1 Introduction
Madagascar is one of the most important countries for biodiversity conservation [1]. This country hosts a great diversity of habitats, and the tropical dry forest, which lies on the western coast of this island, is one of the most threatened forest types of the world [2–4]. The remaining primary vegetation of the dry forest in Madagascar is estimated at 18 900 km2, from which only 4167 km2 (22.1%) are protected [5].
The western dry forest in Madagascar is mainly cleared for agriculture [6], but logging contributes also to the degradation of this ecosystem [7,8]. These practices modify vegetation structure, thereby affecting also microclimate, food resources and foraging opportunities of the animals, especially understorey insectivorous forest birds, which are often considered as sensible to forest disturbance [9–17]. Habitat structure will determine the abundance and distribution of preys for this guild [18,19] and food resources will be the main primary determinant of habitat selection for the insectivorous birds [20,21].
Although there is a strong correlation between bird distribution and vegetation structure [20], the mechanisms and criteria used by a bird to select habitat are little known [22]. Description of habitat structure is scale dependent, and there is a hierarchy of spatial scales at which birds can respond [22,23]. The appropriate scale depends on the goal of the study. To determine how the individuals select their foraging sites and to which aspects of habitat structure they respond, a small-scale (or microhabitat scale) study design is required [21,24]. One can derive information about these processes by comparing the microhabitat sites used by a species in two habitats differing by their structure [19]. Human alteration modifies the habitat structure and has an impact on foraging behaviour and habitat selection. Comparison made in one disturbed and in one undisturbed habitat allows understanding if and how habitat alteration affects a bird species [21].
It is necessary to understand the actual determinants of the microhabitat use, because they can have important applications in conservation biology, if the aim is to assess the consequences of different types of land management and maintain (or maximise) within-habitat diversity. The distribution and abundance of a species can then be described by the availability of its favourite microhabitats [25], and the populations can be managed more efficiently if we know which microhabitat variables are the most important for this species [26].
This paper exposes an analysis of microhabitat use by two insectivorous, terrestrial couas species. These birds are endemic from the dry forest in Madagascar. The aim was to study how the effects of selective logging modified the use of the microhabitat variables into the gallery forest, which lies near the rivers or the ponds. By comparing selection of microhabitat variables in relatively undisturbed and disturbed habitats, I tried to learn which aspects of the vegetation structure are important for the couas, and how disturbance regimes may affect their habitat selection at small scale.
2 Material and methods
2.1 Study site
All the observations were performed during the rainy season in 1999 in the concession of the ‘Centre de formation professionnelle en foresterie’ (CFPF) of Morondava, Madagascar (Fig. 1).
Map of Madagascar showing the situation of the CFPF where the field study was performed.
This station was located in the Menabe region, 60 km north of Morondava, in the Kirindy forest. Rainfall varied from 300 to 1400 mm per year, with a mean of 800 mm [27]. The rainy season extends from January to March. Temperature variability may be very large: mean daily maximum are around 36 °C and the minimum around 19 °C [28]. The Kirindy River crosses the station, and influenced the vegetation structure [29,30]. The gallery forest near the river was tallest, with more evergreen trees, and reached a 25-m height. The forest far from the river tended to be a more deciduous dry forest, with a lower canopy, and a denser understorey. To the west, the forest became gradually lower and more open, as the bush of the southwestern Madagascar [31].
This forest was logged from 1978 to 1991 [32]. Logging was selective, with logging of tree with a diameter > 37 cm, and less than 10 m3/ha were extracted [33]. In addition, the CFPF provided a lot of attention to restore the forest after exploitation [6,34–37].
The station was originally covered by an undisturbed deciduous forest, but this forest has been modified mainly by logging, which led to a vegetation in a mosaic of patches of different degrees of disturbance. Vegetation had thus to be considered as a mosaic of logged/unlogged forests (Fig. 2).
Map of the Kirindy forest (CFPF forest concession). The different forest plots were indicated by letters. The field station was located near the “piste Conoco”. See text for the state (logged or unlogged) of the different forest plots.
2.2 Type of disturbance
Selective logging occurred in Kirindy and it has been shown to have an effect on forest structure [7]. Behaviour of animals in this forest was also affected [7,38], particularly forest birds [39,40].
The main and most common effect of logging on the vegetation structure was an increase of the understorey vegetation density, a lower and more open canopy [39], but natural variation (e.g., distance from a river) could also modify the vegetation structure and alter the number and quality of the microhabitats [7,38].
Measures were performed into the gallery forest, each time in well-defined habitat, by using position of the logged parcels provided by the CFPF [32]. In addition, I searched on the field for indications of logging to confirm the habitat identification (logged/unlogged) of the studied sites.
