | AF094639 | AF094667 |
| Tyrannidae | Tyrannidae | Tyrannus | melancholicus | S. America | MNHN, no 12-33 | AF135051 | AF135058 |
1 The taxonomy presented here follows Sibley and Monroe 〚11〛; the listed tribes belong to Corvidae.
2 The taxonomy follows here Howard and Moore 〚12〛.
3 These two species are under generic name Terpsiphone in Howard and Moore.
4 These two species are under generic name Elminia in Howard and Moore.
5 Family name Petroicidae is used in the text as it is the correct family name for Eopsaltriidae, because of priority.
6 AMNH: American Museum of Natural History, New York, USA; MNHN: National Museum of Natural History, Paris, France; ZMC: Zoological Museum and Institute of Zoology, Copenhagen, Denmark.
7 Genbank accession numbers like AF094xxx correspond to DNA sequences presented by Cibois et al. 〚26〛.
We present molecular data obtained from partial sequences of two mitochondrial genes: cytochrome b (cytb) and the large sub-unit ribosomal RNA (16S rRNA). Numerous previous molecular studies have provided evidence for the phylogenetic effectiveness of cytb at various taxonomic levels 〚e.g. 23〛, whereas the 16S has not often been used for resolving phylogenies among the Passeriformes (see 〚24–26〛).
2 Material and methods
2.1 Species and source of DNA
Our samples (Table 1) of monarchine birds include representatives of all the African and the Asian genera (10 species) and two typical Australasian genera (Myiagra, two species, Pomarea one species) as well as representatives of the related genera Rhipidura (two species) and Dicrurus (one species) 〚1〛. We include also in our analysis one species (or more, as specified) of more or less related passerines families or tribes (following the taxonomy given by 〚12〛): Corvini, Oriolini, Malaconotini (two species), Laniidae, Petroicidae (correct family name for Eopsaltriidae, because of priority 〚27〛), Sittidae, Muscicapidae (five species, including the two Culicicapa), Pycnonotidae, Cisticolidae, Sylviidae (five species), and Passeridae (two species). Tyrannus melancholicus (Tyrannidae) was used as the outgroup; morphological and molecular studies have shown that it is without doubt outside the oscine radiation (e.g. 〚1, 28〛).
Genomic DNA was extracted from either frozen or alcohol-preserved tissues (muscle, liver, blood) or small pieces (0.5 to 1 cm2) of museum skins (labelled MNHN, CG in Table 1), using CTAB buffer containing proteinase K (0.1 mg/ml) 〚29–30〛. In the case of museum skins, protein digestion time was expanded from 2 to 24 h.
2.2 DNA amplification and sequencing
Polymerase Chain Reaction (PCR) was performed on DNA samples for 35–40 cycles. For each cycle, denaturation was done at 93 °C for 30 s, annealing at 50–55 °C for 40 s, and extension at 72 °C for 40 s. Most amplifications were performed with primers 2 and 4 (Table 2); one or two other pairs of primers were used for all other amplifications (see legend of Table 2). Sequencing was performed with amplification primers and internal primers 5 and 6. For phylogenetic analysis, we used two homologous fragments, including 836 bases from cytb and 503 bases located at the 3’-end of the 16S gene. These are positions 15025-15860 and positions 3254-3756 respectively in the chicken mitochondrial genome 〚31〛. For Elminia longicauda, Trochocercus nigromitratus and Pomarea iphis, cytb sequences were 200 bases shorter at the 5’ end, because we could not amplify this section with either L primers number 1 or 2; some other sequences are 1 to 8 bases shorter. Sequencing reactions were performed by direct cycling PCR with the ‘Thermo Sequenase Cycle Sequencing’ kit from Amersham Pharmacia Biotech. Electrophoresis was performed on polyacrylamide gels with manual sequencers. Autoradiographs were read twice independently and managed with the MUST package 〚32〛.
