1 Introduction
Fungal degradation of lignocellulose is probably the most important process for recycling carbon in nature [1]. Lignocellulose is composed primarily of lignin, cellulose, and hemicellulose that are cemented together in wood cell walls as a highly complexed biopolymer, although its true nanostructure is still unknown [2]. Certain basidiomycete fungi, known as white-rot fungi, are able under aerobic conditions to completely mineralise lignin and wood polysaccharides to CO2 and H2O. The process is known to involve a range of physiological and biocatalytic activities during which the fungi colonize the wood structure and secrete a variety of hydrolytic and oxidative enzymes, as well as non-enzymatic steps [1]. Our current detailed knowledge on in-situ decay is however relatively poor, particularly regarding the spatial distribution of enzymes and the non-enzymatic mechanisms involved in relation to the native wood structure. In the present work High Resolution Cryo-Field Emission Scanning Electron Microscopy (HR-Cryo-FE-SEM) and Transmission Electron Microscopy (TEM) immunocytochemistry were used to study the morphological events of lignocellulose degradation by the basidiomycete white-rot fungi Phlebia radiata and mutant (cellulase less) strain P. radiata Cel 26, and localize the spatial distribution of three extracellular fungal oxidoreductases (laccase [EC 1.10.3.2], diarylpropane peroxidase (ligninase, lignin peroxidase, LiP) [EC 1.11.1.14] and manganese peroxidase (MnP) [EC 1.11.1.13] co-operating in lignin degradation. The wild and mutant Phlebia strains were chosen since they show contrasting decay capabilities with the former degrading all major wood components and the latter only the lignin and hemicelluloses, leaving the cellulose. Thus detailed cryo- and immunocytochemical observations were used to reveal novel morphological details on the manner of fibre wall attack and component removal as well as the spatial involvement of the above enzymes. In addition, the study involves the application of Cryo-FE-SEM to wood biodegradation allowing high-resolution SEM observations to be made on cryo-fixed, and frozen hydrated samples thus avoiding the artefacts commonly associated with conventional preparation for SEM.
2 Materials and methods
2.1 Fungal strains
The two strains of Phlebia radiata Fr. used in this study, P. radiata (wild) and its cellulase less mutant P. radiata Cel 26, were obtained from the collection maintained at the Department of Wood Science, Swedish University of Agricultural Sciences. P. radiata Cel 26 was produced by UV-irradiation of spores [3].
2.2 Enzyme production and purification
P. radiata (ATCC 64658) was cultivated in laboratory bioreactors using a modified version of the low-nitrogen media described by Fenn and Kirk [4] by omitting vitamins and mineral salts but including glucose (2.8 mM), guaiacol (0.8 mM) (Merck, Darmstadt, FRG) and Tween 80 (0.05% w/v, Atlas, Essen; FRG). For further details and the enzyme purification see Niku-Paavola et al. [5].
2.3 Production of polyclonal antibodies to oxidoreductases
Antisera to laccase, MnP/LiP and LiP were raised in New Zealand white rabbits immunized monthly over a 6-month period by intracutaneous injections of the purified proteins (1.0 mg) emulsified in 1 ml of Freund's adjuvant (Difco Laboratories). The immunoglobulin (IgG) fraction of the sera was then prepared by ammonium sulphate precipitation using standard techniques and the sera purified by affinity chromatography. Antiserum to laccase showed a single band with laccase and no cross reactivity with purified MnP or LiP enzymes. LiP reacted with only LiP. One antibody cross reacted with both MnP and LiP (for simplicity termed MnP/LiP here) and was used for simultaneous labelling of both enzymes.
2.4 Decayed wood samples
Small wood blocks (
2.5 Transmission electron microscopy (TEM)
Wood samples were fixed directly for 3 h at room temperature (RT) in either: (A) 3% w/v glutaraldehyde containing 2% w/v fresh paraformaldehyde in 0.1-M Na-cacodylate (pH 7.2), or (B) 4% v/v paraformaldehyde containing 0.1% w/v glutaraldehyde in the same buffer. Samples fixed in fixative A were subsequently washed in buffer (
2.6 TEM immunocytochemistry
Ultrathin sections for immunocytochemistry were treated as described by Daniel et al. [7,8]. Labelling was performed on osmicated and non-osmicated samples. Sections were post-immunolabelled using polyclonal antibodies to each of the three enzymes (laccase, MnP/LiP, LiP) using 5- and 15-nm gold-labelled secondary antibodies in single- or double-immunolabelling procedures. Controls included omission of the primary antibody stage, use of rabbit IgG instead of the antibody and the use of antibodies preadsorbed with their respective enzyme. In addition, labelling studies were also performed on undegraded wood.