I tried to study the logging effects by working in the parcel CS7 (the gallery forest parcel close to the camp and left unlogged according to the CFPF annual reports), and the contiguous one CS6, logged in 1980 (Fig. 2). Closeness of the two parcels enhanced the probability of similar vegetation structure, which could be compared as before-and-after control. At first sight, parcel CS6 did not appear logged, because vegetation structure was very similar to the unlogged CS7. However, I found in this parcel some evidence of logging (e.g., stumps). Understorey vegetation structure of the parcel CS5 (logged in 1978) appeared at first sight more disturbed than the parcel CS7, and it was tempting to use this parcel in the study, in order to have extremes of the continuum from unlogged to logged gallery forest, but the distance between CS5 and CS7 prevented from using them as before-and-after control.
2.3 Species
The couas are bird species endemic to Madagascar. Their diet is mainly based on arthropods, but fruits are also recorded [41]. Evolutive radiation among this long isolated taxonomic group has facilitated the diversification and coexistence among these similar species [42]. I studied the two species of terrestrial couas species living in kirindy: the Coquerel's coua (Coua coquereli) and the giant coua (Coua gigas). These birds are medium-size, but the giant coua is twice as long as Coquerel's coua [41]. The giant coua appeared more abundant in the gallery forest with high, closed canopy. The Coquerel's coua was more common in the logged gallery forest than in the unlogged gallery forest, and was also present in the bush, where the giant coua was absent [43].
2.4 Microhabitat variables
I measured some microhabitat variables adapted from Ganzhorn et al. [7] and Hawkins [44], at the feeding sites of each coua species in the two plots studied. I realised the measures during the rainy season in 1998 and 1999. These variables were chosen so that they reflect the habitat features and to be easily measured. They described the immediate environment of any feeding site [45] and they are summarized in Annexe A. Microhabitats were centred on sites where preys were captured on the ground. I used only sites where I observed the birds foraging successfully.
For each coua species, I measured microhabitat variables in 50 feeding sites in the gallery forest (25 in logged and 25 in unlogged forest). For each feeding site, I measured also the same variables in a control site, randomly chosen, at 30 m from the feeding site.
Stems were distributed into two classifications: stems under or above 1-m height (referred to as ‘distribution of the stems’); and dead or live stems (referred as ‘nature of the stems’).
I subdivided the microhabitat variables used in my study into ‘broad-scale’ (including tree size, tree dispersion and canopy characteristics), and ‘fine-scale’ variables (including other variables). Broad scale was similar to that often used to describe avian habitats. These two scales could be considered together as microhabitat scale, but this classification allowed analysing the foraging sites in a hierarchically nested scale, as defined by VanderWerf [21].
To improve the precision of my measurements, I made some preliminary observations until I felt proficient. I made all the observations myself in order to avoid inter-observer variation.
2.5 Analysis
In a first step, I compared an undisturbed area and a disturbed one of the gallery forest, to analyse how logging modified microhabitat availability. Because of the possible selectivity of the couas for foraging sites, measurements can include data not exclusively relevant to the management studied. This is the reason why, to study microhabitat structure between habitat and within habitat, I did not incorporate in my pool of data microhabitat measurements of sites used by the couas. I preferred to keep to the data obtained only at the control sites randomly chosen to compare vegetation between habitats.
In a second step, I compared used sites and control sites in the two plots (logged and unlogged) of gallery forest, for each coua species, to determine which variables the couas may use as structure criteria.
In a third step, I compared the sites used by each coua species in logged and unlogged habitats to determine if the couas altered their selection criteria in disturbed habitat and if they might be limited by logging.
I used two non-parametric tests: a Kruskal–Wallis test and a ranked multiple analysis of variance ANOVA (also called Kruskal–Wallis analysis of rank) when several of the variables were related [46]. I used non-parametric tests, because of the little numbers of measures realised in the different habitats, and because a preliminary analysis of their distribution showed they were not normally distributed, and hence did not allow using parametric tests. However, I indicated the standard deviation to indicate the range of variation of the obtained values.
3 Results
3.1 Effects of the environmental variables on the microhabitat structure
Logging modified the microhabitat structure. Seven variables differed significantly between disturbed and undisturbed gallery forest (Table 1). At broad scale, logging had no effect on tree size and dispersion, but canopy cover was greater in the unlogged forest (Table 1). At fine scale, logging increased the number of live stems and the number of stems under 1-m height (MANOVA, overall , for nature of the stems; , for the distribution of the stems). More lianas were encountered in the logged forest plot. Litter structure was also modified. Proportion of bare soil and quantity of stems on the ground were more important in the logged forest. Proportion of small dead leaves () was more important on the ground in the unlogged habitat, but more medium-sized and big dead leaves were found on the litter of the logged forest.