Primers used in this study. Most of the samples were amplified with primer pair 2 & 7. For Terpsiphone viridis, Rhipidura cyaniceps and Muscicapa striata, we used primer pair 2 & 8; for Hypothymis helenae, Trochocercus cyanomelas and Pellorneum ruficeps, we used primer pairs 1 & 4 and 3 & 7; for Trochocercus nigromitratus, Pomarea iphis and Elminia longicauda, we used primer pair 3 & 7, which delimit a shorter sequence of 636 bases. Primers 5 and 6 were used for internal sequencing.
| Primers | References |
| cytb: | |
| 1. L14827 (ND5) (5’-CCA-CAC-TCC-ACA-CAG-GCC-TAA-TTA-A-3’) | 〚49〛 |
| 2. L14990 (5’-CAT-CCA-ACA-TCT-CTG-CTT-GAT-GAA-A-3’) | 〚50, modified〛 |
| 3. L15206 (5’-CAC-ATC-GGC-CGA-GGA-ATC-TAC-TA-3’) | 〚26〛 |
| 4. H15298 (5’-CAG-CCC-CTC-AGA-ATG-ATA-TTT-GTC-CTC-A-3’) | 〚50, modified〛 |
| 5. L15383 (5’-GGA-CAA-ACA-CTA-GTA-GAA-TG-3’) | 〚26〛 |
| 6. H15487 (5’-GAT-CCT-GTT-TCG-TGG-AGG-AAG-GT-3’) | 〚51〛 |
| 7. H15916 (5’-ATG-AAG-GGA-TGT-TCT-ACT-GGT-TG-3’) | 〚52〛 |
| 8. H16065 (tRNA-thr) (5’-GGA-GTC-TTC-AGT-CTC-TGG-TTT-ACA-AGA-C-3’) | 〚49〛 |
| 16S: | |
| L3214 (5’-CGC-CTG-TTT-ATC-AAA-AAC-AT-3’) | 〚53〛 |
| H3783 (5’-CCG-GTC-TGA-ACT-CAG-ATC-ACG-T-3’) | 〚54〛 |
2.3 16S gene alignment
Like other rRNA, 16S has a specific secondary structure with stems (paired regions) and loops (unpaired regions). Alignment of the sequences was obtained in two different ways. Firstly by eye, minimizing the number of gap positions. Secondly, we used the computer program MALIGN (version 1.9, 〚33〛), which aligns by optimising the length of the sequences with the shortest tree via heuristic searching and branch swapping. We used the following options, quick, iter, score 3, tresswap, alignswap, changecost 2 and internal 3, which gave consistent results 〚see also 26〛. Gaps in 16S sequences were treated as a fifth character, with or without coding the multigaps using BARCOD 〚34〛. All analyses gave very similar results.
2.4 Phylogenetic analyses
For both methods used (neighbour-joining and parsimony), we first analysed cytb and 16S data sets separately and then combined both data sets into a single analysis. The incongruence between cytb and 16S datasets was also evaluated by ILD test 〚35〛. Neighbour-joining (NJ) topologies were obtained with uncorrected distances calculated with PAUP* from full sequences for both separate and combined datasets 〚36〛. Parsimony analyses (MP) were also performed with PAUP* using the heuristic algorithm, TBR swapping, and 100 random addition-sequence replicates. Cytb data were also weighted for transitions versus transversions using a step matrix in PAUP*. The weight applied to transversions was determined via saturation analysis (not shown; 〚37〛). No weighting was used with 16S data, as there was no evidence of saturation (not shown). The robustness of NJ and MP trees was tested by bootstrap analysis with 1000 replicates 〚38〛. The polyphyly of the monarchine birds was evaluated by the Kishino–Hasegawa test 〚39, 40〛 by comparing the resulting optimal tree topologies with the most parsimonious trees using constrained topologies in PAUP*. Sequences were deposited in Genbank (Table 1).
3 Results
3.1 Sequences variation and saturation
As expected for a protein-coding gene, our cytb sequences showed no insertions or deletions and their base composition and pattern of variability are typical of avian cytb sequences, suggesting that they are actual cytb and not nuclear pseudogene sequences.