2.7 High Resolution Cryo-Field Emission Scanning Electron Microscopy (HR-Cryo-FE-SEM)
For HR-Cryo-SEM, small samples (ca 1 mm2) were removed from wood blocks partially degraded by monocultures of the white-rot fungi and rapidly frozen in LN2 slush (
3 Results and discussion
3.1 HR-Cryo-FE-SEM and TEM observations on P. radiata decayed wood
Both Phlebia radiata (wild) and P. radiata Cel 26 hyphae colonized the wood structure and produced typical white-rot decay patterns; the former a simultaneous rot where all cell components (cellulose, lignin, hemicelluloses) were progressively removed from the cell lumen outwards and the latter, preferential attack where the lignin and hemicelluloses were selectively removed across the wall leaving the cellulose [2,3]. Using light microscopy and TEM, decay was seen to occur in either a thin advancing zone across the wall in which all the components were degraded (simultaneous attack) or was reflected by the development of a thick zone (i.e. thickness of the secondary wall) in which the cellulose remains (preferential attack); very few structural details of the degraded wood cell wall were however, evident.
In contrast to previous TEM and conventional SEM observations, HR-Cryo-FE-SEM and deep-etching provides a very different morphological view of the fibre wall decay process. Observations of fractured P. radiata wild degraded birch fibres showed two main morphological features: (1) the birch fibre cell wall was composed of aggregates (i.e. spaghetti-like structures) that were organized into concentric lamellae within the fibre S2 cell wall. During decay the lamellae were progressively separated and attack of the aggregates occurred (Fig. 1a and b); and (2) that the fungal hyphae growing within the cell lumina of fibres were in contact with the surrounding degraded walls, mediated by a complex extracellular “slime” matrix (Fig. 1a and d). The slime matrix had two morphological forms: a more fibrillar type was often organized into discrete concentric bands (2–5 per cell) that surrounded lumina hyphae and which radiated across the cell lumen and was in contact with regions of decay (Fig. 1a and c); and a second type, a ‘gel-like’ slime material present in areas of active wood cell wall decay or in regions where the fibre wall appeared to have been almost totally degraded (Fig. 1d and e). The gel-like material was also frequently arranged in radial patterns (Fig. 1d and e) and often appeared attached and integrated with slime material forming the concentric bands at sites of the previous location of the lumen wall (Fig. 1d, arrows).
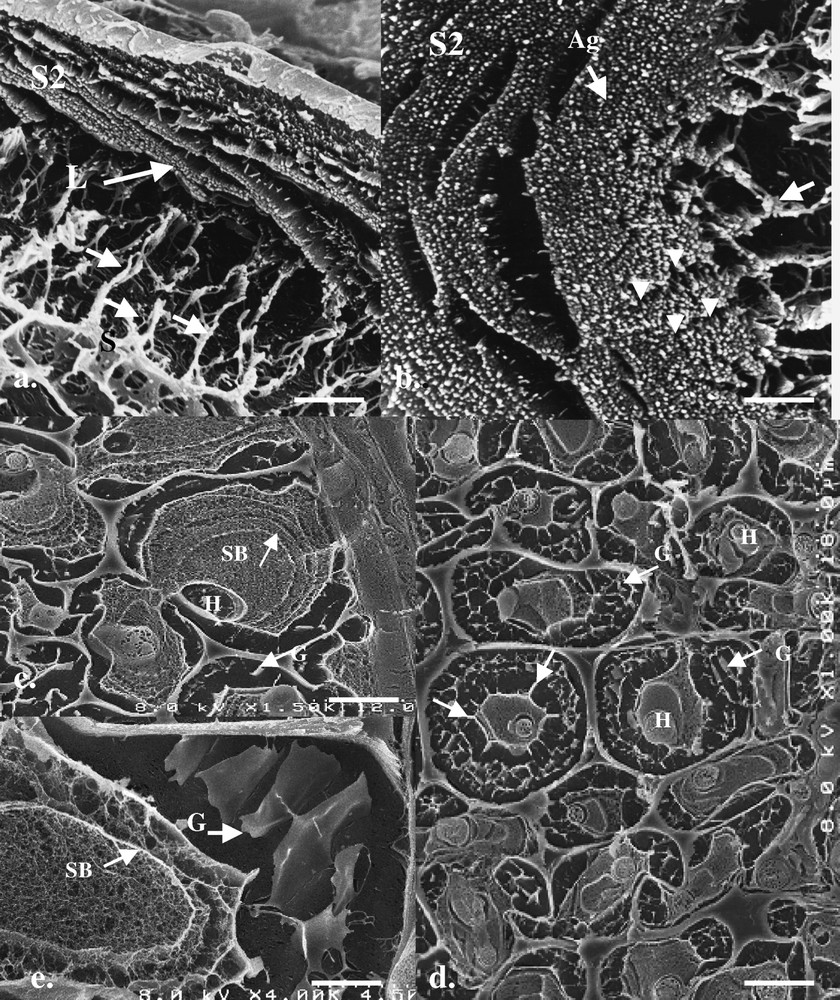
