1 Introduction
The Caenophidia, which are also called advanced snakes, include the aquatic genus Acrochordus and the Colubroidea (Fig. 1). All venomous snakes are found within the Colubroidea, which include the great majority of extant snakes (around 2400 species out of 3000 extant snake species) 〚1〛. The Colubroidea comprise the Atractaspididae (some of them with a front-fanged venom system), the Elapidae and the Viperidae (all of them with a front-fanged venom system), and the paraphyletic ‘Colubridae’ (defined by the absence of a front-fanged venom system), which include the vast majority of Colubroidea (around 1850 species) and therefore the majority of extant snakes (Fig. 1) 〚1–11〛. Interrelationships of most colubroid lineages are still unresolved (Fig. 1) and our aim is first to shed light on these multifurcations using DNA sequences and then to address the following evolutionary question: when did the venom apparatus evolve within Caenophidia? For this purpose, 188 sequences (104 of which are original) were used, obtained from one nuclear and three mitochondrial genes and representing all major caenophidian lineages.
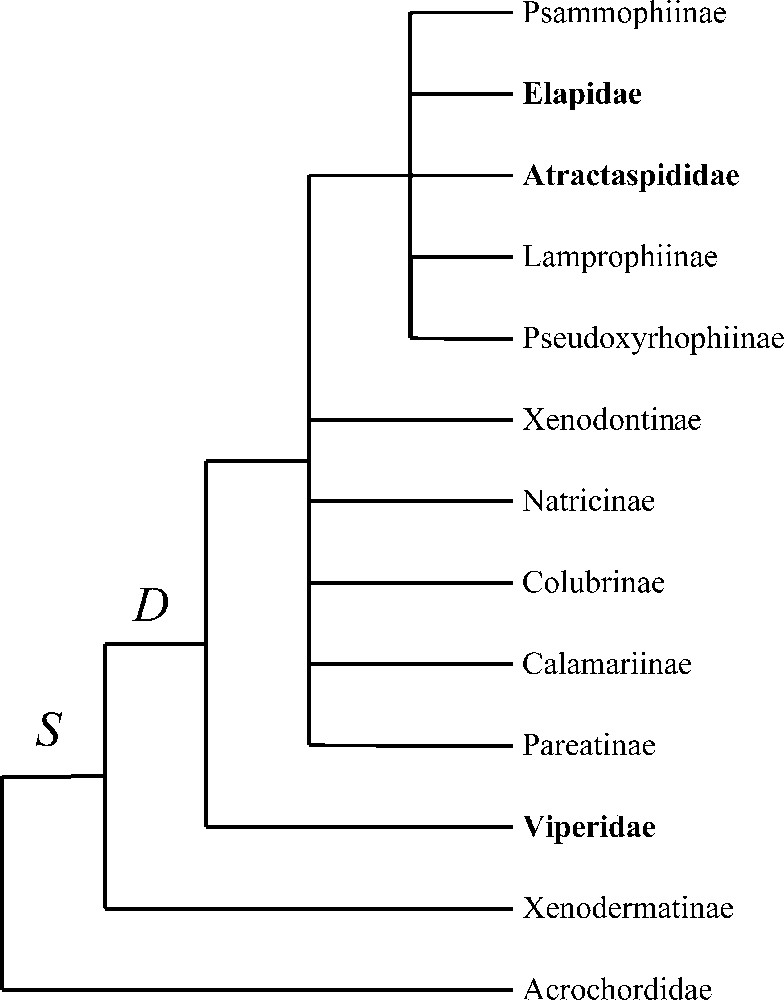
Phylogenetic relationships of Caenophidia based on Cadle 〚2, 3〛, McDowell 〚4〛, Underwood and Kochva 〚5〛, Knight and Mindell 〚6〛, Heise et al. 〚7〛, Kraus and Brown 〚8〛, Zaher 〚9〛, Vidal et al. 〚10〛 and Vidal 〚11〛. Traits: S, supralabial secretory serous cells; D, differentiated maxillary dentition. Terminal taxa written in bold possess a front-fanged venom system. Caenophidia other than Acrochordidae and lacking a front-fanged venom system are traditionally classified as ‘Colubridae’. Pseudoxenodontines and homalopsines are not shown (unknown phylogenetic relationships).