Among the 836 sites analysed, a total of 425 sites (50.8%) were variable and 331 (39.6%) were potentially phylogenetically informative. As with many other cytb studies, comparison of transitions and transversions percentages, plotted for each pair of species, indicated saturation with increasing sequence divergence (not shown). From the initial slope of this transition/transversion distribution, we estimated their ratio to 4:1. This value was subsequently used to weight transversions in the cytb analyses. A few gaps were necessary for the alignment of 16S sequences: manual and MALIGN alignments led to sequence lengths of 503 (see Appendix) and 511 bases respectively (not shown). Recoding manual alignment using BARCOD added 17 extra characters. MALIGN alignment did not need to be recoded as no informative contiguous gaps were obtained. Among the 503 sites of manual alignment, 175 (35%) were variable and 124 (24%) were potentially phylogenetically informative. There was no evidence of saturation in the 16S data (not shown).
Manual alignment of 16S sequences corresponding to amplified segment; parts with insertions (noted *).
3.2 Phylogenetic results
Supported results are presented in Table 3 for separate and combined analyses (nodes bearing bootstrap proportions more than 50%) and full topologies are presented only for NJ and MP combined analyses (Figs. 1 and 2). The cytb analyses (NJ or MP) led to a poorly resolved topology, with only 10 to 14 nodes supported by bootstrap proportions of more than 50% (Table 3). The 16S analyses gave very similar results whatever the method used, NJ or MP (17 nodes at same bootstrap level, Table 3). No incongruence was evident between both datasets (ILD test not significant) and 8 to 13 of the supported nodes were common to analyses of both genes, depending of the method of analysis. The combined analyses resulted in 15 to 20 supported nodes (Table 3).
Bootstrap support indices (only values >50% shown) obtained with various analyses. ‘ Tv*4 ’ indicates that a weight of 4 is given to transversions. The 16S analyses based on different alignments, with coding the gaps or not, gave similar results; only one set of result is presented.