HR-Cryo-FE-SEM micrographs of fractured birch fibres at different stages of decay by P. radiata wild. (a, b) Early and intermediate stages of decay with slime material orientated radially (S, arrows) from the cell lumen against the S2 cell wall. Note that the fibre cell wall is composed of numerous spaghetti-like aggregates (macrofibrils, Ag), which are arranged in concentric lamellae (L) around the fibre cell wall. Radial disruption (gaps, arrowheads, b) in the radial orientation of the remaining wall structure suggests possible infiltration of slime material from the cell lumen. (c, d) Advanced decay of fibre walls with luminal hyphae (H) variously surrounded by concentric bands of fibrillar-like slime (SB) and more ‘gel-like’ (G) slime located in degraded regions of the S2 wall. In some fibres, the slime materials appear to project radially from a band previously lining the cell lumen wall (d, arrows). (e) Concentric bands (SB) and radial gel-like (G) materials within the cell lumen and remaining regions of the highly degraded cell wall. Masquer
HR-Cryo-FE-SEM micrographs of fractured birch fibres at different stages of decay by P. radiata wild. (a, b) Early and intermediate stages of decay with slime material orientated radially (S, arrows) from the cell lumen against the S2 cell wall. ... Lire la suite
HR-Cryo-FE-SEM observations on fractured and deep-etched birch cell walls degraded by P. radiata Cel 26 showed similar features in morphology as for the wild type, but differed in that the majority of the S2 wall material remained in the form of concentric orientated lamellae composed of discrete aggregates. Fig. 2a–d show different views of the remaining aggregates demonstrating both their concentric arrangement, spaghetti-like form and fairly uniform size (ca 40–60 nm); features similar to that reported previously for softwood kraft pulps (i.e. delignified fibres) following rapid freezing and cryo-observations or after application of replica techniques [9]. In addition, fractured radial sections gave indications for characteristic ‘droplet’ material lying in an ordered manner across the aggregates (Fig. 2e, arrows). The chemical composition of the droplet materials is currently unknown, but is thought to reflect remaining hemicelluloses or lignin associated with the aggregates. Their location and spatial distribution strongly indicated a natural occurrence rather than any type of artefact produced during preparation. In contrast, TEM sections through preferential degraded cell walls gave some indications for a concentric orientation of the wood components but no indication for aggregates as seen with cryo-fractures (Fig. 2f).
