1 Introduction
Paratrechina longicornis, native of West Africa, is one of the ‘crazy ant’ species, so-called because foraging workers move quickly along erratic paths. Dispersed by human commerce throughout the tropics, it is now pan-tropical and particularly prevalent in towns, so that it is considered as one of the most frequent ‘tramp species’. Although moist conditions are preferable for reproduction, small and ephemeral P. longicornis nests can be found in plantations, gardens, and buildings where colonies occupy all available cavities in the ground, live or dead plants and walls [1–3]. In spite of the environmental and economic impact noted in areas it has recently occupied, reports on the biology of P. longicornis in its native range are rare. Available scattered information from Cameroon shows that in primary forest, P. longicornis nests in abandoned Cubitermes termitaries or fallen, rotten branches, whereas in plantations, its nests can be noted anywhere and workers attend pest hemipterans [3–5].
Like most invasive ants P. longicornis has a monomorphic worker caste and is omnivorous, feeding on live and dead insects, seeds, honeydew, fruit, plant exudates, and many household foods. Nevertheless, unlike other invasive ant species, P. longicornis is neither territorial nor aggressive toward other ants and its foraging strategy mostly depends on the rapidity of the foragers and their ability to immediately recruit nestmates at short or long range when they find a food source [1,6,7]. This strategy, known as ‘exploitative competition’ is in contrast to ‘interference competition’ used by dominant ants that monopolize resources using repellents or direct aggressiveness toward competing ants [8].
In this study, we hypothesized that the ability of P. longicornis workers to eliminate competing ants may be due in part to the efficaciousness of their predatory behaviour, never studied before. We therefore conducted surveys to (1) verify if P. longicornis workers compete against other ants around the clock, and (2) compare their prey-capture behaviour with those of other ants of economic importance already studied (e.g., dominant ant species; other tramp species). All these latter ant species have populous colonies employing group hunting strategies that permit numerous workers to easily master relatively large prey by spread-eagling them. These strategies are possible because each individual is situated in the vicinity of the others and so can recruit them at short range [9–12].
2 Materials and methods
Field experiments on foraging behaviour were carried out in Yaoundé, Cameroon (1997–1998), those on prey-capture behaviour in Douala (2003–2004).
We firstly verified if P. longicornis foraging workers are competitive vis-à-vis the ant species that most frequently forage in the same areas: Camponotus brutus (Formicinae), Pheidole megacephala (Myrmicinae), and Odontomachus troglodytes (Ponerinae). Experiments, conducted on eight sites (P. longicornis compete with only C. brutus in three sites, P. megacephala in three others, and O. troglodytes in the two remaining sites), were repeated four times every 24 h (8 to 9 AM; 2 to 3 PM; 8 to 9 PM; 1 to 2 AM). They consisted in placing each time one 2.5 cm long numbed cockroach on an area shared by P. longicornis and one of the compared ant species. The number of replicates, limited to 10 when the workers of only one species had retrieved the prey, varied between 12 and 30 in the other cases. For statistical comparisons we used Fisher's exact test (Statistica 5.0 software).
We studied the exploratory behaviour of the workers by comparing the ratio between the width separating the tips of the workers' antennae of 3 to 3.2 mm in length (or their body of 2.5 to 3 mm in length [6]) trained to forage on graph paper (photographs permitted easy evaluation) and compared them to results obtained using for other ant species frequent in the study area. We also calculated the speed of foraging workers by timing them along 50 cm on a horizontal surface: a 4-cm-wide plank serving as bridge interconnecting two natural areas separated by a small stream (the temperature was 28 °C). The workers were in their exploratory phase, different and apparently slightly slower than when they move on a familiar path, when they return to a permanent food source such as a group of hemipterans.
To study prey capture behaviour, we placed plywood planks () perpendicular to the wall or trunks of trees at 50 cm in height and about 2 m from the closest nest entrance (five colonies monitored). During one week, honey and small prey were deposited on these planks that the workers marked as part of their territory. Each time, during experiments conducted from 10:00 to 12:00 and 14:00 to 18:00, we firstly noted the number of workers patrolling on the experimental hunting areas. Then we registered the predatory behaviour of the workers when confronted with live termites (3–4 mm long Microtermes fuscotibialis workers; 50 cases) and grasshopper larvae (5–8 mm long Tettigoniidae; 46 cases) deposited one-by-one on the hunting areas, or groups of 10 Microtermes workers (40 cases). We cut off the tibia of the grasshoppers' posterior legs to prevent them from jumping away. More than 30 min separated two trials.