| Gene | Cytb | 16S | Both genes | ||||||
| Sites | No weigthing | Tv*4 | No weigthing | No weigthing | (Cytb :tv4) | ||||
| Node | Method | NJ | MP | MP | NJ | MP | NJ | MP | MP |
| 1 | Rhipidura albicollis + cyaniceps | 72 | 100 | 97 | 100 | 90 | 77 | ||
| 2 | (1) + Corvus | 72 | |||||||
| 3 | Lanius + Dicrurus | 56 | 61 | 66 | 61 | 57 | 74 | ||
| 4 | Myiagra caledonica + cyanoleuca | 100 | 100 | 100 | 100 | 97 | 100 | 100 | 100 |
| 5 | Trochocercus cyanomelas + nitens | 100 | 100 | 100 | 98 | 86 | 100 | 100 | 100 |
| 6 | Terpsiphone paradisi + viridis | 66 | 85 | 85 | 72 | 66 | 86 | ||
| 7 | Hypothymis azurea + helenae | 57 | |||||||
| 8 | Terpsiphone + Hypothymis | 85 | 50 | 86 | 76 | 77 | 97 | 86 | 96 |
| 9 | (8) + Trochocercus | 60 | 54 | 74 | 50 | 72 | |||
| 10 | (9) + Pomarea | 57 | 59 | 52 | 63 | 62 | 75 | ||
| 11 | Monarchine birds | 65 | 62 | 100 | 94 | 100 | 97 | 87 | |
| 12 | Phoenicurus + Muscicapa | 93 | 82 | 79 | 93 | 74 | 100 | 97 | 86 |
| 13 | Anthus + Passer | 84 | 90 | 79 | 73 | 74 | 99 | 99 | 92 |
| 14 | Culicicapa ceylonensis + helianthea | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 15 | Trochocercus albonotatus + nigromitratus | 85 | 69 | 59 | 94 | 86 | 98 | 94 | 76 |
| 16 | (15) + Elminia | 90 | 90 | 100 | 100 | 96 | 100 | 100 | 100 |
| 17 | Camaroptera + Orthotomus | 66 | 80 | 100 | 100 | 100 | 99 | 99 | |
| 18 | Erythrocercus + Andropadus | 55 | 56 | 74 | |||||
| 19 | Garrulax + Pellorneum | 68 | 74 | 70 | 70 | ||||
| 20 | (19) + Sylvia | 60 | 57 | ||||||
| 21 | Sylvii-Timalii-Pycnonotidae clade | 63 | 57 | 84 | 82 | ||||
| 22 | (21) + (16) + (14) | 52 | 55 | ||||||
| Number of nodes > 50% | 14 | 10 | 14 | 17 | 17 | 20 | 17 | 15 | |
| Number of MP trees | 5 | 1 | 14 | 4 | 1 | ||||
| Length of the MP trees | 2275 | 4997 | 717 | 3024 | 5748 |
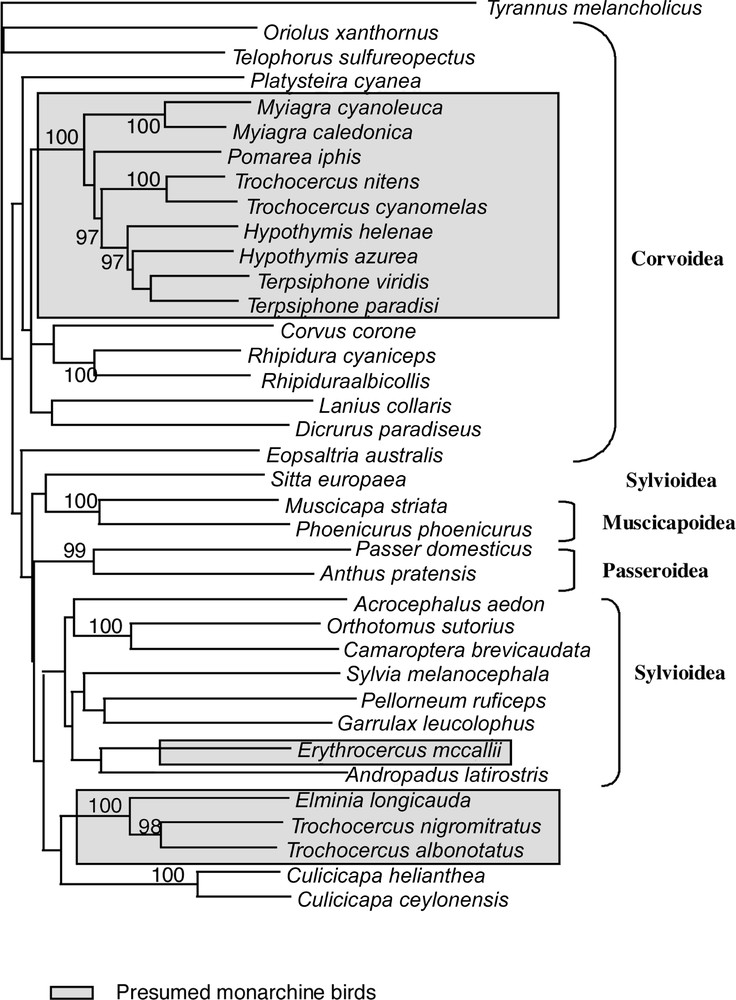
Unweighted NJ tree obtained with NJ analysis of combined datasets (cytb and 16S). Bootstrap values shown are those higher than 90%.
