Version française abrégée
L'acuité gustative des singes anthropoı̈des vis-à-vis des nutriments solubles et des métabolites secondaires communs des végétaux est presque totalement méconnue. Afin de préciser si les tendances alimentaires distinctes des orangs-outans, des chimpanzés et des gorilles reposent sur des caractéristiques sensorielles différentes, nous avons mesuré les seuils gustatifs de ces espèces vis-à-vis d'une substance sucrée et d'un composé astringent.
Les mesures ont été réalisées à l'aide de la méthode du two-bottle test, dont la pertinence a pu être démontrée par comparaison des seuils obtenus sur différentes espèces de primates avec ceux issus des enregistrements des potentiels évoqués sur l'un des nerfs périphériques de la gustation. Dans une première série d'expériences, l'enregistrement des réponses ingestives des individus isolés en présence de concentrations variables de fructose en solution, proposées simultanément à de l'eau lors de tests de courte durée, ont permis de déterminer les seuils de préférence pour le fructose. Des différences significatives de consommation sont observées à partir des concentrations minimales de [10–20] mM chez Pongo ; de [40–50] mM chez Pan ; et de [70–80] mM chez Gorilla. Dans une seconde série d'expériences, une solution de fructose légèrement attractive est proposée simultanément à une solution identique à laquelle est ajoutée, au cours de chaque test, une concentration variable d'acide tannique. L'inhibition exercée par le tannin sur la consommation de la solution sucrée est observée à partir d'un seuil de concentration de [2,9–3,5] mM chez Pongo ; de [2,9–5,9] mM chez Pan, et supérieur à [8,8–14,7] mM chez Gorilla. De manière inattendue, cette dernière espèce manifeste une préférence significative pour des solutions mixtes dont la concentration d'acide tannique est comprise entre 0,59 et 5,9 mM. Les différences de seuils pour le fructose, dont les valeurs se situent parmi les plus basses (Pongo) et les plus élevées (Gorilla) enregistrées chez les primates humains et non humains, contrastent avec la faible sensibilité des trois espèces vis-à-vis du tannin. Les capacités gustatives des grands singes ne semblent donc pas refléter un effet de l'inertie phylogénétique. Elles pourraient avoir été sélectionnées en relation avec la nécessité pour ces espèces de grande taille de diversifier leur régime alimentaire, notamment à travers une tolérance gustative élevée vis-à-vis des aliments riches en polyphénols. La poursuite des expériences avec d'autres primates permettra d'analyser la perception gustative de l'espèce humaine dans le contexte de l'évolution des systèmes sensoriels et des comportements alimentaires.
1 Introduction
Several studies have identified interspecific differences of taste acuity, observed in non-human primates, as one proximal cause underlying the variation of food choices of wild animals. Primate species tested to date, including humans, exhibit various combinations of taste sensitivities toward sugars, alkaloids and tannins [1–3]. Some authors consider polyphenols as the main category of chemical substances that have shaped the perceptive world of primates, including early hominids, because these secondary metabolites occur in many plant taxa in all environments where primates live [4]. But, whereas taste responses to sugars and alkaloids have been studied in a wide range of species within the primate order, thresholds for tannins are still available for a limited number of primates. In particular, little is known at present whether great apes differ in their perception of sugars or secondary metabolites, nor whether they share some common pattern of taste sensibilities following possible effects of phylogenetic inertia.
In this study, we carried out taste tests on orang-utans, chimpanzees and gorillas. Taste thresholds for fructose and tannic acid were determined using a behavioural procedure previously validated by electrophysiological records of the activity of the peripheral taste nerve in response to various concentrations of sugar and tannin in a few primate species [5,6]. Data presented here are regarded as preliminary owing to the small size of our test sample. However, given the paucity of data on ape taste thresholds and the observation, in both our study and that of Remis and Kerr [7], of a low sensitivity of gorillas toward tannic acid and a broadly similar threshold for fructose, we believe that these results bring a contribution to the knowledge of ape taste perception.
2 Materials and method
Experiments were carried out at La Palmyre zoological park (France) on gorillas (Gorilla g. gorilla), orang-utans (Pongo p. pygmaeus) and chimpanzees (Pan troglodytes verus) between May and August 2001. Animals were routinely maintained in outdoor enclosures during summer days to interact within their social group and they normally entered indoor individual cages in the afternoon. Experiments were carried out in the morning before animals were fed and prior to their release in outdoor enclosures. Except for chimpanzees, all individuals were born in zoos including La Palmyre. The first experiment with fructose used gorillas: two males born in 1989; orang-utans: two males born in 1984 and 1991, respectively, and two females born in 1975 and 1976; chimpanzees, one male born in 1975 and one female born in 1980. Of this sample, one orang-utan male and one chimpanzee female could not be tested with tannic acid during the second experiment owing to their scheduled transfer to another ape colony. Animals were fed fresh vegetables and fruits, barks, biscuit mixed with cereal and milk, pellets (Own Chunks E Banana) and water ad libitum.
