Version française abrégée
La cleptobiose (également connue comme « kleptoparasitisme » ou « piraterie ») concerne le vol d'aliments entre individus de même espèce ou d'espèces différentes. Cette forme de compétition par interférence constitue un moyen important par lequel de nombreuses espèces parviennent à obtenir des ressources alimentaires limitées, tout en réduisant les coûts liés à leur recherche et à leur obtention. Ce type de stratégie alimentaire, largement répandu dans le monde animal et abondamment décrit chez de nombreux vertébrés, a également été rapporté chez divers invertébrés et notamment des fourmis, où le cas de la ponérine néotropicale Ectatomma ruidum est un des exemples le mieux étudié. L'observation fortuite au Panama, il y a une quinzaine d'années, d'une relation de type parasitique entre une autre espèce d'Ectatomma (E. tuberculatum) et la petite myrmicine Crematogaster limata, au cours de laquelle les Crematogaster pénétraient à l'intérieur des nids d'Ectatomma et grimpaient sur le dos des ouvrières pour les lécher sans subir d'agression, nous a incité à étudier de plus près ce genre d'association, afin de vérifier si une relation de type cleptobiotique n'était pas en jeu et d'en détailler les différentes phases. Les observations, réalisées en saison des pluies en Guyane (Petit Saut), ont concerné 36 nids d'E. tuberculatum, dont au moins 10 présentaient l'association avec Crematogaster. La majorité des données a été collectée sur le nid le plus actif. Les enregistrements ont porté sur divers paramètres, liés, d'une part, à l'activité de fourragement des deux espèces (nombre de fourrageuses d'Ectatomma sortant et entrant du nid, flux journalier de Crematogaster près des entrées des nids d'Ectatomma, rythme journalier d'exploitation des sources sucrées produites par des nymphes de membracides, taux comparatif de présence des fourmis au niveau des nectaires extrafloraux d'Inga thibaudiana et sur 30 groupes de nymphes de Membracidae) et, d'autre part, à leurs interactions (proportion de fourrageuses d'Ectatomma arrêtées lors du retour au nid, nombre d'ouvrières de Crematogaster explorant les ouvrières d'Ectatomma et parties du corps concernées par l'exploration, durée des interactions). Bien que présentant un pic d'activité plus important en période nocturne, E. tuberculatum est active toute la journée et exploite en continu les nectaires extrafloraux et les membracides. Comme chez la plupart des autres ponérines, les échanges trophallactiques n'existent pas chez E. tuberculatum, et les fourrageuses qui récoltent des liquides les transportent entre leurs mandibules (ces liquides adhèrent grâce aux forces de tension superficielle) avant de les redistribuer par pseudo-trophallaxie à d'autres fourrageuses assurant un relais dans le transport (29,9 % des cas) ou directement aux congénères à l'intérieur du nid. Jusqu'à 61,8 % des ouvrières rentrant au nid transportent ainsi des liquides sucrés (N=377). Sur les sites alimentaires, E. tuberculatum rentre en compétition avec d'autres espèces de fourmis pour la récolte des sources sucrées, notamment avec les C. limata parabiotica, qui exploitent les mêmes espèces de membracides, sur les mêmes arbres et durant les mêmes créneaux horaires, mais généralement sur des branches différentes. Ectatomma tuberculatum est connue pour être une prédatrice efficace d'un grand nombre d'autres fourmis parmi lesquelles le genre Crematogaster est largement représenté. De jour, les C. limata parabiotica évitent manifestement tout contact direct avec les Ectatomma – utilisant les mêmes hémiptères, mais en alternance, ou bien exploitant les individus les plus éloignés des ouvrières d'Ectatomma et s'écartant dès que l'une d'elles se rapproche – et servent même fréquemment de proies aux Ectatomma. Les interactions comportementales entre les deux espèces changent de façon drastique pendant la nuit, l'agressivité normalement présentée par les Ectatomma vis-à-vis des Crematogaster disparaissant totalement. Les Crematogaster n'évitent plus alors le contact avec les Ectatomma et même l'initient, l'interaction se réalisant en deux phases distinctes. Dans un premier temps, en début de période nocturne, les Crematogaster effectuent de véritables raids à l'intérieur des nids de la ponérine. Elles en ressortent plus tard, sans avoir apparemment volé de nourriture, restent près de