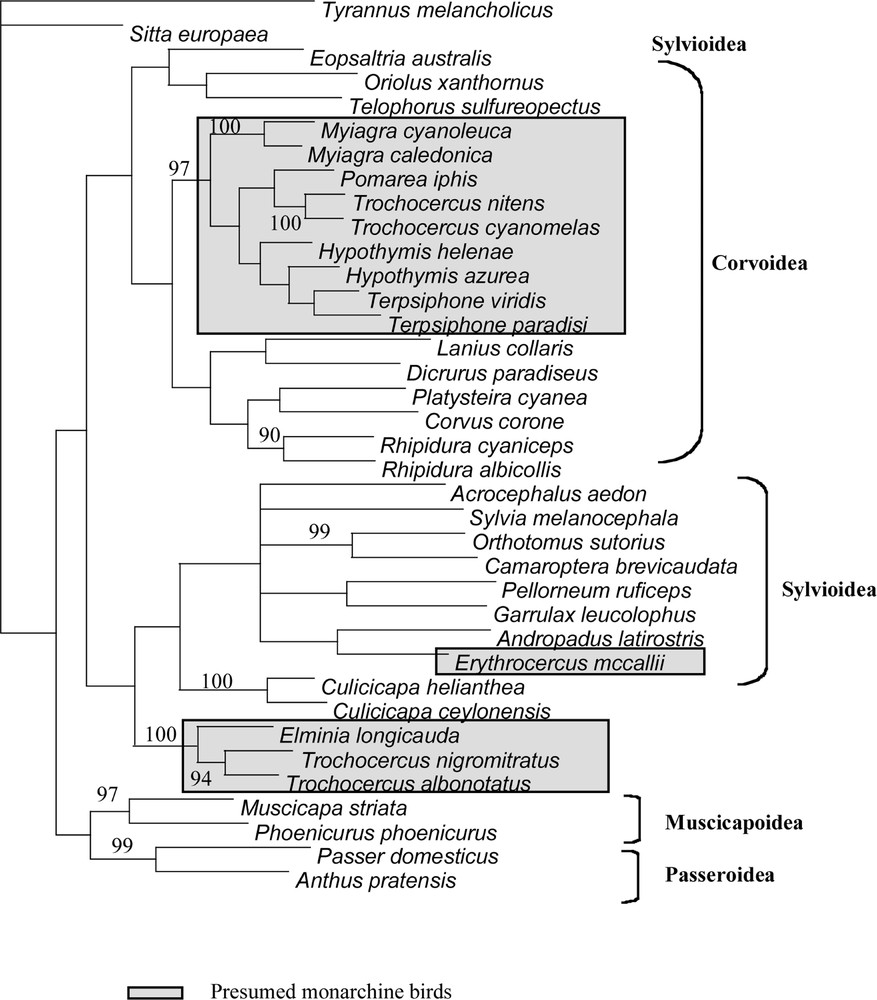
Strict consensus of 4 MP trees obtained from combined datasets (cytb and 16S). Bootstrap values shown are those higher than 90%.
All phylogenetic results present the following well defined clades. Node # 11 (Table 3) includes Terpsiphone, Hypothymis, Myiagra, Pomarea and two of the four Trochocercus, T. nitens and T. cyanomelas (both members of the superspecies T. 〚cyanomelas〛). Inside this clade, the representatives of each genus are often linked in terminal positions, but the two species of genus Hypothymis are in a paraphyletic or unresolved position in most of the analyses. Terpsiphone and Hypothymis form a clade (# 8 in Table 3), which is consistent in all analyses. When Trochocercus, Pomarea and Myiagra species are added, this clade #8 is included within deeper clades in paraphyletic positions. The second well-defined clade (# 16 in Table 3) is composed of Elminia and the two other Trochocercus (T. albonotatus and T. nigromitratus, both members of the superspecies T. 〚albonotatus〛). Members of this clade do not appear closely related to the Trochocercus or any member of clade # 11, nor to any of the Corvoidea or other super-family representatives, but instead this clade is sister-group to Culicicapa, which is not closely related to Eopsaltria, monarchine birds or Muscicapoidea. We tested the monophyly of Trochocercus species for the most parsimonious trees of the combined analyses (all unweighted sites: length 3024; weighting cytb by Tv4: length 5748; see Table 3) with constrained topologies (Kishino and Hasegawa test): the monophyly of the Trochocercus is rejected for both topologies (p < 0.0001), either when Elminia is included or not. The monophyly of the monarchine birds (clade # 11 + Erythrocercus + clade # 16) is also rejected (p < 0.0001) in both topologies. If we exclude Erythrocercus from this test, the monophyly of monarchine birds is still rejected for both topologies, albeit weakly (p = 0.0926 and p = 0.0024). Erythrocercus is not closely related to any other presumed monarchine birds (from clade # 11 or clade # 16), but belongs to the main Sylvioidea clade (clade # 21 in Table 3).