The test procedure, the ‘two-bottle test’, has been detailed in previous studies [5] and is briefly as follows: individuals were initially provided with two feeding bottles containing tap water versus water with an attractive concentration of fructose (200 mM). Bottles were left for 2 min, and their relative position was determined at random in each test to avoid side-preference effects. This habituation phase lasted until individuals displayed a marked preference for the sweet solution during four consecutive tests. In a first experiment, the concentration of the fructose solution was varied at random in each trial within a range of concentrations that varied according to animal responses: between 50 and 800 mM in gorillas (11 concentrations), between 5 and 200 mM in orang-utans (11 concentrations), and between 20 and 800 mM in chimpanzees (12 concentrations). The amount of liquid consumed from the sugary solution divided by the total amount of liquid consumed from the two bottles yielded various percentages of ingestion according to each concentration, of which 66.7% was used as a conservative criterion of preference [3]. Alternatively, the taste threshold for fructose was determined statistically using the paired-sample t-test in order to assess the similarity of results obtained from different data processing. In this case, data were grouped as successive concentration intervals, and the threshold was defined as the lowest concentration interval (with minimum width) for which the mean difference of consumption between the sugar solution and tap water was significant (P<0.05; [5]).
In a second experiment, animals were offered the choice between a moderately sweet solution (⩽ twice the threshold for fructose, as determined in the first experiment) and the same solution mixed with tannic acid (Fluka, MW: 1701.23). The reference fructose concentration was 20 mM in Pongo, 50 mM in Pan, and 150 mM in Gorilla. The concentration of tannic acid was varied at random within the following range: between 0.58 and 14.7 mM in gorillas (six concentrations), and between 0.58 and 8.82 mM in orang-utans and chimpanzees (both with seven concentrations). The criterion of avoidance (based on the consumption of the mixture relative to the total amount of fluid consumed) was set at 33.3% [3]. As for fructose, an additional analysis was performed to determine the inhibition threshold statistically, defined as the lowest tannin concentration interval for which the mean difference of consumption between the mixture and the pure fructose solution is significant (P<0.05 with a paired-sample t-test).
The onset of the experiments depended on the time each individual required to reach the criterion for full habituation. Accordingly, during the two series of experiments, some individuals could not be tested with the full range of concentrations. However, in the course of the experiments, the provisional estimation of the threshold led us to provide all individuals with concentrations that proved retrospectively to be close to the threshold. As a result, individual data were generally available in the range of just below- and above-threshold concentrations and, in agreement with the conditions of application of the statistical test, entered equally into the calculation of P when clustering the results for these blocks of concentrations.
3 Results
Responses to fructose are shown in Fig. 1. The 66.7% criterion of preference is reached for a concentration of 20 mM in orang-utans, of 40 mM in chimpanzees, and of 70 mM in gorillas (in the latter two species, results are not consistent between concentrations lower than 40 and 70 mM, respectively). The statistical preference threshold is [10–20] mM in orang-utans (P<0.01; n=8), [40–50] mM in chimpanzees (P<0.01; n=4), and [70–80] mM in gorillas (P<0.05; n=4), with lower intervals yielding non-significant results in each species.


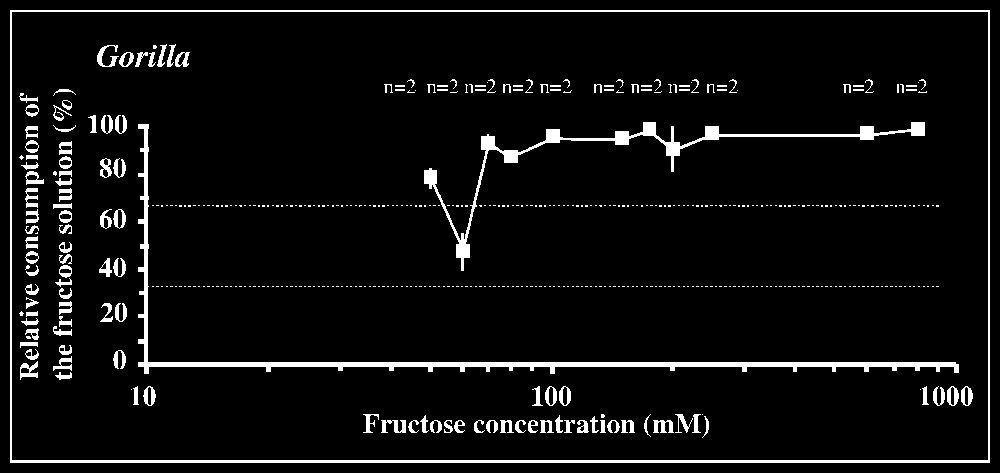
Results of the two-bottle test using fructose solutions versus tap water. The mean percentage consumption of the sugar solution (± standard deviation) relative to the total amount of liquid ingested in each test is plotted against fructose concentrations. The number n of individuals tested is indicated for each concentration. Dotted lines correspond to the criteria of preference and rejection, set at 66.7 and 33.3%, respectively.
