1 Introduction
The black-footed ferret (Mustela nigripes) is one of the most endangered mammals in North America. Not coincidently, it also is a quintessential specialist among carnivores. The extreme dependence of this 1-kg mustelid on similarly-sized prairie dogs (Cynomys) made it especially vulnerable to extinction as its prey were persecuted as agricultural pests during most of the 20th century. Conversions of native grasslands to crops destroyed vast areas of highly productive prairie dog habitat (where the highest densities of prairie dogs may have been), and the perception that these rodents were incompatible with grazing of livestock motivated an effective campaign to rid rangelands of prairie dog colonies.
As the attack on prairie dogs was spreading from east to west across the Great Plains with increasingly extensive and intensive farming and ranching activity, a second threat to prairie dogs, sylvatic plague, was advancing from the west. The disease, caused by the bacterium Yersinia pestis, was introduced to several seaports in North America, but became established in wild rodents near San Francisco, California, early in the 20th Century. By the 1950s, it had invaded the entire range of the Utah prairie dog (C. parvidens), now classified as a threatened species, the white-tailed prairie dog (C. leucurus), and Gunnison prairie dog (C. gunnisoni), and had colonized the western two-thirds of the much larger range of the black-tailed prairie dog (C. ludovicianus). The distributions of the latter three species of Cynomys comprise the historic range of the black-footed ferret. Plague often decimates entire colonies of prairie dogs, and has extirpated prairie dog populations from areas where original populations were geographically isolated [1]. Many carnivores show partial resistance to plague. However, multiple deaths of captive black-footed ferrets resulting from accidental exposure to Y. pestis in their prairie dog food supply (D.E. Biggins, unpublished), and other plague-caused deaths of ferrets and hybrids [2,3], underscore the exceptional sensitivity of this mustelid to plague, exacerbating an already serious conservation challenge.
An understanding of the causes of decline in populations of an organism is a prerequisite to planning for recovery. We identified above several anthrogenic changes that placed the black-footed ferret in jeopardy, and highlighted the habitat and prey specialization that increased its vulnerability to altered conditions. Before adding to the list of challenges to ferret recovery, however, we will briefly consider the question of what conditions may have led to such extreme specialization by the black-footed ferret. Its closest living ancestor, the Siberian polecat (Mustela eversmannii) is not so specialized. Although the black-footed ferret and Siberian polecat have been labeled “ecological equivalents” [4,5], studies have suggested behavioural differences between these two species [6], and differences in life histories have been noted [7]. In addition to its specialization on a single prey, the black-footed ferret differs from the Siberian polecat in having smaller litters. What differences in selective pressure on the steppes of the two continents promoted divergence of ferrets and polecats? Plague may provide an explanation [8].
2 Black-tooted ferret specialization
Siberian polecats have been historically subjected to the potentially strong influences of plague even if Y. pestis itself has a fairly short evolutionary history [9]. A varying environment favors generalist predators [10] by preventing consistent advantage of preying on any single prey species, (assuming incomplete synchrony in fluctuations of prey species). Plague causes oscillations in populations of susliks and gerbils of central Asia (Fig. 1), and polecat populations appear to be affected [11]. The few polecats remaining after an epizootic may be forced to shift to less preferred prey species, similar to seasonal shifts in prey use by polecats in some areas [13] and to shifts in prey use by other generalist mustelids [14].
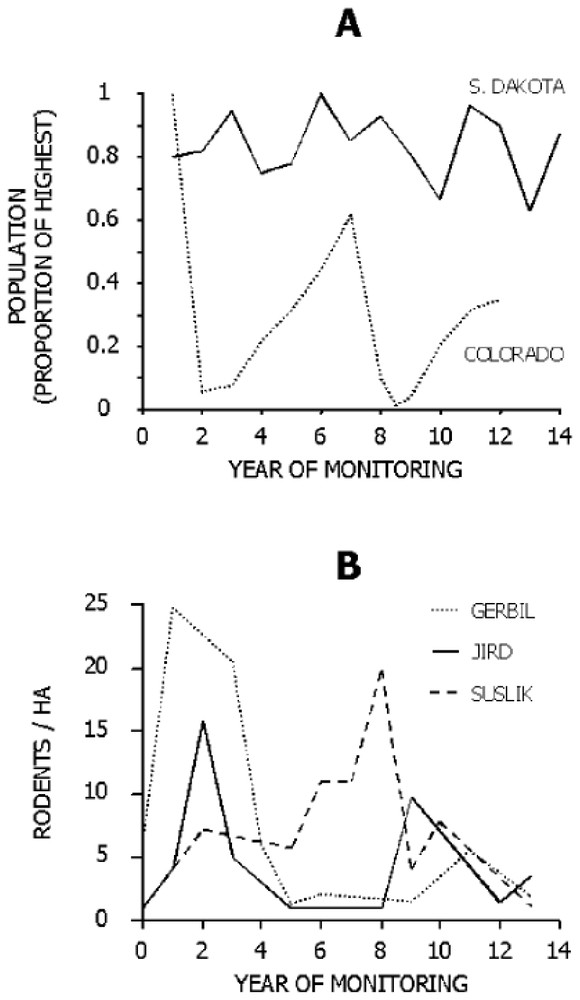
Fluctuations in rodent populations in North America (A) and Asia (B). (A) Black-tailed prairie dogs at Wind Cave National Park, South Dakota [16], a site without plague, and at the Rocky Mountain Arsenal National Wildlife Refuge, Colorado, a site with plague (data provided by D. Seery, U.S. Fish and Wildlife Service). (B) Great gerbils (Rhombomys opimus), Libyan jirds (Meriones libycus), and large-toothed susliks (Spermophilus fulvus) in a central Asian focus of plague [12].