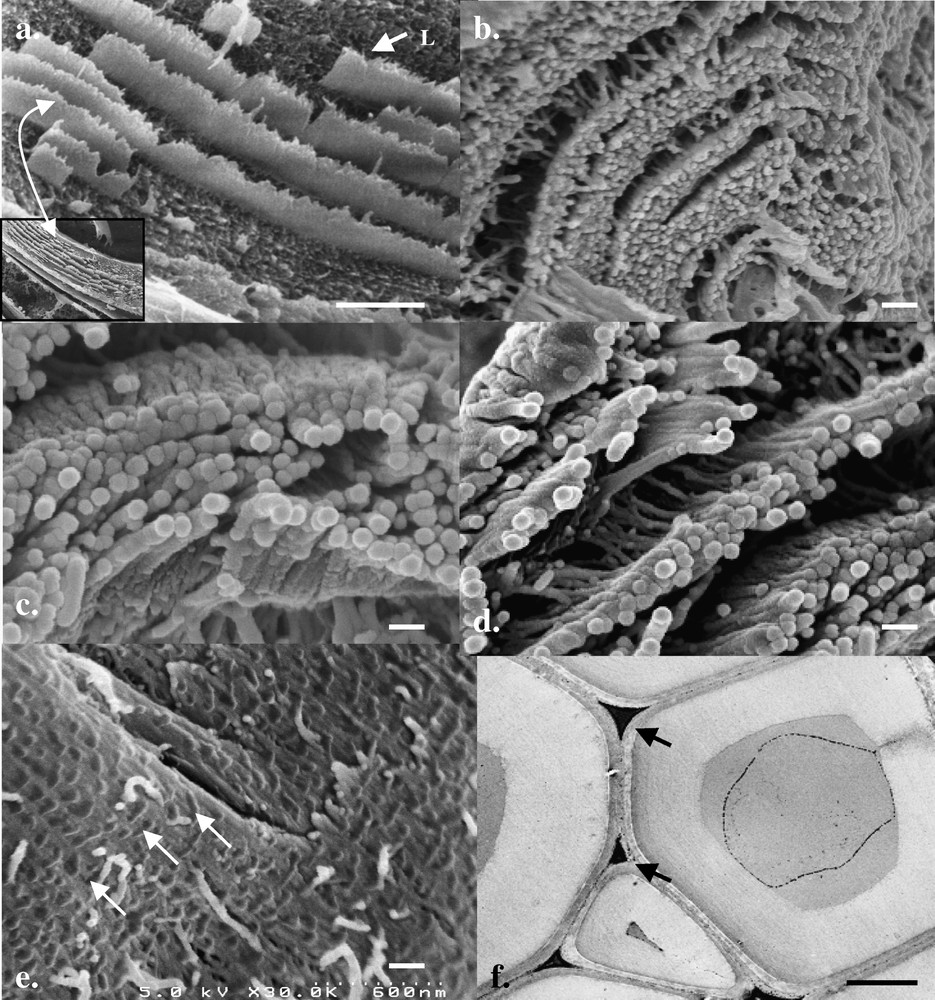
HR-Cryo-FE-SEM and TEM micrographs of birch fibres degraded by P. radiata Cel 26. (a–d) Fractured degraded (i.e. delignified) birch fibres show the S2 wall composed of concentrically orientated lamellae which at high magnification are composed of characteristic spaghetti-like aggregates of fairly uniform size and morphology. (e) Fractured sections through fibre walls gave indications for the presence of radial orientated droplet-like structures aligned across the aggregates (arrows). (f) TEM micrographs of highly degraded birch fibres (only middle lamella cell corners remain, arrows) gave weak indications for concentric alignment of the wall structure but no indication for the presence of aggregates. Masquer
HR-Cryo-FE-SEM and TEM micrographs of birch fibres degraded by P. radiata Cel 26. (a–d) Fractured degraded (i.e. delignified) birch fibres show the S2 wall composed of concentrically orientated lamellae which at high magnification are composed of characteristic spaghetti-like ... Lire la suite
Using chemical fixation and conventional SEM preparation techniques (i.e. fixation, dehydration, critical point drying) shrinkage and coagulation of the slime resulted in the matrix lying closely adpressed to the lumen cell wall and no indication for a temporal distribution was noted (not shown). TEM observations of P. radiata wild degraded wood also showed the extracellular slime material to be arranged in a characteristic concentric fashion and in highly degraded wood fibres, the only remaining wood material was located in middle lamellae and middle lamellae cell corner regions, the slime matrix completely filling the cell lumina and engulfing the remaining wood cell wall (e.g., Fig. 3a and b). TEM also revealed that the slime material was fibrillar in form and that the concentric bands were also interconnected by slime material (Fig. 3a and b). Detailed observations showed the slime material in the thin concentric bands to have a tripartite-type structure (Fig. 3c, inset) similar to that produced by other fungi (e.g., both brown and white rots) during decay of wood [10,11]. Cryo-SEM further revealed the presence of numerous calcium oxalate crystals and deposits of manganese (confirmed by TEM energy dispersive X-ray microanalysis) in the cell lumina that were also embedded in the extracellular slime matrix.