Responses to tannic acid/fructose mixtures versus pure fructose solution are shown in Fig. 2. The 33.3% criterion of avoidance is reached when tannic acid concentration is 3.5 mM in orang-utans and 2.9 mM in the chimpanzee male. The mean difference between consumption of the mixture and consumption of fructose by orang-utans is significant (P<0.05; n=6) for the concentration interval [2.9–3.5] mM, but not for lower intervals. In the chimpanzee male, a significant difference (P<0.05; n=3) is found for the interval [2.9–5.9] mM. In gorillas, the lack of clear avoidance of tannic acid mixture at the highest concentration offered (14.7 mM) was observed for one animal, while the other individuals displayed an aversion, according to the criterion used, for concentrations ⩾11.8 mM (Fig. 2). The low-responsive individual nevertheless consumed much lower amount of the mixture at 14.7 mM (76.8 ml) than of less concentrated solutions (e.g., 184 ml at 11.8 mM; 190 ml at 8.8 mM; 170 ml at 5.9 mM), suggesting that this concentration was probably close to the criterion of avoidance. No statistical inhibition threshold could be determined in gorillas, as grouping the results for the highest concentration interval ([8.8–14.7] mM) yielded non-significant differences (P=0.572; n=6). In contrast, the tannin/fructose mixtures within the concentration interval [0.59–5.9] mM or even [0.59–2.93] mM were significantly preferred over fructose (P<0.01, n=6 or P<0.05, n=4).
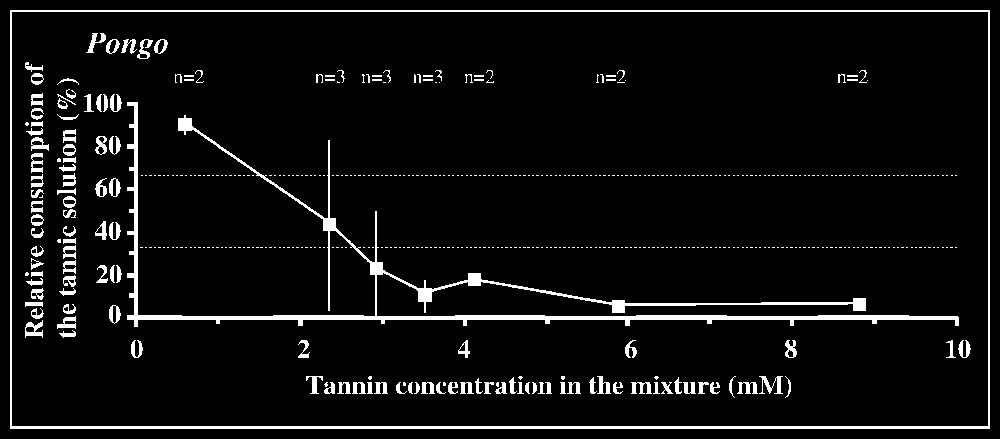

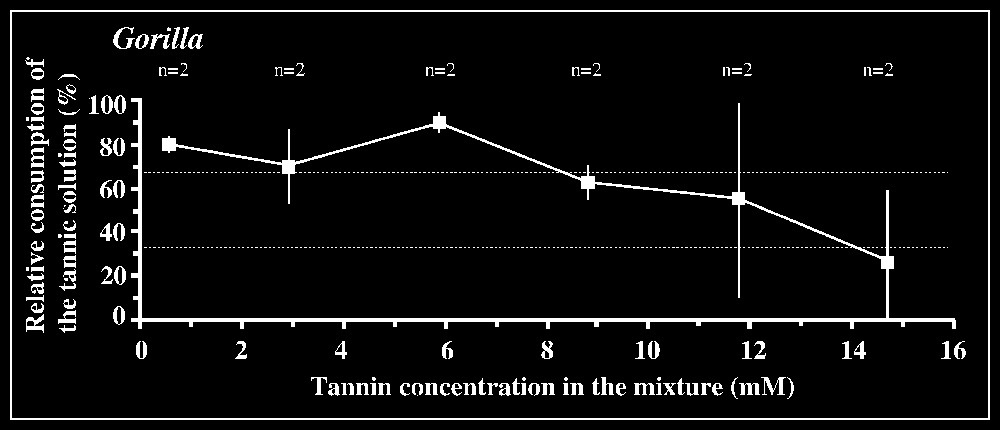
Results of the two-bottle test using mixtures of tannin/fructose solutions versus control fructose solution. The consumption of the binary solution relative to the total amount of liquid ingested in each test is plotted against tannic acid concentrations. The control sugar solution is 20 mM in Pongo, 50 mM in Pan, and 150 mM in Gorilla (see § Materials and method). The number n of individuals tested is indicated for each concentration. Dotted lines correspond to the criteria of preference and rejection, set at 66.7 and 33.3%, respectively.