The behavioural sequences were recorded through direct observation from the introduction of the prey into the centre of the hunting areas (on the plank of plywood) until their capture and retrieval to the nest. A full repertoire of behavioural sequences was first established during preliminary experiments. Referring to this complete list, we recorded each behavioural act performed vis-à-vis the prey (e.g., detection by contact, antennal palpation, attack, seizure, immobilization, spread-eagling, cutting up, and retrieval) as well as nestmate recruitment. This allowed us to build flow diagrams with transition frequencies between each behavioural act.
Throughout the text, values are given as mean ± SD. Percentages (transition frequency between behavioural acts) were calculated from the overall number of cases. Raw data were compared using Fisher's exact-test. The total durations of captures of different prey were recorded as the time separating the detection of the prey to its retrieval. Finally, we compared the number of workers that cooperated in spread-eagling the prey using the Mann–Whitney rank sum test (normality and equal variance tests failed).
3 Results
The survey on competition illustrates that P. longicornis workers supplanted or were as efficacious as compared species during the daytime and the beginning of the night, but were supplanted by C. brutus and P. megacephala in the middle of the night (Fig. 1). Workers from the four compared ant species foraged around the clock, but their density varied. Note that these data illustrate the confrontations between workers of the compared species. Most of the cockroaches were discovered first by P. longicornis individuals unable to retrieve them before they were discovered in turn by foragers of the competing species. During the daytime, the P. longicornis actively defended their prey, while C. brutus or P. megacephala workers were not nearly as insistent, although they stole prey from P. longicornis at night. Reciprocally, when preys were discovered by C. brutus or P. megacephala, P. longicornis workers were sometimes able to steal them during the daytime, but never in the middle of the night. The situation was different vis-à-vis O. troglodytes workers as the P. longicornis readily competed for prey during the daytime, but never at night (Fig. 1). Also, O. troglodytes workers were able to retrieve the cockroaches singly, dragging them backward, but releasing them from time to time in order to get their bearings. This permitted the P. longicornis to discover the cockroaches in turn and, sometimes, steal them.
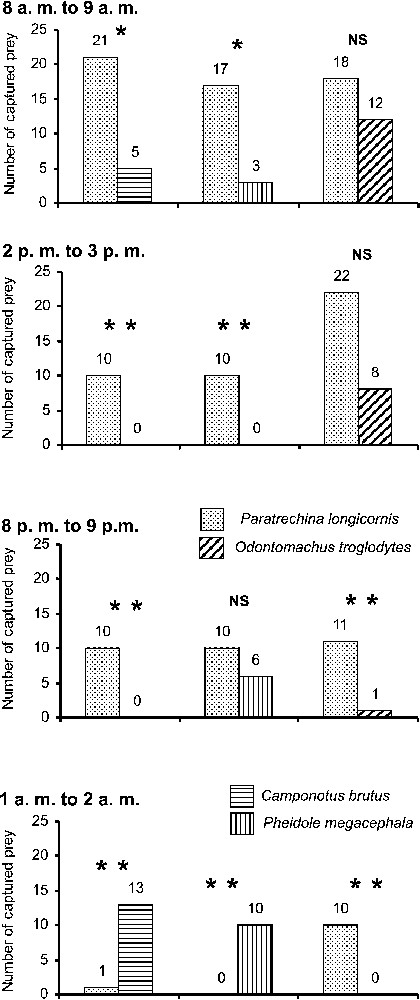
Competition between ant species for 2.5-cm-long cockroaches during four periods of the nychthemeron. We deposited the cockroaches on areas where Paratrechina longicornis workers competed with workers of Camponotus brutus, Pheidole megacephala or Odontomachus troglodytes, respectively. Fischer's exact test: ; ; NS = non-significant.
While exploring, each P. longicornis worker moved quickly, its long antennae wide open, so that their extremities were separated by 3.5 to 5 mm (median: 4 mm; mean: ; cases). This value, which corresponds to 1.17 to 2.00 times the body length (median: 1.45), is greatly superior to those noted for other monitored species (Fig. 2). The speed of the P. longicornis workers was during exploratory paths ( for the return path; and 57 cases, respectively), so that the surface explored per second, around 264 mm2, is relatively vast compared to the size of the workers. Also, workers foraged in a group since we noted the permanent presence of 10 to 20 of them on the experimental hunting arenas during the survey periods (presence of workers per hunting arena; n= 136 cases).