2 Materials and methods
Tissue samples (tissue homogenate, liver, blood, tail tip, or shed skin) were obtained from the tissue collections of Nicolas Vidal and S. Blair Hedges (see Appendix 1). All protocols (from DNA extraction until sequence analyses) are identical to those described in our companion paper on higher-level snake phylogenetic relationships and only those corresponding to the ND4 mitochondrial gene are described here. Amplification of the ND4 fragment was performed using the following set of primers: ND4, 5’–TGA–CTA–CCA–AAA–GCT–CAT–GTA–GAA–GC–3’ 〚12〛 and LEU, 5’–TAC–TTT–TAC–TTG–GAT–TTG–CAC–CA–3’ 〚12〛. Sequence data obtained from Genbank or the references are listed in Appendix 2. Alignment of the ND4 sequences was straightforward as there were no indels. Alignments will be deposited in EMBL alignment database and the corresponding sequences will be deposited in GenBank upon publication. In some cases, different lower-level taxa (species or genera) were used as exemplars for higher-level taxa (subfamilies or families) in different genes (C-mos and 12/16S rRNA on the one hand, ND4 on the other hand). In those cases, we constructed hybrid sequences after having checked, with separate analyses, that those lower ranked taxa belonged to monophyletic higher ranked taxa.
3 Results and discussion
3.1 Models of evolution selected
For the models used in the 12S-16S rRNA analysis and the C-mos/12-16S rRNA combined analysis (both covering the diversity of snake and caenophidian lineages), see our companion paper on higher-snake phylogenetic relationships (this issue).
The C-mos caenophidian dataset includes 435 bp for 50 taxa (187 variable sites, 110 of which are informative for parsimony). The model selected is the HKY+G model (base frequencies: A (0.308), C (0.185), G (0.202), T (0.305); TS/TV ratio: 2.39; G: 0.64). The ND4 caenophidian data set includes 694 bp for 46 taxa (442 variable sites, 394 of which are informative for parsimony). The model selected is the TVM+I+G model (base frequencies: A (0.406), C (0.355), G (0.048), T (0.192); rate matrix: 〚AC〛: 0.16, 〚AG〛: 4.81, 〚AT〛: 0.52, 〚CG〛: 0.59, 〚CT〛: 4.81, 〚GT〛: 1; I: 0.26; G: 0.41). The combined C-mos/12S-16S rRNA/ND4 caenophidian data set includes 1968 bp for 32 taxa (955 variable sites, 742 of which are informative for parsimony). The selected model is the GTR+I+G model ((base frequencies: A (0.363), C (0.277), G (0.145), T (0.216); rate matrix: 〚AC〛: 2.79, 〚AG〛: 4.91, 〚AT〛: 2.17, 〚CG〛: 0.57, 〚CT〛: 16.35, 〚GT〛: 1; I: 0.36; G: 0.52).
3.2 Phylogenetic results
In addition to the bootstrap consensus ME tree obtained from the combined analysis including four genes (C-mos/12S-16S rRNA/ND4), the bootstrap consensus ME tree obtained from the C-mos alone analysis is also presented as it includes additional lineages. Results discussed below are based on the tree resulting from the combined analysis except otherwise mentioned.