Other reasonably supported associations between different genera confirm previous studies and will not be further discussed: a) Anthus and Passer 〚see 1, 41〛, b) Pellorneum and Garrulax (see 〚26〛), c) Orthotomus and Camaroptera (see 〚26, 42–44〛), and d) Muscicapa and Phoenicurus (see 〚1〛).
4 Discussion
Our results clearly illustrate the polyphyly of the African monarchine birds and that none of them is closely related to Muscicapoidea and Passeroidea. The genus Trochocercus in particular appears polyphyletic and, on the basis of body shape, proportions, skull characters and etho-ecological arguments 〚e.g., 18–22, 45〛, was already divided previously into two groups: Trochocercus-1 (cyanomelas and nitens) and Trochocercus-2 (albonotatus, nigromitratus and albiventris). Our molecular results confirm this division and that Hypothymis, Terpsiphone and Trochocercus-1 are monarchine birds (in Corvoidea), as proposed by Sibley and Ahlquist 〚1〛, who based their study only on Terpsiphone paradisi, Hypothymis azurea, Trochocercus cyanomelas, but with a larger Australasian taxonomic sampling. These species belong to a clade including typical monarchine taxa like Myiagra or Pomarea. The generic name Trochocercus given by Cabanis (1850) to Muscicapa cyanomelas Vieillot, 1818 (type species by monotypy), but used subsequently for the five species cyanomelas, nitens, nigromitratus, albiventris and albonotatus, must now be restricted to the species cyanomelas and nitens (i.e. Trochocercus-1). These latter Trochocercus species were already believed to be close to Terpsiphone on the basis of similar skull osteology, and it was even suggested that both genera could be merged 〚21, 22〛. But most taxonomists 〚see particularly 46〛 maintained Trochocercus as a distinct genus, and Erard et al. 〚47〛 eventually adopted this conservative position. General habits of Hypothymis, as well as calls and songs (C. Chappuis, pers. comm.), are also quite similar to those of Terpsiphone, suggesting that these three genera are closely related. Our molecular results show that Terpsiphone is closer to Hypothymis than to Trochocercus-1 and that, unless all three genera are merged, Trochocercus should be maintained as a distinct genus.
Species from Trochocercus-2 were believed to be related to Elminia 〚20, 21, 45〛, and this is confirmed by our study. General behaviour, breeding habits, and vocalizations supported this close relationship 〚see 21, 47 for details〛. However, differences exist in plumage patterns and body proportions, so one may wonder whether maintaining two separate genera would be appropriate, particularly in the light of the clear molecular divergence, but unclear relationship, existing between Elminia longicauda and the pair albonotatus/nigromitratus. We propose here to merge nigromitratus, albiventris and albonotatus into Elminia (s.l.), as it was done by Howard and Moore 〚12〛, but not by Sibley and Monroe 〚11〛.
Because of our large non-monarchine taxonomic sampling, the values of the tests and the general pattern of supported nodes, we can also conclude that Trochocercus-2 and Elminia, as well as Erythocercus, are not monarchine. Elminia (s.s.) and Erythrocercus have previously always been considered to be monarchine. Species now included in the genus Elminia (s.l.) are known to share a characteristic behaviour with Rhipidura; continuously they fan their tail, droop their partly open wings, and progress with jerky pivoting movements. Because of these striking similarities in behaviour and also some similarity in external morphology and breeding habits, including nest characteristics, some authors (e.g., 〚20, 47, 48〛) have speculated about a possible relationship between them. Present molecular data do not provide support for such a close relationship. Likewise they do not support the very close relationship between Rhipidura species and monarchs suggested by some authors, who lumped them in the same family. Ames 〚5〛 and Olson 〚22〛 have stressed the fact that Elminia (s.l.) and Erythrocercus lack the corvine configuration of the humerus (unlike corvids, they have a non-pneumatic humerus, with a double tricipita fossa) and the amphirhinal condition of ossification of the nostril, characters present in true monarchs. Elminia (s.l.) and Erythrocercus are thus neither monarchine, nor rhipidurine, nor muscicapid birds (they also lack the ‘turdine thumb’ pattern of the syrinx 〚5〛, and spotted juvenile plumage). They do not belong to the Corvoidea, but seem close to the Sylvioidea.