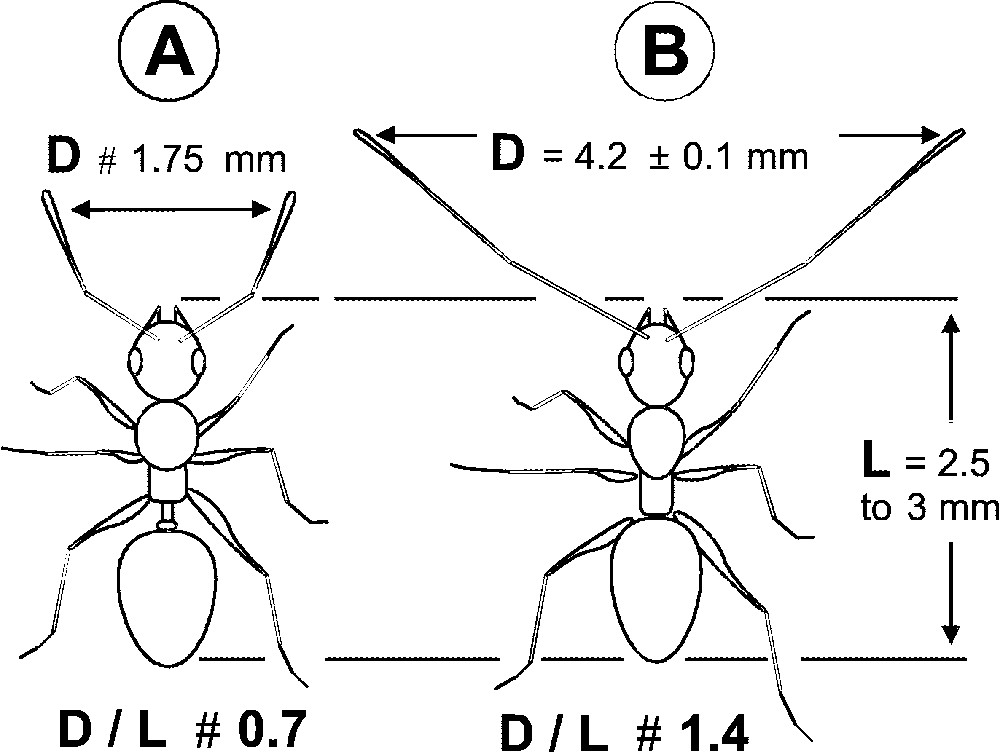
(A) Schema of a theoretical myrmicine ant during its exploratory paths; the distance between the tips of its antennae is shorter than the length of its body (D/L=0.7). (B) The case of Paratrechina longicornis for which D/L is about 1.4 (median value: 1.45). During exploratory paths, D/L was inferior to ‘1’ for all other tested ground foraging ant species: Camponotus spp.: 0.70 to 0.85; Monomorium sp.: 0.5; Odontomachus troglodytes: 0.70 to 0.85; Pachycondyla soror: 0.6 to 0.7; Pheidole spp.: 0.38 to 0.65 (10 cases monitored for each species).
In all cases, P. longicornis workers detected prey when contact occurred with one of their antennae. They were able to stop ‘on a dime’, although they were moving at full speed, and immediately attacked the prey, seizing it without any antennation (Fig. 3). There was a solitary phase in the behavioural sequences where workers discovering the prey attacked and seized it unaided, pulling it backward over a short distance (the same was noted for the 2.5-cm-long cockroaches studied above) and a short-range recruitment phase where they released the prey, and, seemingly very excited, moved very quickly around the prey in a looping pattern. They then repeated these behaviours. The discovering worker probably emits a recruiting pheromone as nestmates situated on the experimental hunting arenas stopped moving along their own fast erratic paths and went toward the prey, in turn adopting the behaviour of the discovering worker. This triggered a chain reaction permitting the recruitment of enough individuals to immobilize the prey. The recruitment behaviour ended when the recruited workers simultaneously seized the prey (by the head, an appendage or the abdomen) and pulled backward, spread-eagling it. Five minutes after, the prey was discovered by the first worker; the number of recruited nestmates (in the process of spread-eagling the prey), varying from 2 to 8 workers, was significantly lower for small termite workers ( ants; ) than for the larger grasshopper larvae ( ants; ; Mann–Whitney test: ), while we noted ants () after the groups of 10 termites were discovered.