l'entrée et se mettent à arrêter les fourrageuses d'Ectatomma lors de leur retour au nid, tout en faisant vibrer leur abdomen, recrutant ainsi de nombreuses congénères. Sur 322 ouvrières d'Ectatomma rentrant ainsi au nid, 75,2 % ont été arrêtées, dont près de la moitié reprend rapidement le chemin vers leur nid. Dans tous les autres cas (126 sur les 242 arrêts), les petites ouvrières de Crematogaster grimpent sur le dos de la ponérine, la lèchent et, lorsqu'une gouttelette de miellat est présente entre ses mandibules – ce qui est le cas pour 88,1 % des ouvrières ainsi explorées –, la volent. Ces interactions, de type « cleptobiose interspécifique », présentent ainsi l'originalité de concerner des aliments sucrés et non des proies, contrairement aux autres cas rapportés dans la littérature. De plus, son expression est temporelle, car limitée à la période nocturne, et facultative, les Crematogaster ne dépendant pas strictement de cette stratégie pour s'approvisionner. Cette temporalité du phénomène pourrait s'expliquer par la forte augmentation d'activité des Ectatomma en début de période nocturne, provoquant une grande confusion à l'entrée des nids, ce qui réduirait ou même annulerait le filtrage réalisé par les gardiennes et autoriserait ainsi les raids de Crematogaster avec un minimum de risques d'agression. Cette phase permettrait à ces dernières d'acquérir un camouflage chimique par absorption passive d'hydrocarbones cuticulaires, camouflage qui serait par la suite intensifié lors des léchages des fourrageuses chargées de liquides sucrés.
1 Introduction
Cleptobiosis, kleptoparasitism, food robbing, thievery and piracy concern the stealing of food by individuals of the same or different species. This widespread form of interference competition is an important means by which many animals obtain limited resources while reducing the costs of searching for and handling food [1–3]. This foraging strategy has been extensively described for a large range of vertebrates, including fishes [4,5], birds [2,6,7], and mammals [8,9]. Numerous cases of intra- and interspecific cleptobiosis have also been reported in marine invertebrates [10], spiders [11,12] and insects such as thrips [13], wasps [14], bees [15], and ants. In ants, since the first descriptions by Wroughton in 1892 (in [16]) and the first use of the term ‘cleptobiosis’ in its current sense by Forel [17], numerous cases have been reported [18–21], particularly that of the Neotropical ponerine ant Ectatomma ruidum [22–27].
Aiming to further the understanding of the daily foraging rhythms of Ectatomma tuberculatum, Wheeler [28] provided a fortuitous account of a parasitic-like association between this species and Crematogaster limata. She observed, particularly at dawn and dusk, that hundreds of C. limata file into the nest entrances of E. tuberculatum. When encountering an E. tuberculatum worker, the smaller C. limata climbed up one of the larger ant's legs and onto its thorax and head, while the E. tuberculatum did not react aggressively to the intruders. As Wheeler [28] did not note any aggressiveness, we decided to conduct a study on this relationship, hypothesizing that the intriguing behaviour of the Crematogaster workers could be related to cleptobiosis.
2 Materials and methods
Data were collected in Petit Saut, French Guiana, mostly in July 2000. The mean temperature during the daytime was 28.5 °C, falling to 22.8 °C at night. The mean relative humidity was 85–90% during daytime all throughout the periods of observation.
Ectatomma tuberculatum is a monogynous (or occasionally polygynous) ponerine ant, widely distributed in Neotropical regions. Colonies have polydomous nests excavated at the base of a tree trunk. Nest entrances are characterized by an external tunnel (made with rough vegetal fibres) leaning against the base of the supporting tree. The opening of the tunnel is generally guarded by specialized workers. Workers concentrate most of their foraging activity on the tree situated above their nest plus neighbouring trees and shrubs. They solitarily gather a wide variety of small invertebrates, and exploit different sugary sources. As for most Ponerinae, trophallactic exchanges cannot occur in Ectatomma. So, foragers carry between their outstretched mandibles droplets of liquid substances that adhere thanks to surface tension strengths [22,28–33].