In contrast, the historical lack of plague in North America [15] seems to have allowed greater stability in populations of prairie dogs (Fig. 1), favoring specialization on them by ferrets. In part, the suitability of prairie dogs as prey for ferrets may stem from the high degree of coloniality and sociality in prairie dogs [16]. The prairie dogs' anti-predator warning system of alarm calls operates most efficiently when the prairie dogs are living at high densities. Although this defense works admirably against many predators, ferrets hunt mainly at night and usually kill prairie dogs below ground. Coloniality of prairie dogs benefits ferrets by providing high densities of prey, and the burrows dug by prairie dogs protect the ferrets against intraguild predators. Perhaps most importantly, the combination of locally abundant prey and shelter allows ferrets to minimize the need to move on the surface where they are exposed to predators [6].
Relative stability of resources may affect evolution of life histories as well as behaviours. These closely related Mustela provide an opportunity to contrast the relative predictions of r-K selection theory [17–19]. The prediction of greater fecundity in the r-selected form subjected to an unstable environment seems met by the larger average litter size of the Siberian polecat (; [13]), compared to the black-footed ferret (, [20]). In a stable environment, optimal litter size would be expected to be more constant, and greatest efficiency over the long term would be achieved by conceiving the number of young that could be consistently reared. The smaller litters of the black-footed ferret (compared to the polecat) thus suggest the K-selected maximization of energetic efficiency. As emphasized by Ricklefs [21], “The importance of r-and-K selection theory, relative to other sources of variation in life histories, depends on demonstrating a direct link between differences in population fluctuations and life history traits in pairs of otherwise similar organisms”. The direct link has not been proven, but circumstantial evidence is compelling for these ferrets and polecats.
Thus, an exotic disease and the dependence of black-footed ferrets on prey that were deemed to be pests resulted in declines to the point where the species became a charter member of the endangered species list after passage of the U.S. Endangered Species Act of 1973. At that time, a remnant population of ferrets existed, and was being studied, in southwest South Dakota [20,22]. However, the free-ranging population continued to decline, and had disappeared by the late 1970s. The first captive breeding of black-footed ferrets was attempted using nine ferrets taken from that South Dakota population during 1971–1973 [23], an effort which resulted in production of a few litters, but no surviving offspring. The last member of that captive population died in 1978.
3 Captive breeding
Hope for black-footed ferrets was revived in 1981 [24], with the discovery of a new population living on white-tailed prairie dog colonies near Meeteetse, Wyoming. Intensive studies on this poorly understood carnivore began immediately, using radio-telemetry and other techniques [25–28], following recommendations from researchers in South Dakota. Canine distemper and plague simultaneously decimated the population in 1985 [29,30] transforming a somewhat tardy attempt to begin a captive breeding program into an emergency rescue effort [31]. During winter of 1985–86, ferret numbers dwindled to 10 known individuals (6 of which already were in captivity); the final free-ranging adults and their offspring were captured by March, 1987, bringing the total to 18 captives. The prairie dogs at Meeteetse continued to decline over a 10-year period due to the effects of plague [32], and the area is no longer suitable habitat for black-footed ferrets.