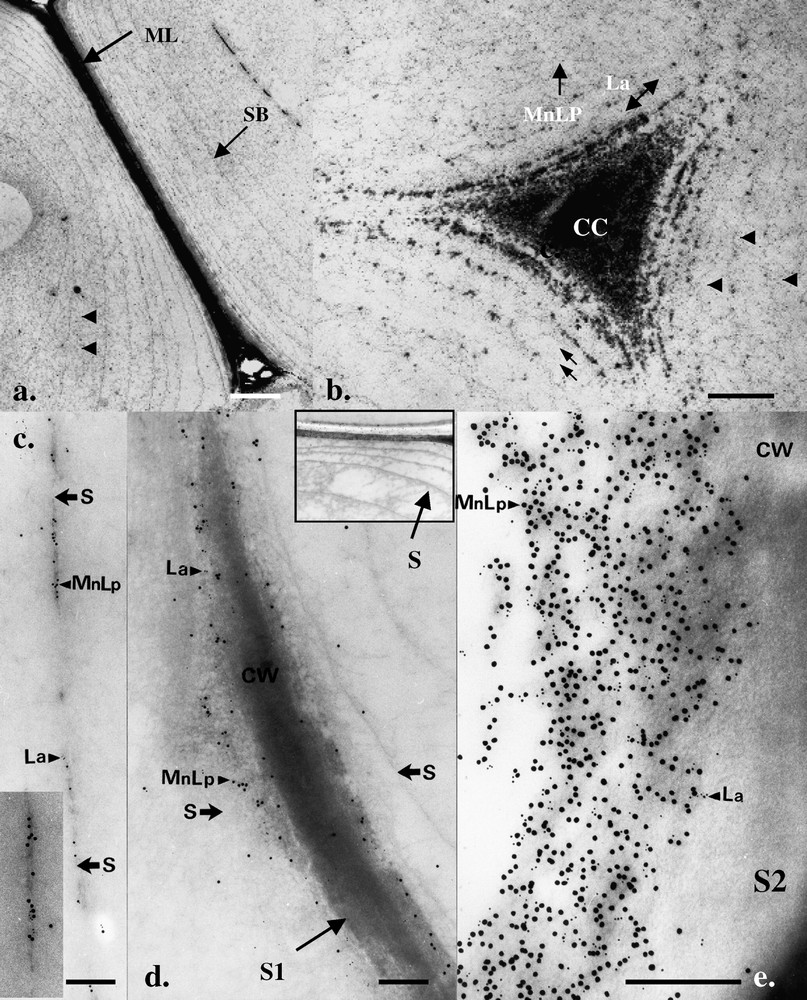
TEM micrographs showing morphological aspects of birch cell wall decay by P. radiata wild and localization of laccase and MnP/LiP. (a, b) Advance fibre cell wall attack with only parts of the S1 and middle lamella region (ML) and ML cell corners (CC) remaining. Extracellular slime is arranged in concentric bands (SB) in the region of the previous S2 wall layer and is apparent between both the bands (arrowheads) and between the remaining delaminated S1 layer surrounding the ML cell corner (double arrows). (c–e) Double labelling with laccase (La, 5 nm Au) and MnP/LiP (15 nm Au) antibodies showed evidence for the enzymes associated with the tripartite slime bands (c) and the outer regions of the degraded fibre S2 and S1 cell walls (arrows). For orientation, an inset in (d) shows a low-magnification micrograph of the slime bands from a region similar to (d). Masquer
TEM micrographs showing morphological aspects of birch cell wall decay by P. radiata wild and localization of laccase and MnP/LiP. (a, b) Advance fibre cell wall attack with only parts of the S1 and middle lamella region (ML) and ... Lire la suite
3.2 Immunolocalization of oxidative enzymes in degraded wood
Using immunogold cytochemistry and specific antibodies, the spatial distribution of the oxidative enzymes was determined. Observations revealed the association of the three enzymes (laccase, MnP/LiP, LiP) with the degraded birch wood cell-wall regions in P. radiata wild, as well as the extracellular slime material (Fig. 3c–e). Consistent with the known molecular size of the enzymes (42–64 kDa) and limited porosity of the undegraded wood cell walls, the ligninolytic enzymes were restricted to degraded wood cell wall regions (Fig. 3e) and did not penetrate into the unmodified (i.e. ‘unopened’) wood structure. Some differences were noted in the labelling pattern of the two fungi. Strong labelling of regions of active decay was shown for P. radiata wild while only weak labelling of highly degraded walls was noted for P. radiata Cel 26. TEM also revealed a periplasmic distribution (frequently with periplasmic vesicles) of the enzymes within the luminal hyphae from where these glycoproteins were presumably secreted into the cell lumina in close proximity of the wood cell wall, or possibly conveyed by the slime material onto the wood cell wall (Fig. 4a–f). The enzymes were also localized on the tripartite membranes within the cell lumen (Fig. 3c). Both laccase, MnP/LiP and LiP (not shown) were found localized in highly degraded middle lamellae (Fig. 3d) and middle lamellae cell corner regions distant from the original lumen hyphae indicating some type of transport or diffusion mechanism in operation. Labelling results were more intense for non-osmicated (fixative B) samples but the spatial distribution was similar with osmicated samples. All controls were negative.