We noted the position of 36 Ectatomma nests thanks to their characteristic entrance tunnels. Nevertheless, due to problems of accessibility and great variation in the colonies' levels of activity, detailed observations were focused on the most active colony that had four nest entrances. Ten out of the 36 nests had relationships with Crematogaster limata parabiotica, a myrmicine species very frequent in pioneer formations with polydomous nests, built in hollowed structures or ant gardens [34,35]. The difference in size between the species was great (mean Ectatomma worker length±SE: 9.5±0.8 mm; N=20; Crematogaster: 3.5±0.06 mm; N=20).
As the Ectatomma circadian rhythm of activity varies seasonally [31], we conducted a series of 10-min scans over an 8-day-long period until we had covered each hour out of the 24. During each scan, we checked the number of workers entering and leaving the nest of one colony. We also estimated the flux of Crematogaster workers around the Ectatomma nest entrances by using a grid ranging from 0 to 4 (0: total absence of workers; 1: one or two workers near the nest entrance; 2: up to ten workers; 3: 10 to 50 workers; 4: more than 50 workers).
We also studied the rhythm of the exploitation of sugary sources. Firstly, we conducted 10-min scans per hour concerning Ectatomma workers attending membracid nymphs (Hemiptera) on a small tree, as well as Crematogaster workers on several sites in the area where we also registered the presence of Ectatomma (six 3-h time periods between 08:00 and 02:00). Secondly, we noted the presence of ants on 100 leaves of 20 juvenile Inga thibaudiana (Mimosoideae) and 30 groups of membracid nymphs in the studied area during three periods: at night, in the morning and in the afternoon.
We quantified the cleptobiotic events performed by Crematogaster workers through the proportions of Ectatomma foragers stopped while returning to their nest, the number of Crematogaster individuals exploring an Ectatomma worker, the body part concerned (i.e., mouthparts, legs, abdomen and thorax), and the duration of the interaction.
3 Results