The initial failure to produce any young ferrets during the first season of the new captive breeding program (1986), considered in light of the failed program a decade earlier, was disconcerting. During subsequent years, however, breeding success improved as knowledge increased [33]. Although kit production rates recently have reached their highest point (P. Marinari, pers. comm.), the number of young reared per female in the captive population of about 250 adults at 6 zoos and a federal facility (1.9 kits) remains significantly lower (P=0.004) than the historic production by free-ranging females at Meeteetse (3.2 kits per female). Production in the captive population is hampered by females that either (1) do not breed, (2) become pseudopregnant, or (3) do not successfully rear their litters; the average size of captive litters is similar to the average litter size of their wild ancestors. Finding a solution could greatly increase the efficiency of captive propagation.
Another important question raised early in the program was whether or not the kits produced in captivity would be suitable for release (behaviourally and physically). Initially, we addressed this question using Siberian polecats as surrogates to investigate the development of behaviours [34]. Rearing conditions and learning affected predator avoidance behaviours [34]. Also, effects of rearing polecats in cages were cumulative, with decreasing cautiousness through four generations [6]. The apparent transmission of boldness (or lack thereof) seemed to be mediated by social learning between dam and offspring, interacting with environmental conditions. A single generation of rearing in quasi-natural outdoor pens partially reversed the process, an effect that was measurable through two subsequent generations (D. Biggins, unpublished data). These Lamarckian phenomena are not a recent discovery [35], but socially-mediated attributes are receiving renewed attention in regard to adaptive significance and evolution [36,37]. Experimental releases of Siberian polecats confirmed that rearing environments had significant effects on behaviours and that behavioural differences measured in laboratory trials were relevant to survival in the wild [6]. Studies on captive black-footed ferrets confirmed effects of environment on behavioral development [38–40] Further experimentation with quasi-natural rearing environments in the form of outdoor pens demonstrated significant effects on behaviours (Fig. 2) [41] and survival (Fig. 3) [42] of released black-footed ferrets. Predation has accounted for 93% of the losses in released polecats and ferrets monitored via radio-telemetry (n=130), underscoring the critical importance of development of predator avoidance behaviours.

Maximum postrelease dispersal (distance between release site and most distant telemetric fix) of black-footed ferrets moving northeasterly into poorer habitat and southwesterly into relatively good habitat [41]. Habitat quality provided by these white-tailed prairie dogs was evaluated by sizes of prairie dog colonies, distances between colonies, and densities of prairie dogs within colonies (medians, ranges, 25th–75th percentiles).

Minimum short-term (1-month) and long-term (9-month) survival of young black-footed ferrets reared in cages and outdoor pens (means and upper bounds of 95% confidence intervals) [42]. Producers raised cage-reared kits in traditional cages and we released them without experience in outdoor pens. The pen-resident kits were born in pens or were born at facilities with pens and moved with dams to the pens when kits were <60 d old.
The issue of quality versus quantity of animals released was debated vigorously in the early years of the black-footed ferret program. It is relatively cost-efficient to mass-produce animals in relatively simple environments, but efficiency must take into account post-release survival rates. An example taken from the first several seasons of reintroductions at the Charles M. Russell Wildlife Refuge in Montana illustrates the net effect of releasing animals of varying backgrounds on a single site. Because of differences in short-term and long-term survival [42], and reproductive success during the first two breeding seasons post-release, the proportion of the population with pen-experience in their lineage became amplified and the cage-reared representation nearly disappeared (Fig. 4).

Changes in proportionate composition of a Montana population of black-footed ferrets, 1994–1996, illustrating the amplification effect of releasing high-quality animals that survive and reproduce. Wild-born kits in summer, 1996, were classified according to the captive experience of their released maternal ancestors (mothers or grandmothers).
Although the generational changes in behaviour of captive ferrets seemed to be mostly due to cultural evolution coupled with an environment providing unnatural stimuli, genetic change also is possible. Even if we are avoiding unintended directional selection for phenotypes that flourish in captivity (which is doubtful), we have removed the captive population from stabilizing selection, allowing variation to expand. Rapid evolution in captive populations of vertebrates is common, including changes wrought by artificial selection during the process of “domestication”. These processes are overlaid upon a foundation of low genetic variability in black-footed ferrets [43] compared to Siberian polecats and many other carnivores, perhaps due to repeated genetic bottlenecks in the isolated remnant wild populations [44]. The final bottleneck was defined by about 8 founder equivalents represented in the 18 individuals taken from the Meeteetse population. Furthermore, it is unlikely that the Meeteetse population, even in its historic condition, would have well-represented the genetic variability of a species whose range spanned 20 degrees latitude and 2500 m elevation.