3.2.1 Higher-level caenophidian relationships
Caenophidian relationships have been reviewed recently by Vidal 〚11〛 (Fig. 2) and the reader is referred to this paper for additional references. Earlier concepts of snake evolution placed the Caenophidia at the top of a stepwise progression of branching lineages going from the most primitive snakes to the most derived. However, a previous analysis of a smaller gene dataset 〚7〛 and our current sequence analyses do not support this model, but instead show that the Caenophidia and the Henophidia are closest relatives (see companion paper). This result also agrees with the recent discovery of colubroid fossils in the earliest snake assemblage, dating from mid-Cretaceous 〚13〛. Caenophidia are monophyletic (bootstrap value: 66%, see companion paper) and the position of Acrochordus as sister-group to the remaining Caenophidia (traditionally recognised under the name of colubroids) is confirmed (bootstrap value: 60%). Within Caenophidia, most basal nodes (corresponding to splits separating subfamilies or families) are supported by bootstrap values between 60 and 100%. Caenophidia have an Asiatic origin as the following basal lineages, all of Asiatic range and monophyletic (100% bootstrap values), display a paraphyletic successive branching pattern: acrochordids, xenodermatines, viperids, pareatines and homalopsines. Xenodermatines are the most basal lineage of colubroids (bootstrap value: 100%), followed by vipers or pareatines. Within vipers, Azemiops is the sister-group to pitvipers (combined C-mos/12-16S rRNA analysis, bootstrap value: 51%, data not shown) and Causus is the sister-group to viperines (combined C-mos/12-16S rRNA analysis, bootstrap value: 84%, data not shown). The lineages of Asiatic range being primarily nocturnal (with the exception of some viperids), Caenophidia have a nocturnal origin (all the other snake lineages being also of nocturnal origin, snakes have a nocturnal origin). All the remaining colubroids form a monophyletic group (bootstrap value: 78%). All diurnal colubroids with an active foraging mode (and a high metabolic rate) are found within this clade. It comprises two main clades, one comprising elapids and African colubroids (bootstrap value: 100%) and the other comprising three main groups: American xenodontines, cosmopolitan colubrines (associated with the African genus Grayia) and cosmopolitan natricines (bootstrap value: 81%). Elapids form a monophyletic group (bootstrap value: 90%) which is sister-group to African colubroids (comprising atractaspidids (Atractaspis), psammophiines (Mimophis, Psammophylax), ‘lamprophiines’ (often incorrectly called ‘boodontines’) (Lamprophis, Mehelya) and ‘pseudoxyrhophiines’ (Leioheterodon). We increased the taxonomic sampling within this large African group using nine C-mos sequences deposited in Genbank by Slowinski and Lawson and four 16S rRNA sequences deposited in Genbank by Vences and performed corresponding separate analyses. The C-mos analysis (Fig. 3) shows that psammophiines are monophyletic (Malpolon, Mimophis, Rhamphiophis, Psammophylax) (bootstrap value under 50%), as are atractaspidids (Atractaspis, Homoroselaps, Aparallactus) (bootstrap value: 58%). The inclusion of Homoroselaps (which clusters with Atractaspis; bootstrap value: 81%) within atractaspidids is in agreement with morphological studies 〚14〛 and its position in recent molecular studies as sister-group to elapids is due to inadequate taxonomic sampling 〚15〛. The remaining African colubroids are classically recognised under the terms ‘lamprophiines’ (continental genera) and ‘pseudoxyrhophiines’ (Madagascan genera). ‘Lamprophiines’ (Bothrophthalmus, Duberria, Lamprophis, Lycophidion, Mehelya, Pseudaspis) do not appear to be monophyletic in our analysis (Fig. 3). Moreover, the Asiatic genus Psammodynastes (classically considered to be a natricine) belongs to the African colubroid clade, while Macroprotodon (classically considered to be a ‘lamprophiine’) does not belong to this African clade but to the colubrines (Fig. 3). The 16S rRNA analysis shows that the Madagascan colubroid fauna (‘pseudoxyrhophiines’) consists of at least two lineages (Leioheterodon, Madagascarophis, Stenophis, Micropisthodon and Liopholidophis on the one hand and Dromicodryas on the other hand) in addition to the endemic psammophiine genus Mimophis (data not shown). The last higher-level colubroid clade includes American xenodontines (monophyletic, bootstrap value: 94%), cosmopolitan natricines (monophyletic, bootstrap value: 99%) and cosmopolitan colubrines (including the African genus Grayia) (monophyletic, bootstrap value: 98%). In addition to the combined analysis presented here, the C-mos and ND4 separate analyses bring information on phylogenetic relationships of calamariines and pseudoxenodontines, two Asiatic lineages otherwise missing from our taxonomic sampling (C-mos analysis including one calamariine (Calamaria) and one pseudoxenodontine (Pseudoxenodon), sequences deposited in Genbank by Slowinski and Lawson; ND4 analysis including one calamariine (Oreocalamus), sequence deposited in Genbank by Kraus and Brown). Both analyses show that calamariines are associated with colubrines while the C-mos analysis shows that pseudoxenodontines are the sister-group to xenodontines (Fig. 3), a result confirming the inference by Vidal et al. 〚10〛 that xenodontines have an Asiatic/North American origin.