3.1 Circadian rhythm of activity
Although continuously active, Ectatomma workers presented two peaks of activity at night, around 20:00 and 03:00. Nocturnally, between 19:00 and 05:00, the number of workers returning to their nest surpassed that of workers exiting the nest (Fig. 1A). During a continuous 24-h-long observation period, we noted that 61.8% of the workers entering the nest transported sugary liquids between their mandibles (N=377). On the foraging sites, Ectatomma competed with other ant species, including Crematogaster, for the exploitation of membracid honeydew and the extrafloral nectar of Inga. In spite of a reduced level of activity in the morning, Ectatomma workers attended membracids all day long (Fig. 2A and C). The nocturnal increase in the number of ants exploiting Inga extrafloral nectar corresponds to the increase in the production of nectar at night [36].
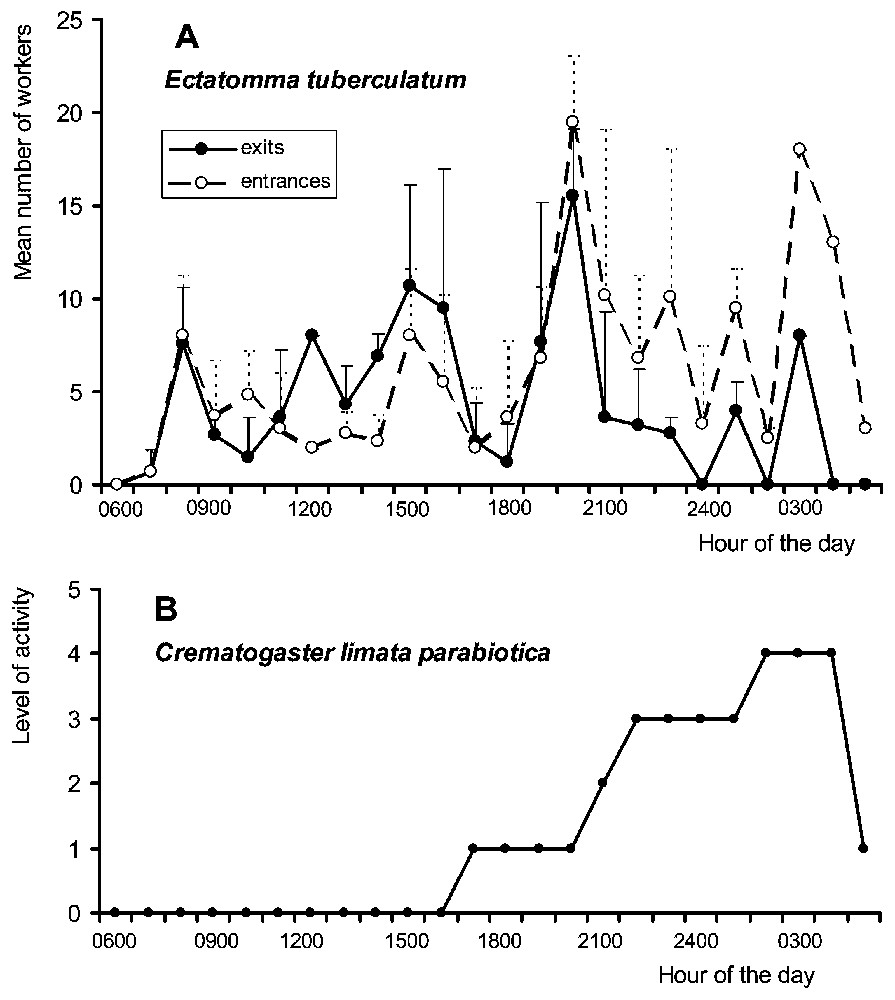
(A) Circadian rhythm of activity of Ectatomma tuberculatum through the mean number of workers entering and exiting the nest per 1-h time periods (mean ± SE). (B) Estimation of the flux of Crematogaster limata parabiotica workers at the E. tuberculatum nest entrance by using a grid ranging from 0 (absence of Crematogaster workers) to 4 (more than 100 Crematogaster workers).
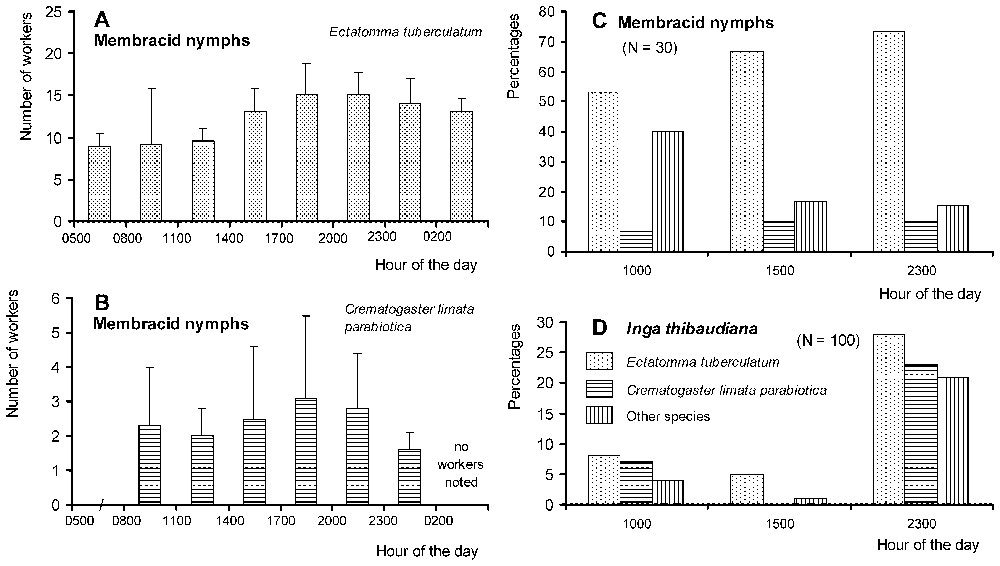
(A) Dynamics of the exploitation of membracid nymphs by Ectatomma tuberculatum workers at one site during a 24-h cycle. (B) The same for Crematogaster limata parabiotica workers recorded at different sites between 08:00 and 02:00. (C) Percentages of groups of membracid nymphs attended by ants during three periods of the day (other species: Camponotus spp.; 100% of the groups attended by ants at 10:00 and 23:00; 93.3% at 1500 hours). (D) Percentages of leaves of Inga thibaudiana (Mimosoideae) noted with ant workers exploiting the extrafloral nectaries during three periods of the day (other species: mostly Camponotus spp., but also some Gigantiops destructor and Cephalotes sp. in the morning; total of 39%, 7% and 72% of the leaves with ants at 10:00, 15:00, and 23:00, respectively).