4 Discussion
The thresholds for fructose measured here lie in the range of taste sensitivities previously determined in non-human primates (between 15 and 66 mM; [1–3,7]) as well as in different human populations (especially those living in tropical rain forests, savannas or even Arctic environments: 20–30 mM; [8]). It is noticeable that the threshold for fructose determined in gorillas ([70–80] mM) is congruent with that found by Remis and Kerr (50 mM; [7]).
In contrast, clear inhibitory effects of tannic acid on ingestive responses were observed for high tannin concentrations compared with other primates tested using similar mixtures (0.1–0.5 mM; [3,7,9]) and humans (0.16 mM; [10]). Gorillas appeared to have the highest level of tolerance of tannins of all primates tested so far, in agreement with previous results [7]. Like other experimental designs investigating individual ape responses, especially taste perception, our study is not proof against sampling biases. Accordingly, hypothesis relating nutrient/allelochemicals-based food selection in great apes with taste abilities are only tentative. Contrasting data for fructose, in which great apes exhibited both the lowest and the highest threshold found across primate species, and tannic acid, where apes all exhibited high tolerance levels, do not support the hypothesis of a major influence of phylogenetic inertia on their taste abilities. Instead, ape taste perception may have been shaped by selective pressures in relation to their eclectic diets adapted to meeting large nutritional requirements. On the one hand, the lower the taste sensitivity for tannins, the wider the range of foods that is potentially perceived as edible. On the other hand, the higher the taste sensitivity for sweet substances, the wider the array of sugar-containing foods perceived as palatable. The combination of a low sensitivity for tannins and a high sensitivity for fructose (with species differences) would partly account for the generally large dietary repertoire observed in these species, especially in terms of nutrient and allelochemical contents of foods (e.g., [11–13]).
An intriguing result was obtained in our tests: gorillas significantly preferred a range of low concentrations (0.59–5.9 mM), partly overlapping the inhibition thresholds of other apes and non-hominoid primates. The fact that the control fructose solution provided simultaneously with this set of tannin/fructose mixtures was systematically ingested in substantial amounts (mean: 31.1 ml, sd: 17.4 ml; n=6) argues against an experimental artefact (e.g., side-preference effect). Accordingly, this range of concentrations, especially the interval [0.59–2.93] mM (see statistical significance of results) would be considered as the threshold limit to detecting tannin. While these data compare well with the threshold of 4 mM published by Remis and Kerr [7], the fact that their study found no preferential consumption of tannin suggests that one might be in the presence of idiosyncratic responses.
All primates tested so far, including chimpanzees, orang-utans and humans, display a stereotyped facial expression of rejection in response to bitter stimuli, especially newborns (the gusto-facial reflex of Steiner and Glaser [14]). In humans, the pleasantness of moderately bitter, pure solutions or of astringent tastes elicited by some foods has been reported ([15]; F. Cousin, comm. pers.), but this may simply be the result of sociocultural effects. One presently cannot determine whether mixtures provided to gorillas were immediately perceived as pleasant by naive animals or whether this corresponds to a sensory preference acquired through individual's own alimentary experience.
While preference and inhibition taste thresholds appear to be generally consistent for studies using similar procedures [9,16, this study], differences may arise when dealing with responses to above-threshold concentrations, in which the hedonic dimension may be preponderant. Selection for foods containing moderate amounts of tannins, while high concentrations are avoided, has recently been experimentally demonstrated in one herbivorous mammal (the roe deer [17]). In this respect, additional studies may help determining whether the preferential consumption of low tannin concentrations, which are distasteful to other primate species, is limited to a subset of individuals within a primate population and if this might have a functional significance for food selection.
Acknowledgements
We are indebted to Claude and Patrick Caillé, and T. Petit, for giving permission to carry out the experiments at La Palmyre. We also thank the staff for help during the tests. We are grateful to A. Schilling for construction of the drinking apparatus and to E. Leigh Jr., who improved the manuscript.