Phylogenetic relationships of Caenophidia based on C-mos, 12-16S rRNA and ND4 sequences (bootstrap ME consensus tree, 2000 replicates, values above 50% are shown). Traits: S, supralabial secretory serous cells; D, differentiated maxillary dentition. Terminal taxa written in bold possess a front-fanged venom system.

Phylogenetic relationships of Caenophidia based on C-mos sequences (bootstrap ME consensus tree, 2000 replicates, values above 50% are shown). Traits: S, supralabial secretory serous cells; D, differentiated maxillary dentition. Terminal taxa written in bold possess a front-fanged venom system.
3.2.2 Evolutionary implications: the evolution of the venom apparatus
The evolution of the venom apparatus was recently reviewed by Vidal 〚11〛. The original data presented here confirm for the most part the inferences made in this review. Two possible indicators of the venomous nature of Caenophidia are the presence of a differentiated maxillary dentition and the presence of supralabial secretory serous cells. It should be noted that some alethinophidians other than caenophidians have differentiated maxillary dentition, but in those cases, the enlarged maxillary teeth are the anterior ones, which aid in prey capture 〚16〛. When these two indices are mapped on our phylogeny, several significant facts appear. First, the sister-group to colubroids, the aquatic genus Acrochordus, lacks both differentiated maxillary dentition and supralabial secretory serous cells 〚5, 17〛. Second, the most basal lineage of colubroids, the xenodermatines, lacks a differentiated maxillary dentition, but has alternating cords of mucous and serous cells along the supralabial region 〚5, 17〛. All the other lineages of colubroids have a Duvernoy’s gland or a venom gland (both of dental origin), with the exception of the pareatines, which are malacophagous 〚5〛. Third, the differentiation of the maxillary dentition appears with another basal family among colubroids, the vipers, and is retained in all the other subfamilies (with the exception, once again, of the pareatines in the case they are more derived than vipers). Among the species studied by Taub 〚17〛, Kochva and Wollberg 〚18〛 and Underwood and Kochva 〚5〛, apart from the pareatines, those lacking both a differentiated maxillary dentition and supralabial secretory serous cells belong to seven genera from two subfamilies (colubrines and xenodontines) that are in a derived position compared to xenodermatines and vipers. So, the absence of indicators of venom systems in a few species of colubroids, such as the pareatines, some colubrines, and some xenodontines, is satisfactorily explained from a parsimonious point of view if we postulate a few secondary losses. This hypothesis had already been favoured by several authors, such as Underwood 〚19〛, McDowell 〚4, 20〛, Savitzky 〚21〛, and Underwood and Kochva 〚5〛. The venom apparatus, indicated primarily by the presence of supralabial serous secretory cells and of a differentiated maxillary dentition, is then a character defining the colubroids, a shared derived character of the group. We wish to make it clear that as we know little about the biological role of extant venoms and Duvernoy’s secretions 〚22〛, the term venom apparatus is here used without any implied meaning concerning its initial function(s). On the other hand, we can reasonably infer that the basic components of the venom apparatus were present since the origin of colubroids between 60 and 100 Myr ago 〚13, 23〛 and have experienced extensive evolutionary tinkering 〚24〛 during this period, resulting in many kinds of toxic secretion associated with diverse delivery systems in extant forms. This key evolutionary innovation 〚25〛 liberated caenophidians from the major prey-killing mechanism used by their henophidian sister-group, constriction, permitting the uncoupling of the locomotor and feeding systems 〚21, 26〛. New modes of locomotion (including rapid ones), diets and ecological niches were then available. A wonderful illustration of this evolutionary tinkering is brought by the evolution of the front-fanged venom system, which appeared three times independently within caenophidians: once with viperids, once within atractaspidids (common ancestor of the genera Homoroselaps and Atractaspis) and once with elapids (Figs. 2 and 3).