Crematogaster workers also displayed both diurnal and nocturnal activity on the foraging sites (Fig. 2B–D). Around the Ectatomma nest entrances, their activity level was high at night, mostly between 21:00 and 04:00, when Ectatomma foragers returned to their nest (Fig. 1B).
3.2 Ants attending membracids
In the studied area, both Crematogaster and Ectatomma foragers attended membracid nymphs on the same trees during the same time periods, but generally on different branches. They shared the same parts of the branches on only 25 occasions out of 194 noted during the study of their circadian rhythm of activity. In such cases, the Crematogaster attended the membracid individuals the most distant from the Ectatomma foragers, and they moved when an Ectatomma approached. The latter frequently tried to capture the Crematogaster workers: 15 attempts occurred on the 25 branches shared by both species. Although most of them successfully escaped, we noted during a series of observations that 14 prey out of 27 retrieved by E. tuberculatum foragers were ants, 10 of them Crematogaster. Also four retrieved prey were membracids originating from attended groups, two complete predatory sequences being observed.
During trophobiotic relationships, Ectatomma workers palpated the lateral parts of the membracid nymphs with their antennae in an up and down movement, eliciting the secretion of honeydew. As they stood above the nymphs with their mandibles slightly open, they recuperated the honeydew that adhered to their mandibles thanks to surface tension strengths. While repeating this behaviour, the workers opened their mandibles wider and wider as the droplets of honeydew increased in size (Fig. 3A). Then, in 70.1% of the cases (N=127), each Ectatomma forager retrieved the droplet of honeydew accumulated between its mandibles, after having been replaced or not by a nestmate (25.2% and 44.9% of the cases, respectively). In the other situations (29.9%), Ectatomma workers specialized in honeydew transport shuttled between the individuals that collected the honeydew from the membracids and the nest (one transporter for 4–7 collectors). The transfer of honeydew from the collector to the transporter followed an antennation initiated by the transporter that also made a series of 3–4 back and forth movements with its body. Then, the collecting worker again attended the membracids while the transporter looked for other collectors. It returned to the nest when the droplet of honeydew completely filled the space separating its wide-open mandibles.
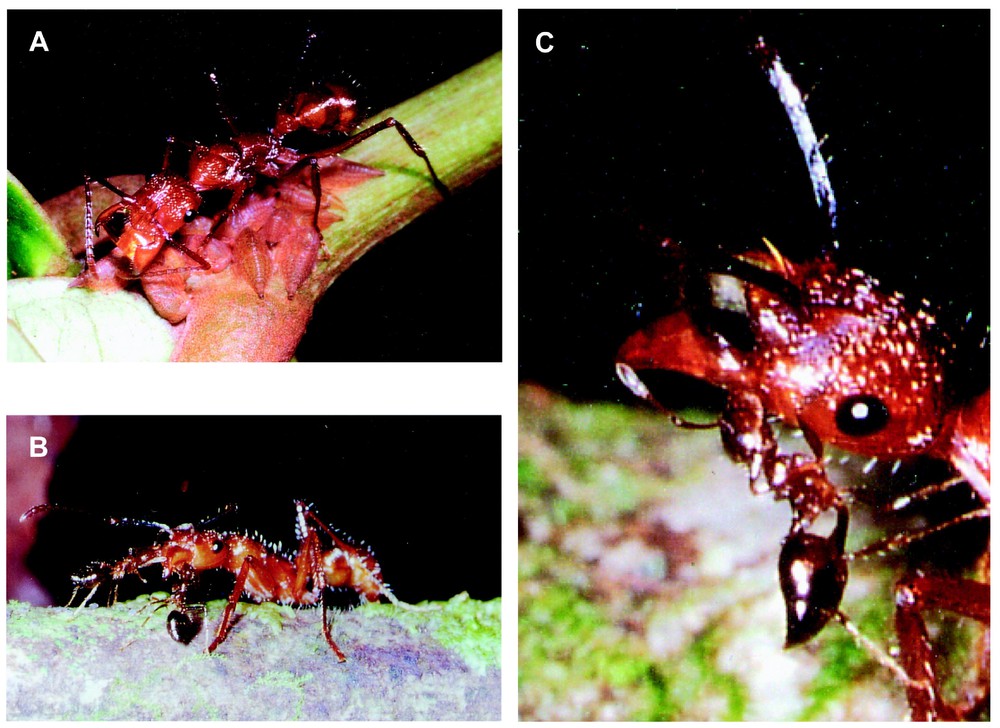
(A) Ectatomma tuberculatum worker attending membracid nymphs and gathering a honeydew droplet between its outstretched mandibles. (B) E. tuberculatum worker stopped by two Crematogaster limata parabiotica workers inspecting its mouthparts. (C) Worker of C. limata parabiotica licking a part of the honeydew droplet from a E. tuberculatum forager.