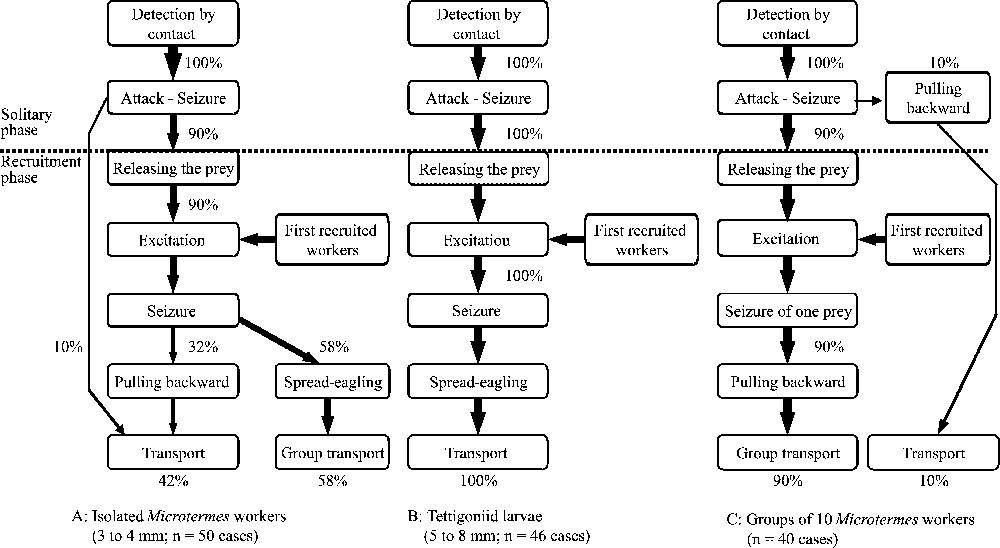
Flow diagrams of the behavioural events recorded during prey capture by Paratrechina longicornis workers.
During the capture of isolated prey, the first attacking workers indifferently seized termite workers by an accessible body part (32.0% by the head, 28.0% by an appendage; 40.0% by the abdomen; n= 50 cases), while they preferentially seized grasshopper larvae by an appendage (13.0% by the head; 65.2% by the appendages; 21.7% by the abdomen; cases) resulting in a significant difference (appendages versus body; Fisher's exact test: ). Venom spraying and gaster bending were never noted and both kinds of prey were retrieved whole by a group of workers (never cut up on the spot). For the groups of 10 termites, after detection by contact of one termite, the first workers in 10% of the cases seized, pulled backward and/or lifted it and retrieved it unaided. This behaviour, nevertheless, was accompanied by the recruitment of nestmates at short-range. In all other situations the behaviour of workers when discovering a group of 10 termites was similar to when they discovered single prey.
Small termite workers were retrieved by one or several workers (58.0% for group retrieval; ), whereas grasshoppers were mostly retrieved by a group of workers (97.8% of the cases; ; comparison with termites using Fisher's exact test: ). The number of workers involved in the group transport varied according to prey size (termites: workers; ; grasshoppers: workers; ; Mann–Whitney rank sum test: , ).
All tested preys were successfully captured and the duration of capture varied from 2 to 8 min for isolated prey and 6 to 15 min for groups of termites. It was significantly lower for termites than for the larger grasshopper larvae (termites: , ; grasshoppers: , ; Mann–Whitney rank sum test: , ) and reached for groups of 10 termites.
4 Discussion
We recorded variations in the behaviour of competing ant species according to their nychthemeron, something noted between species sharing the same territory when one is nocturnal and the other diurnal: the morning's aggressors become the expulsed at dusk [13]. Also, Crematogaster workers that steal liquid food from Ectatomma tuberculatum workers nightly avoid them during the daytime as they can be killed [14]. As a result, in their native range, P. longicornis workers are confronted with competitors able to supplant them during certain times of the day, while in areas where it has been introduced, P. longicornis can completely expulse other ant species [6].