Acknowledgements
This work was supported for its most part by the “Service de systématique moléculaire”, “Institut de systématique” (CNRS FR 1541), directed by Simon Tillier. N.V. thanks C. Bonillo, K. Daoues, P. David, R. Debruyne, A. Dubois, A. Halimi, G. Lecointre, O. Pauwels, F. Pleijel, J.-C. Rage, A. Tillier, S. Tillier for their help during the course of this work. S.B.H. thanks D. Rabosky for technical assistance and NSF and NASA for partial support. N.V. and S.B.H. thank the following persons for contributing most of the tissue samples used in this work: M. Boulay, R.M. Burger, B.N. Campbell, L. Chirio, K. Daoues, I. Das, P. David, S. Delahaie Thourin, J.-C. de Massary, H.G. Dowling, C. Gans, A. Halimi, S. Imbott, I. Ineich, U. Kuch, P. Lacour, S. Lavoué, O. Le Duc, D. Mebs, T. Moncuit, P. Moret, O. Pauwels, J. Reynes, C. Skliris, A. Teynié, W. Wüster, H. Zaher.
Version abrégée
Les Caenophidia, appelés aussi serpents « avancés », comprennent le genre aquatique Acrochordus et le clade des Colubroidea. Tous les serpents venimeux appartiennent aux Colubroidea, qui représentent la grande majorité des serpents actuels, avec environ 2400 espèces sur les 3000 espèces de serpents. Les Colubroidea sont constitués des Atractaspididae (certains présentant des crochets à venin en position avancée sur le maxillaire), des Elapidae et des Viperidae (tous présentant des crochets à venin en position avancée sur le maxillaire), et des «Colubridae», paraphylétiques, qui sont définis par l’absence de crochets à venin en position avancée sur le maxillaire. Les « Colubridae » représentent la grande majorité des Colubroidea, avec environ 1850 espèces. Les relations de parenté entre la majorité des lignées de Colubroidea demeurent irrésolues. Le but de ce travail consiste d’abord à éclaircir ces points en utilisant des séquences d’ADN, puis à étudier l’évolution de l’appareil venimeux au sein des Colubroidea. Pour cela, 188 séquences (dont 104 d’entre elles sont originales) ont été utilisées, obtenues à partir d’un gène nucléaire (C-mos) et de trois gènes mitochondriaux (ARNr12S et 16S, cytochrome b), et représentant toutes les lignées de Caenophidia.
56 modèles alternatifs d’évolution moléculaire ont d’abord été testés de façon statistique pour chacun des gènes utilisés à l’aide d’une approche de maximum de vraisemblance. Le modèle choisi a alors été utilisé pour l’estimation des phylogénies en utilisant le critère d’optimalité du minimum d’évolution. La robustesse des nœuds a été estimée à l’aide de la technique du bootstrap avec 2000 réplicats. Les analyses séparées ne montrant pas de non-congruence topologique significative (aucun nœud contradictoire soutenu par des valeurs de bootstrap supérieures à 70%), nous avons réalisé des analyses combinées en répétant la procédure décrite ci-dessus. La technique de bootstrap ne mesurant que la robustesse interne d’un jeu de données, la fiabilité des nœuds a été estimée en utilisant le critère de congruence taxinomique entre jeux de données indépendants.