Traylor 〚16〛 suggested a close relationship between Erythrocercus and Culicicapa, but Erard et al. 〚47〛 found the call and songs of Erythrocercus reminiscent of those of Elminia species. Molecular results obviously indicate that Erythrocercus is not a close relative of these birds. Moreover, all analyses place Erythrocercus within the Sylvioidea clade with clear though not strong support (clade #21, 82–84%, see Table 3).
The systematic position of Culicicapa has been much debated. For a long time, Culicicapa was believed to be either a member of Muscicapidae 〚2〛, or a close relative of Rhipidura 〚15〛, of Erythrocercus 〚16〛, or a member of the Petroicidae 〚11〛. Unfortunately, Sibley and Ahlquist 〚1〛 provided no data. A relationship between Elminia (s.l.) and Culicicapa is only suggested by our molecular data and together they may constitute the sister-group of the Sylvioidea considered in the present study. However, Culicicapa and Elminia differ greatly in general postural and vocal behaviour, as well as in foraging behaviour 〚47, Pasquet pers. obs.〛. Culicicapa, like Elminia, belongs neither to Muscicapidae nor to Petroicidae. Though one may be tempted to introduce new family names for them, the exact relationships of Erythrocercus, Culicicapa and Elminia need further detailed studies with more material for comparisons.
5 Conclusion
Though African Terpsiphone and part of Trochocercus (i.e. Trochocercus-1) are really monarchs and belong to the Monarchidae, it is sure that other Trochocercus (i.e. Trochocercus-2), Elminia and Erythrocercus are not. Trochocercus-2 and Elminia belong to the same family, but this family will remain indeterminate as long as the exact relationships with Culicicapa and the various families of the Sylvioidea are unresolved. Erythrocercus belongs to another family, which also requires further study before it is clearly identified. More material and wider taxonomic coverage will probably show that new family names are necessary for these birds.
Morphological (e.g. bill and tail shape, plumage texture and pattern, body proportions), ecological and behavioural (e.g. foraging location and behaviour, nesting habits) similarities that these birds share with monarchs reflect more convergent fine-tuned adaptations to habitat and resource exploitation than relationships.
Obviously oscine families require revisions and better definitions. More comparative phylogenetic studies, based on large samples and wide taxonomic coverage, are needed before it can be envisioned a reliable picture of the evolution of passerines and of the relationships among families in this order, which gathers more than half of extant bird species.
Acknowledgements
We are grateful to colleagues who provided tissues: J. Fjeldså and J. Garcia-Moreno (Institute of Zoology, Copenhagen, Denmark), J. Cracraft (American Museum of Natural History, New York, USA), M. Combrexelles (MNHN, Paris, France), J.-C. Thibault (PNR, Corse, France) and to O. Khobkhet (Kasetsart University, Bangkok, Thailand) and Han Lian Xian (South West Forestry University, Kunming, P.R. China) who helped É. Pasquet in Asian field work. This work was conducted at, and supported by, the ‘Service de systématique moléculaire’, CNRS–FR 1541, MNHN. Other financial support was provided by IRD (ex-ORSTOM), by the ‘Pluriformations Asie du Sud-Est’ Programme and by the ‘Réseau national de biosystématique’ (ACC-SV7). A. Cibois was supported on a Chapman postdoctoral fellowship at the American Museum of Natural History while working on this project. We are also grateful to Jon Fjeldså, John Harshman, Simon Tillier, anonymous referees, and particularly Frederick H. Sheldon, who provided us with many valuable comments and suggestions on earlier drafts of the manuscript, and to Raoul Mulder, who amended the English writing.