TEM micrographs of transverse sections through P. radiata wild (a–c, f) and mutant (d, e) lumenal hyphae and the presence of laccase (La) and MnP/LiP in the periplasmic space, fungal cell wall and extracellular slime material. (a) Periplasmic vesicles (pv), and (b) Au labelling of vesicles using laccase (La) antiserum. (c) Labelling of MnP/LiP in periplasmic vesicles. (d, e) Double labelling of La (5 nm) and MnP/LiP (15 nm) and single labelling (e) of MnP/LiP in the periplasmic space and hyphal wall. (f) Single labelling of the outer hyphal cytoplasm showing presence of LiP. Masquer
TEM micrographs of transverse sections through P. radiata wild (a–c, f) and mutant (d, e) lumenal hyphae and the presence of laccase (La) and MnP/LiP in the periplasmic space, fungal cell wall and extracellular slime material. (a) Periplasmic ... Lire la suite
3.3 The importance of extracellular slime materials during wood cell wall degradation
The present observations show evidence for the involvement of extracellular slime materials during the degradation of wood fibres by P. radiata, particularly the wild type. Both HR-Cryo-FE-SEM with the rapid freezing of samples and TEM using conventional fixation and processing techniques showed evidence for the presence of slime materials during cell wall decay, while the immunocytochemical approach further showed the association of the enzymes with extracellular slime. The fact that the slime has a high molecular weight with substantial polysaccharide composition is consistent with its retention during the rapid freezing and deep-etching process where it presumably behaves as a cryo-protectant. The association of extracellular slime materials with fungal hyphae during wood decay has been known for many years and it has been variously implicated in wood decay processes [2,10–16]. Previous immuno- and cytochemical studies with the white-rot fungi Phanerochaete chrysosporium and Trametes versicolor [10] has shown the association of LiP and the H2O2 producing enzyme pyranose 2-oxidase [14] with extracellular tripartite membranes and slime lining the fibre lumen wall of partially degraded fibres [10,12] while laccase has also been shown localized in extracellular slime associated with Rigidoporus lignosus and Lentinus edodes hyphae [16–18]. More recent work has also shown enzymes associated with tripartite membranes in brown rot fungi (Daniel & Volc, unpublished). Previous NMR studies have also shown the extracellular slime of P. chrysosporium as a β-1,3–1,6-d-glucan [15,19], a polymer similar to that found in fungal cell walls.