Les Caenophidia apparaissent comme le groupe frère des Henophidia rompant avec une conception des Henophidia, groupe souche des Caenophidia. Ce résultat est en accord avec des données paléontologiques récentes qui montrent que les Caenophidia sont présents dans les plus vieilles faunes fossiles connues de serpents, datées du milieu du Crétacé. Les Caenophidia sont monophylétiques avec le genre Acrochordus groupe frère des Colubroidea.
Les lignées asiatiques de Caenophidia (Acrochordidae, Xenodermatinae, Pareatinae, Viperidae, Homalopsinae) formant un groupe paraphylétique basal, les Caenophidia ont une origine asiatique. Toutes les autres lignées de Colubroidea forment un groupe monophylétique auquel appartiennent tous les Colubroidea diurnes présentant un mode de chasse actif associé à un métabolisme élevé. Ce groupe comprend deux grands clades : un clade incluant les Elapidae et les Colubroidea africains et un clade incluant les Xenodontinae, américains, les Pseudoxenodontinae et les Calamariinae, tous deux asiatiques, et les Natricinae et les Colubrinae, tous deux cosmopolites. Les Elapidae, monophylétiques, sont le groupe frère d’un clade africain comprenant les Atractaspididae, les Psammophiinae, les « Lamprophiinae » et les « Pseudoxyrhophiinae ». Les Atractaspidididae (incluant le genre Homoroselaps) et les Psammophiinae sont tous deux monophylétiques, contrairement aux « Lamprophiinae », continentaux, et aux « Pseudoxyrhophiinae », malgaches. Les Calamariinae sont associés aux Colubrinae, tandis que les Pseudoxenodontinae sont le groupe frère des Xenodontinae, confirmant l’origine asiatique/nord-américaine de ce dernier groupe.
Deux indices de la venimosité des Caenophidia sont la présence de cellules sécrétrices séreuses supralabiales et d’une denture maxillaire différenciée. D’après nos arbres, si le groupe frère des Colubroidea, le genre Acrochordus, ne possède ni denture maxillaire spécialisée ni cellules sécrétrices séreuses supralabiales, la lignée la plus basale des Colubroidea, les Xenodermatinae, à la denture maxillaire uniforme, possède des cordons alternés de cellules muqueuses et séreuses au niveau supralabial. Toutes les autres lignées de Colubroidea possèdent une glande séreuse supralabiale d’origine dentaire (sous la forme d’une glande de Duvernoy ou d’une glande à venin), à l’exception des Pareatinae, malacophages. La différenciation de la denture maxillaire apparaît aussi avec une lignée basale de Colubroidea, les Viperidae, et est conservée chez toutes les lignées plus dérivées (à l’exception des Pareatinae dans le cas où ils seraient plus dérivés que les Viperidae). En dehors des Pareatinae, les quelques espèces de Colubroidea dénuées de tout indice de venimosité appartiennent à deux lignées dérivées par rapport aux Xenodermatinae et aux Viperidae : les Colubrinae et les Xenodontinae. Cette absence complète d’appareil venimeux chez certains Colubroidea s’explique ainsi parcimonieusement par quelques pertes secondaires. L’appareil venimeux, dont nous ignorons les fonctions initiales, est donc une synapomorphie des Colubroidea. Son apparition dès l’origine des Colubroidea il y a environ 100 millions d’années a été suivie d’un bricolage évolutif intense aboutissant à la présence actuelle de nombreux types de sécrétions toxiques associés à des modes d’injection variés. Cette innovation évolutive clé a permis aux Caenophidia d’adopter un mode de neutralisation des proies différent de celui utilisé par leur groupe frère hénophidien (constriction unimodale), rendant ainsi possible le découplage des structures de locomotion et de nutrition.