Paratrechina longicornis workers explore a relatively large area thanks to their long, wide open antennae and their high speed. Along with their ability to ‘stop on a dime’ these two factors contribute strongly to their efficaciousness in finding prey (or other non-permanent food sources), even though they detect prey by contact. Note that the ratio between the distance separating the tip of their antennae and their body length, greatly superior to that of the compared species (Fig. 2), is probably one of the largest among ants, and their speed is clearly superior to that of all other ants, except for desert-dwelling species (Table 1). In fact, their speed and their very long antennae compensate their inability to detect prey at a distance like workers of most previously studied ants that detect prey at a short distance (i.e., 0.2 to 0.8 cm), with variations depending on the size of the workers, or size and number of prey [9–12]. Exceptions concern workers of arboreal species that detect prey visually, up to 1 m away for Gigantiops destructor [15].
Speed of different ant species compared to that of Paratrechina longicornis (from http://www.woodcow.org/teachers/esi/2001/CostaRicaLla_selva/atta2/; http://www.biorobotics.; L. Passera, G. Beugnon and A.D., pers. commun.)
| Ant species | Speed in cm/s | Ant species | Speed in cm/s |
| Pachycondyla berthoudi | 2.60 | Pheidole spp. | 0.5 to 0.7 |
| Pachycondyla spp. | 1.5 to 2.5 | Camponotus herculeanus | 3.03 |
| Dorylus laevigatus | 2.02 | Formica fusca | 2.73 |
| Atta cephalotes | 1.5 to 3 | Formica rufa | 1.88 |
| Atta colombica | 1.62 | Formica sp. | #3.00 |
| Decamorium decem | 0.70 | Gigantiops destructor | 1.8 to 3.8 |
| Leptothorax albipes | 1.04 | Lasius niger | 2.4 to 3.7 |
| Messor sancta | 1.6±0.7 | Paratrechina longicornis | 6.30±0.75 |
| Ants living in deserts | |||
| Pogonomyrmex rugosus | 3.39 | Cataglyphis albicans | 20.00 |
| Myrmecocystus mendax | 4.00 | Cataglyphis fortis | 100.00 |
| Myrmecocystus mexicanus | 4.20 | Cataglyphis bombycina | >100.00 |
Because exploration by each P. longicornis worker is combined with that of nestmates foraging in a similar pattern within the range of a recruitment pheromone, all together these workers perform a kind of group hunting that has not previously been reported. Note that group hunting with short-range recruitment is considered to be a more ‘evolved’ strategy than solitary hunting because it implies cooperation between workers and enables a species to rapidly exploit a greater range of prey sizes or food sources [16]. Also, short-range recruitment, firstly demonstrated in Oecophylla [17], has been noted in several other ant species with populous colonies that spread-eagle prey [9–12]. Moreover, P. longicornis workers are able to retrieve small prey (i.e., termites) unaided, but, like Oecophylla [9], group hunting predominates.
Paratrechina longicornis workers limit prey escape by immediately attacking then running around a newly discovered prey while recruiting nestmates at short-range. Also, like Oecophylla [9], P. longicornis workers spread-eagle their prey apparently without using venom and retrieve them whole in all cases (the same was true for larger prey such as the tested 2.5-cm-long cockroaches). Nevertheless, the slender P. longicornis workers seem very weak compared to Oecophylla workers, so that we cannot deny the possibility that P. longicornis workers produce chemical compounds responsible for their success. Indeed, with the exception of Oecophylla, other ant species that master prey by spread-eagling them use venom and generally cut them up the spot [10–12].
In conclusion, because P. longicornis is a feeble competitor against most frequent ground-dwelling species such as Pheidole megacephala and Camponotus spp. in Africa, or fire ants in areas where it has been introduced, its foraging strategy depends on rapidly discovering and depleting food sources [1,2]. The same is true for its prey hunting behaviour that can be considered as a new kind of group foraging strategy well adapted to detecting prey by contact and, after short-range recruitment, spread-eagling it, then rapidly and collectively retrieving it whole.
Acknowledgements
We are grateful to Barry Bolton (Museum of Natural History, London, where voucher specimens were deposited) for the identification of the ants, and to A. Yockey-Dejean for useful comments and English corrections on the manuscript. This research was supported by a project of the French ‘Ministère des Affaires étrangères’ (CORUS program, research agreement No. 02 412 062).