The quality of the habitat at potential ferret reintroduction sites attracted considerable attention before releases began, and a system for ranking sites, based on ferret energetics and prey density, was developed [45]. We later discovered that prairie dog density seemed to effect movements and dispersal of released black-footed ferrets [41], but many questions remain unanswered regarding the effects of habitat and diseases on the success of ferret reintroduction. Canine distemper and plague are particularly perplexing, but perhaps it does not happen by chance that the most vigorous populations of black-footed ferrets have been those released in South Dakota where plague is absent and prairie dog densities are highest (Fig. 5). Vaccines are being developed and tested for both diseases, but some biologists had not envisioned a level of management of reintroduced ferret populations that includes delivery of vaccines in perpetuity.

Reintroduction sites receiving black-footed ferrets during 1991–2001, with corresponding population estimates in summer of 2001 followed by cumulative numbers of animals released.
4 Translocation
The foregoing discussion highlights a sample of biological challenges to ferret conservation. Several socio-political problems also have surfaced during this conservation program. Even among those individuals whose motivations are altruistically ferret-oriented, opinions differ on the best means to reach widely-accepted goals. Individual working groups are understandably concerned primarily with growth rates of their own populations and protection of those populations from risks. Although those concerns should also be important to individuals and agencies responsible for the overall recovery program, the perspectives are understandably somewhat different. For example, in 1983, the cost in terms of risk to the Meeteetse population of removing ferrets for translocation or captive breeding were deemed to be too high to warrant that action. Conservation and protection of the Meeteetse population took such high priority that alternative options necessary for recovery could not be considered [31]. We do not suggest that the nearly disastrous result of that philosophy early in the program portends the program's future in any way, but simply that perspectives of scale have tremendous influence over decisions. The Fish and Wildlife Service, which oversees the recovery program, must look at recovery over the long term and maximum geographic scale. We have made this argument for research activities that were perceived to have some costs at the local and short-term scales, but could have potentially large benefits for the program as a whole. Translocation of ferrets is similar from the standpoint of perspective and priority.
Translocation is an example of a recently-tested tool that is biologically attractive but poses political problems. Data from South Dakota suggest that about 40% more wild-born kits than captive-born kits remain 30 days after groups of both are released onto new habitat. This difference (or even a less dramatic difference) could result in considerable monetary savings given the cost of producing a ferret in captivity (ca. $4000 U.S.). We do not imply that money is the only measure of efficiency (or even the best measure). Other considerations include the probability of establishing a self-sustaining population in a time frame that will not test the patience of working groups, getting populations back under the influence of natural selection, and reducing time spent in a genetic bottleneck.
Game managers have long held to the notion that highest productivity of a population occurs well before its numbers reach the carrying capacity of the habitat. Although years of experience has taught us a great deal more about the complexities of ecological systems, that basic tenet remains generally valid. Managing ferrets at carrying capacity of the habitat (if we can figure out what that is) may provide a buffer against losses, but probably does not make most efficient use of animals produced as long as there are new populations to be established. Local managers will doubtless be protective of the populations they have reestablished, especially if translocations cross jurisdictional boundaries. Like the Meeteetse example, the risks will be impossible to articulate exactly, leaving much room for debate. Nevertheless, high security of individual populations must be balanced against achieving the highest growth rates possible in the program as a whole.
Even after all parties come to agree on the point in population growth at which removals may begin, the financial burden of those operations must be equitably distributed. Costs include monitoring the donor population to annually assess its status and capability to supply ferrets, capture of animals selected for translocation, and quarantine/transport of ferrets. Fairness dictates that the group managing the site receiving ferrets (the immediate beneficiary) and the recovery program (the secondary beneficiary) should find ways to collectively participate in these tasks to further the cause of the black-footed ferret (the ultimate beneficiary). The group or agency managing the donor site should not be expected to support the entire operation, even if that agency has a mandate to contribute to recovery of endangered species.
5 Conclusions
Other socio-political problems are long-standing (e.g., attitudes of the agricultural community regarding prairie dogs) or have surfaced during various phases of the recovery program (e.g., conflicts over use of “experimental nonessential” provisions of the Endangered Species Act), and are described elsewhere [31,46,47]. Despite these biological and social challenges, recovery of black-footed ferrets has progressed. The captive population reached the capacity of facilities (200–250 adults) in 1995, and has been supplying an expanding reintroduction program (Fig. 5). Rapidly-increasing populations of naturally-reproducing ferrets have been reestablished in South Dakota (Fig. 5). The future of ferrets is less secure in parts of the range impacted by plague, but research is addressing possible tools for plague management including vaccines and pulicides.