De nouveaux modes de locomotion (dont la locomotion rapide), modes de chasse, régimes alimentaires et niches écologiques étaient alors disponibles. Ce bricolage évolutif de l’appareil venimeux est particulièrement évident au niveau de la position avancée des crochets à venin sur le maxillaire qui est apparue trois fois indépendamment : une fois avec les Viperidae, une fois avec les Elapidae et une fois au sein des Atractaspididae.
Appendix 1. Tissue samples used
Tissue samples were obtained from the tissue collection of Nicolas Vidal for the following species (sequences produced: C: C-mos, 12/16: 12/16S rRNA, N: ND4):
Acrantophis madagascariensis (Madagascar; C, 12/16), Acrochordus granulatus (〚MNHN 1997.6576〛, Ko Mai Phai Island, Muang District, Phang-Nga Province, Thailand; C, 12/16), Alsophis cantherigerus (Cuba; C), Aplopeltura boa (〚MZUSP 12187〛, Malaysia; C, 12/16), Atheris squamigera (20 km north of Kinshasa, Democratic Republic of Congo; C, 12/16), Atractaspis micropholis (Togo; C, 12/16), Azemiops feae (unknown origin; C), Boa constrictor (Petit Saut, French Guiana; C), Bothriechis schlegelii (Costa Rica; C), Bungarus fasciatus (‘Malaysia’; C), Causus resimus (‘Tanzania’; C, 12/16), Cerastes cerastes (captive born; C, 12/16), Dendroaspis angusticeps (Southeast Tanzania; C, 12/16), Diadophis punctatus (USA; C, 12/16), Elapsoidea semiannulata (Central African Republic; C, 12/16), Enhydris enhydris (〚CUB MZ R 1998.12.11.25〛, Ban Pho Yai, Ban Lat District, Phetchaburi Province, Thailand; C), Erpeton tentaculatum (Malaysia; C, 12/16), Grayia ornata (〚MNHN〛 1997.6517, Ivindo river, Ogooué, Gabon; C, N), Hapsidophrys smaragdina (〚MNHN 1997.6516〛, Ivindo River, Ogooué, Gabon; C), Homalopsis buccata (〚CUB MZ R 1998.12.11.26〛, Ban Sala Moo Sri, Ban Lat District, Phetchaburi Province, Thailand; C, 12/16), Lamprophis fuliginosus (Burundi; C, N), Laticauda colubrina (〚MNHN 1999.7692〛, Ko Khai Nai Island, Ko Yao District, Phang-Nga Province, Thailand; C), Leioheterodon madagascariensis (Madagascar; C, 12/16), Leptodeira annulata (Kaw, French Guiana; C), Mehelya capensis (Burundi; C, 12/16, N), Micrurus surinamensis (RN1 road, pk 68, French Guiana; C, 12/16), Mimophis mahfalensis (〚MZUSP 12188〛, Madagascar; C, 12/16, N), Natriciteres olivacea (Burundi; C, 12/16), Natrix natrix (Forêt de Carnelle, Viarme, Val d’Oise, France; C), Pareas carinatus (〚MZUSP 12186〛, Malaysia; C, 12/16), Psammophylax variabilis (Burundi; C, 12/16, N), Pseudoboa nigra (Brazil; C, 12/16), Rhamphiophis oxyrhynchus (〚MNHN 1990.4336〛, Dielmo, close to Toubakouta, 15 km from the Gambia border, Senegal; C), Rhabdophis subminiatus (〚CUB MZ R 2000.2〛, Ban Salakern, Ban Lat District, Phetchaburi Province, Thailand; C, 12/16), Rhinobothryum lentiginosum (Petit Saut Hydroelectric Plant, French Guiana; C), Sibon nebulata (10 km from Hill Bank, Orange Walk, Belize; C, 12/16), Sinonatrix annularis (〚MNHN 1999.9016〛, Guangxi, China; C, 12/16), Stoliczkaia borneensis (Crocker Range National Park, Sabah, East Malaysia; C, 12/16), Xenochrophis flavipunctatus (〚CUB MZ R 1998.12.11.16〛, Ban Had Sai, Ban Lat District, Phetchaburi Province, Thailand; C, 12/16), Xenodermus javanicus (Maninjau Lake, Sumatera Barat Province, Sumatra; C, 12/16).