Version abrégée
Les caractéristiques morphologiques et éco-éthologiques des gobe-mouches ne reflètent pas nécessairement les relations de parenté entre les espèces. Cependant, les gobe-mouches de l’Ancien Monde (du sous-ordre des Oscines) sont souvent réunis dans un nombre variable de groupes (familles ou sous-familles), dont la définition et le contenu changent au gré des auteurs. Les monarques (Monarchidae) occuperaient l’Australasie, l’Asie et l’Afrique. Dans ce dernier continent, ils sont censés être représentés par le genre Terpsiphone, qui est répandu jusqu’en Asie, et par trois autres genres endémiques : Trochocercus, Elminia et Erythrocercus. Les données morphologiques, écologiques et éthologiques disponibles suggèrent l’existence de deux groupes au sein des Trochocercus, dont un se rapprocherait des Elminia et l’autre des Terpsiphone. Par ailleurs, Erythrocercus se singulariserait par un certain nombre de traits biologiques.
Pour éclaircir cette apparente hétérogénéité des monarques africains, nous avons établi une phylogénie moléculaire basée sur les séquences partielles (déposées dans GenBank) de deux gènes mitochondriaux (cytochome b et ARNr 16S) d’un échantillon représentatif des Monarchidae africains, asiatiques et australasiens, des familles actuellement considérées comme étant leurs plus proches parents, ainsi que des principales familles de passereaux caractérisant les grands groupes reconnus chez les Oscines: les Corvoidea, les Sylvioidea, les Muscicapoidea et les Passeroidea. Les résultats sont figurés par deux arbres phylétiques établis par l’analyse combinée de jeux de données sur les deux gènes considérés. L’un a été obtenu par la méthode des distances (neighbour-joining, NJ), l’autre par la méthode de parcimonie (MP) avec enracinement à l’aide d’un gobe-mouche néotropical (Tyrannidae, Sub-Oscine); leur robustesse a été testée par bootstrap analysis avec 1000 réplicats.
Les monarques africains sont indiscutablement polyphylétiques. Aucun n’apparaît proche des Muscicapoidea, ni des Passeroidea. Le genre Trochocercus est clairement divisé en deux groupes. L’un comprend les deux espèces cyanomelas et nitens; il est étroitement associé aux Terpsiphone et autres Monarchidae (genres Hypothymis, Pomarea, Myiagra) dans les Corvoidea. L’autre, représenté par les espèces nigromitratus et albonotatus, est le groupe-frère de Elminia. Ce second clade 〚deuxième groupe de Trochocercus + Elminia〛 est très nettement séparé des premiers. Les espèces qui le composent ne sont donc pas des monarques. Ce clade apparaît comme le groupe-frère des Sylvioidea, pris en compte dans cette étude. Il n’est non plus aucunement proche parent des Rhipiduridae (qui sont des Corvoidea asiatiques, sans doute pas aussi proches des monarques, comme on a pu parfois le croire), comme certains morphologistes ou éco-éthologistes l’ont suggéré. Le genre Erythrocercus, quant à lui, ne s’inscrit pas non plus parmi les monarques, mais se place dans les Sylvioidea et, curieusement, près des bulbuls (Pycnonotidae). Les gobe-mouches asiatiques du genre Culicicapa, que certains taxinomistes ont rapproché des monarques africains, se placent plutôt près du clade incluant les Elminia.
Il est donc certain qu’une partie des Trochocercus, les Elminia et les Erythrocercus ne sont pas des Monarchidae. Si les deux premiers sont bien de la même famille, qui reste d’ailleurs à déterminer, les Erythrocercus appartiennent à une autre qui, elle aussi, requiert des études complémentaires pour son identification. Il est probable qu’un matériel plus conséquent et une couverture taxinomique plus large montreraient la nécessité de caractériser des familles nouvelles pour ces oiseaux. Les ressemblances morphologiques et étho-écologiques qui existent entre ces oiseaux et les monarques relèveraient de phénomènes de convergence dans l’occupation du milieu et l’utilisation de ses ressources. Manifestement, les familles de passereaux Oscines demandent des révisions. D’autres études comparatives, basées sur un échantillonnage plus important dans un éventail taxinomique beaucoup plus large, sont nécessaires avant que l’on puisse prétendre commencer à posséder une image fiable de l’évolution et des liens de parenté entre les familles qui constituent cet ordre, qui regroupe la moitié des espèces d’oiseaux actuels.