3.3 Relationships between Crematogaster limata parabiotica and Ectatomma tuberculatum
While completely absent in the vicinity of the Ectatomma nest entrances during the daytime (Fig. 1B), at dusk Crematogaster workers entered the Ectatomma nests without any opposition, as the guards were absent. As already reported [28], the column of Crematogaster passed through the entrance tunnels in a raid-like fashion. The beginning of such raids occurred between 18:00 and 22:45, which corresponds to the peak of nocturnal activity of Ectatomma (Fig. 1A). After these raids, the Crematogaster workers exited the tunnel and remained around the nest entrance almost all night long (Fig. 1B). They never had a swollen abdomen nor any evidence of having filled their crop. Then, they stopped returning Ectatomma foragers at the base of the supporting tree trunk around the nest entrance or on surrounding branches. Crematogaster workers waved their abdomens at the approach of a returning Ectatomma forager, but no liquid droplet was noted at the tip of their abdomens. They also tapped their abdomens on the substrate. These behaviours attracted nestmates that immediately arrived and rapidly aggregated around the Ectatomma. Certain Crematogaster individuals stayed in front of the Ectatomma that remained immobile, while up to 12 others climbed on the Ectatomma (3.2±2.4; N=98).
A study conducted on 322 Ectatomma foragers returning to their nest permitted us to note that only 80 (24.8%) were not intercepted by the Crematogaster workers prior to the tunnel entrance. Among the 242 Ectatomma intercepted, 116 (36% of all returning foragers) were stopped, and remained immobile during a short time before resuming their return trip to the nest. The remaining 126 individuals (39.1%) were stopped, explored and licked during a relatively long time (>1 min) by the Crematogaster workers. This included 111 individuals carrying a droplet of liquid food that was stolen (34.5%).
Of the 316 Crematogaster workers thoroughly observed after the interception of an Ectatomma forager laden with a droplet of liquid food, 165 (52.2%) focused their exploration on the Ectatomma mouthparts (see Fig. 3B and C). Each individual licked the honeydew only during a few seconds (6.6±5.4 s, N=70). When the honeydew droplet was depleted, the Ectatomma workers remained motionless, mandibles open, maxilo-labial mouthparts extended, allowing the Crematogaster workers to lick them during a long time (25±14.5 min; N=15).
4 Discussion
We confirm therefore the existence of the unusual relationship between E. tuberculatum and C. limata as reported in central Panama [28]. Nevertheless, some new information and significant differences deserve to be pointed out.
First, Ectatomma workers constantly attended groups of membracid nymphs thanks to two strategies. Workers collected honeydew that they transported to the nest once they were replaced by a nestmate, or certain workers collected the honeydew that was then transported to the nest by other workers. The latter cooperative behaviour, known in the Formicinae [3], presents similarities with the ‘relay transport’ and the ‘prey chain transfer behaviour’ noted for other ponerine ants during predation [37,38].
Second, we noted a dramatic difference in Crematogaster-Ectatomma interspecific relationships between day and night. Although sharing branches with them, Crematogaster workers maintained a ‘safe distance’ from Ectatomma in the foraging areas during the day, the latter being able to prey on them (see also [33]). On the contrary, at night the Crematogaster workers no longer avoided direct contact with E. tuberculatum, but initiated the interactions. They conducted their raids on the Ectatomma nest entrances, then engaged in contact with the Ectatomma foragers returning to their nest, stopping and exploring them.