To explain the decay features of P. radiata wild, the following working hypothesis is presented:
- (i) the extracellular slime is produced progressively by lumina hyphae and conveys the enzymes (in this case oxidoreductases) involved in the decay process onto the fibre wall (initial stages involving coating of the lumen wall);
- (ii) slime material accumulates on the wall and penetrates into the fibre cell wall following the native fibre architecture causing progressive decay across the wall; with simultaneous decay, cell wall thinning occurs, and with preferential attack selected decay of lignin and hemicelluloses occurs in the wall;
- (iii) enzymes associated with the slime carry out local decay of the fibre wall components causing both delamination of lamellae and aggregate mineralisation;
- (iv) other components associated with the slime (e.g., Mn(II) or oxalate) possibly provide a means for initial non-enzymatic attack and ‘opening’ of the fibre wall for enzymatic attack.
Such a process would be advantageous in that the wall degrading enzymes may be progressively transported and retained at sites of fibre wall attack thus overcoming the known problems for the penetration of high molecular weight proteins (such as enzymes) into the unmodified wood cell wall. In addition, as the fibre wall becomes more distant to the lumenal hyphae in advanced stages of decay (i.e. during middle lamella decay), the slime will provide the medium for retaining contact between the fungus and the fibre wall. Slime has several other advantages in that it can also retain moisture in the substrate and it can also act as a storage material representing a by-product of lignocellulose mineralisation [20].
Previous studies have also implicated slime as a major component of wood decay systems for a range of other wood decay fungi [10–18,20]. However, a perplexing problem in this study is why strong labelling of the enzymes has not so far been recognized in preferentially degraded fibre cell walls. Possibly this reflects a more highly developed non-enzymatic mechanism directed from lumen hyphae by preferential white-rot fungi like P. radiata Cel 26. However, it is also possible that the TEM approach with inherent resin embedding of samples is not the optimal method for showing this process. Interestingly our, cryo-fixed and FE-SEM observations show the wall of P. radiata Cel 26 preferentially degraded birch fibres to have a macrofibrillar aggregate structure, a feature which is consistent with the morphological cell wall structure of delignified fibres in which the cellulose is retained and most of the other components have been removed [21,22]. However this is not evident from TEM observations of the wall structure from delignified P. radiata Cel 26 fibres (Fig. 2f). Additional problems include shrinkage of the fibre wall material during resin embedding and polymerisation, and possibly inherent problems with steric hindrance during immunolabelling studies. It is noteworthy that cellulose within the fibre wall is considered to be arranged in aggregates in a concentric or possibly concentric-radial arrangements (see [2] for references) a feature that is reflected by the present cryo-observations. Lignin, which is expected to complement the cellulose organization of the fibre wall, has been shown elegantly by Ruel an co-workers [23] using permanganate staining to have a concentric orientation using scanning-transmission techniques (STEM) at very high magnifications, but the same pattern has not so far been recognized using specific lignin antibodies in TEM studies, an aspect which may also reflect the problems with the TEM technique. An aspect that also needs further study is to distinguish between the slime material actively secreted by the fungus (presumably the tripartite membranes) and that produced or transformed from the wood polymers during decay. Slime is produced by decay fungi in situations of low nitrogen and high glucose, and represents a way by the fungus for controlling nutritional conditions and the local microenvironment [20]. In this case, the enzymes responsible for glucan synthesis/hydrolysis are also high molecular weight proteins and should also be located at sites of fibre wall decay.
4 Conclusions
HR-Cryo-FE-SEM has been used to reveal new details on the manner birch cell walls are degraded by white-rot fungi – in this case P. radiata wild and its cellulase less mutant P. radiata Cel 26. Novel features on the architecture of the partially degraded fibre wall were shown, in particular its construction of concentric orientated lamellae with aggregate structure. The lamellae and aggregates were degraded progressively from the cell lumen by the wild-type fungus while with the cellulase-less mutant, materials (lignin, hemicelluloses) between the aggregates were removed. Abundant extracellular slime provided close contact between luminal hyphae and the degrading cell walls at all stages of attack. A periplasmic distribution of laccase, LiP and MnP were found in hyphae and the enzymes were extracellularly associated with tripartite membranes, slime and the attacked fibre walls in P. radiata wild suggesting all enzymes were involved in the decay process. It is hypothesized that slime plays a major role in the decay process by providing a medium for conveying enzymes to sites of cell wall attack.
Acknowledgments
This work was supported by grants from the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), by the Czech Republic Grant, AV0Z5020903 and by exchanges financed by the Czech and Swedish Academies of Sciences.



Vous devez vous connecter pour continuer.
S'authentifier