Tissue samples were obtained from the tissue collection of S. Blair Hedges for the following species:
Dendrelaphis caudolineatus (RH 56536, ‘Thailand’; 12/16), Phyllorhynchus decurtatus (RH 60813, locality unknown; C, 12/16), Psammodynastes pulverulentus (RH 57759, ‘Thailand’; 12/16).
Appendix 2. Sequence data obtained from Genbank or the references
Sequence data for the following genes and species were obtained from Genbank or bibliography.
C-mos gene: Aparallactus werneri (AF471116), Bothrophthalmus lineatus (AF471129), Calamaria pavimentata (AF471103), Duberria lutrix (AF471138), Homoroselaps lacteus (AY058931), Lycophidion ornatum (AF471144), Macroprotodon cucullatus (AF471145), Malpolon monspessulanus (AY058936), Psammodynastes pulverulentus (AF471157), Pseudaspis cana (AY058942), Pseudoxenodon karlschmidti (AF471102).
12S and/or 16S rRNA gene: Alsophis cantherigerus (AF158475, AF158405), Azemiops feae (AF057187, AF057234), Boa constrictor (Z46470, Z46495), Bothriechis schlegelii (AF057213, AF038888), Bungarus fasciatus (U96793, Keogh 〚27〛), Dromicodryas bernieri (AF215268), Enhydris enhydris (Z46492, Z46458), Grayia ornata (AF158503, AF158434), Hapsidophrys smaragdina (AF158504, AF158435), Lamprophis fuliginosus (Z46489, Z46457), Laticauda colubrina (U96799, Keogh 〚27〛), Leptodeira annulata (AF158473, AF158404), Liopholidophis lateralis (AF215270), Micropisthodon ochraceus (AF215271), Natrix natrix (AF158530, AF158461), Rhamphiophis oxyrhynchus (Z46738, Z46443), Rhinobothryum lentiginosum (AF158535, AF158465), Stenophis cf. betsileanus (AF215269).
ND4 gene: Achalinus rufescens (U49319), Acrantophis madagascariensis (Forstner et al. 〚12〛), Acrochordus granulatus (U49296), Alsophis portoricensis (U49308), Aplopeltura boa (U49312), Atractaspis bibroni (U49314), Azemiops feae (U41865), Boa constrictor (Forstner et al. 〚12〛), Boiga dendrophila (U49303), Bothriechis schegelii (U41874), Bungarus fasciatus (U49297), Causus rhombeatus (U41866), Cerberus rhynchops (U49327), Chilomeniscus cinctus (U49305), Coluber constrictor (U49300), Dendrelaphis pictus (U49304), Dinodon semicarinatum (AB008539), Dispholidus typus (U49302), Elaphe flavolineata (U49301), Enhydris plumbea (U49328), Farancia abacura (U49307), Helicops pictiventris (U49310), Heterodon nasicus (Forstner et al. 〚12〛), Hypsiglena torquata (U49309), Lampropeltis mexicana (Forstner et al. 〚12〛), Leioheterodon madagascariensis (U49318), Lycodon capucinus (U49317), Macropisthodon rudis (U49326), Madagascarophis colubrina (U49313), Malpolon monspessulanus (AY058989), Micrurus surinamensis (AF228444), Nerodia taxispilota (U49322), Oligodon octolineatus (U49316), Oreocalamus hanitschi (U49306), Pareas nuchalis (U49311), Pelamis platurus (U49299), Rhabdophis subminiatus (U49325), Storeria occipitomaculata (U49323), Thamnophis butleri (U49324), Xenochrophis trianguligerus (U49321), Xenodermus javanicus (U49320).