Third, Wheeler [28] noted that the behaviour of Crematogaster workers during raids was limited in time and consisted of a kind of ‘shampooing’ deriving nutrition from the cuticular secretions and/or the excess fluids (nectar and honeydew) trapped on the mandibles of Ectatomma workers. This author noted that such behaviour, first described between an inquiline ant and its host [39], could account for a mutualistic association of the ‘cleaner-fish’ type where both species derive benefits. Even if we again noted that Crematogaster workers licked the different body parts of the Ectatomma, they mostly stopped and explored the Ectatomma retrieving a honeydew droplet (88.1% of the explored E. tuberculatum foragers) and recruited nestmates in order to rob this droplet. This resulted therefore in a parasitic relationship of the ‘cleptobiotic’ form. This nocturnal robbing behaviour only complements the food supply of the Crematogaster that gather most of their sugary food by attending hemiptera or exploiting extrafloral nectaries.
Fourth, other cases of cleptobiosis in ants concerned prey robbing, with nevertheless a certain similitude with the present study. For example, Myrmecocystus mimicus workers thoroughly inspect returning foragers of Pogonomyrmex spp. before robbing their prey [20]. The originality of the cleptobiotic activity of C. limata parabiotica consists in exploiting the kind of liquid transport limited to arboreal ponerine ants. Indeed, they carry droplets of liquid between their mandibles, which are then imbibed by nestmates, an adaptation to the lack of trophallaxis in most Ponerinae [40,41].
Finally, we questioned the origin of the lower nocturnal aggressiveness of Ectatomma workers towards Crematogaster. The immobility of the Ectatomma workers could be triggered by a secreted chemical, while the Crematogaster wave their abdomens. Indeed, secretions from the mandibular, metapleural or Dufour glands act as repellents in several Crematogaster species [42–44], and such an effect was noted in the lestobiotic Solenopsis fugax [45]. Similarly, a pacifying action on the host occurs when an individual of the parasitic Formicoxenus provancheri raises its abdomen and extrudes the sting producing a droplet of volatile liquid before engaging in ‘shampooing’ behaviour with Myrmica incompleta [46].
Nevertheless, why does this not work during the daytime? As the Crematogaster ‘raids’ coincide with the dusk peak in activity of Ectatomma (19:00–21:00; Fig. 1A), another hypothesis can be formulated. During this period, the heavy flow of Ectatomma foragers entering and leaving the tunnel drives the guards back into the nest. This permits the small Crematogaster workers to creep inside the tunnel without being attacked. In this situation, they can modify the composition of their cuticular hydrocarbons enough to be accepted by the next Ectatomma foragers returning to their nest, and so to lure them. A similar deceptive mechanism, based on chemical camouflage acquisition, was proposed to explain intraspecific cleptobiosis in E. ruidum [27]. Later, new Crematogaster individuals can enter the tunnel, or they can acquire cuticular compounds directly from an intercepted Ectatomma worker by climbing on it and licking its cuticle, this corresponding to the ‘shampooing’ behaviour described by Wheeler [28]. This hypothesis might be supported by the fact that C. limata parabiotica has the ability to share nests and trails with different formicine and ponerine ants, in a phenomenon called ‘parabiosis’, while these ants never share nests between them [34,35,46].
In conclusion, we noted a new kind of cleptobiosis in ants concerning the robbing of sugary substances, and not prey robbing as previously known. Temporally limited to night time and facultative, it implies the presence of Ectatomma nests on Crematogaster territories.
Acknowledgements
We are grateful to Dr. Jacques H.C. Delabie (U.P.A. Laboratório de Mirmecologia, Convênio UESC/ CEPLAC, Itabuna, Bahia, Brazil) for the identification of the ants and to Andrea Dejean for proofreading the manuscript. This work was supported by the French « Ministère de l'Écologie et du Développement durable » (SOFT program, research agreement GIF ECOFOR No. 98, and the « Tropical Ecosystems » program No. 02-E2002).