Effects of selective logging on the microhabitat structure in the gallery forest in Kirindy
| Unlogged forest | Logged forest | ||
| Broad scale | |||
| Understorey tree size (m) | 6.69 ± 0.88 | 6.91 ± 0.83 | |
| Understorey tree dispersion (m) | 2.74 ± 0.65 | 3.01 ± 0.74 | |
| Overstorey tree size (m) | 20.07 ± 7.90 | 21.55 ± 7.51 | |
| Overstorey tree dispersion (m) | 4.14 ± 1.80 | 3.97 ± 1.08 | |
| Ht of canopy (m) | 19.06 ± 4.24 | 18.41 ± 4.21 | |
| Canopy cover (%) | 84.4 ± 16.1 | 84.0 ± 8.66 | ** |
| Fine scale | |||
| Number of live stems (/2 m2) | 6.04 ± 4.72 | 6.96 ± 5.19 | * |
| Number of dead stems (/2 m2) | 2.00 ± 3.85 | 1.72 ± 1.86 | |
| Number of stems under 1-m height (/2 m2) | 5.76 ± 3.99 | 6.68 ± 4.36 | * |
| Number of stems above 1-m height (/2 m2) | 2.28 ± 2.35 | 2.00 ± 2.22 | |
| Depth litter (cm) | 2.00 ± 0.53 | 2.13 ± 0.68 | |
| % of SDL | 94.40 ± 6.51 | 84.0 ± 20.21 | * |
| % of MDL | 5.60 ± 6.50 | 10.80 ± 12.56 | |
| % of BDL | 0 | 5.20 ± 1.58 | |
| % of bare soil | 4.00 ± 10.40 | 10.00 ± 14.72 | * |
| % of stem cover on the ground | 21.6 ± 14.05 | 26.0 ± 19.6 | * |
| Number of lianas (/4 m2) | 0.44 ± 0.51 | 0.60 ± 0.76 | ** |
** ;
* .
3.2 Microhabitat variables used by the different coua species
In the unlogged gallery forest, the Coquerel's coua foraged more often in the microhabitats with greater overstorey tree size and smaller overstorey tree dispersion than control sites (Table 2, see also Table 1 and Fig. 3). In addition, canopy cover and canopy height of the foraging sites used by this species were smaller compared to the control sites (Table 2). There were more stems (live and dead; under or above 1-m height) around the foraging site compared to the control sites (MANOVA, overall , for nature of the stems; , for the distribution of the stems). The litter used to forage had a greater proportion of medium-size dead leaves and a greater proportion of bare soil around the feeding site (Table 2).
Comparison of the microhabitats used by the two couas and the microhabitats randomly chosen, in logged and unlogged forest. See Table 1 for statistical analysis
| Microhabitat variables | Coua coquereli | Coua gigas | ||
| unlogged | logged | unlogged | logged | |
| Broad scale | ||||
| Understorey tree size | 1.33 ns | 1.40 ns | 0.003 ns | 0.018 ns |
| Understorey tree dispersion | 2.15 ns | 2.75 ns | 2.32 ns | 1.20 ns |
| Overstorey tree size | 8.31** | 0.76 ns | 0.26 ns | 0.36 ns |
| Overstorey tree dispersion | 5.56* | 1.36 ns | 0.01 ns | 0.01 ns |
| Canopy height | 17.44*** | 6.23* | 0.20 ns | 6.88** |
| Canopy cover | 14.23*** | 0.29 ns | 6.67** | 1.89 ns |
| Fine scale | ||||
| Number of Live stems | 7.56** | 9.08** | 17.10*** | 6.90* |
| Number of dead stems | 5.20* | 6.56* | 0.43 ns | 0.32 ns |
| Number of stems under 1 m height | 6.22** | 4.70* | 14.16*** | 1.38 ns |
| Number of stems above 1-m height | 26.77*** | 25.89*** | 2.62 ns | 7.18* |
| Depth of the litter | 0.95 ns | 3.24 ns | 0.28 ns | 0.29 ns |
| Small dead leaves on the ground | 11.54*** | 0.27 ns | 3.31 ns | 0.89 ns |
| Medium dead leaves on the ground | 9.98** | 0.01 ns | 3.16 ns | 0.05 ns |
| Proportion of bare soil | 21.82*** | 18.71*** | 1.94 ns | 3.70 ns |
| Stem cover on the ground | 0.11 ns | 2.79 ns | 5.17* | 1.63 ns |
| Number of lianas | 0.52 ns | 14.07*** | 6.22* | 0.22 ns |
* ;
** ;
*** .
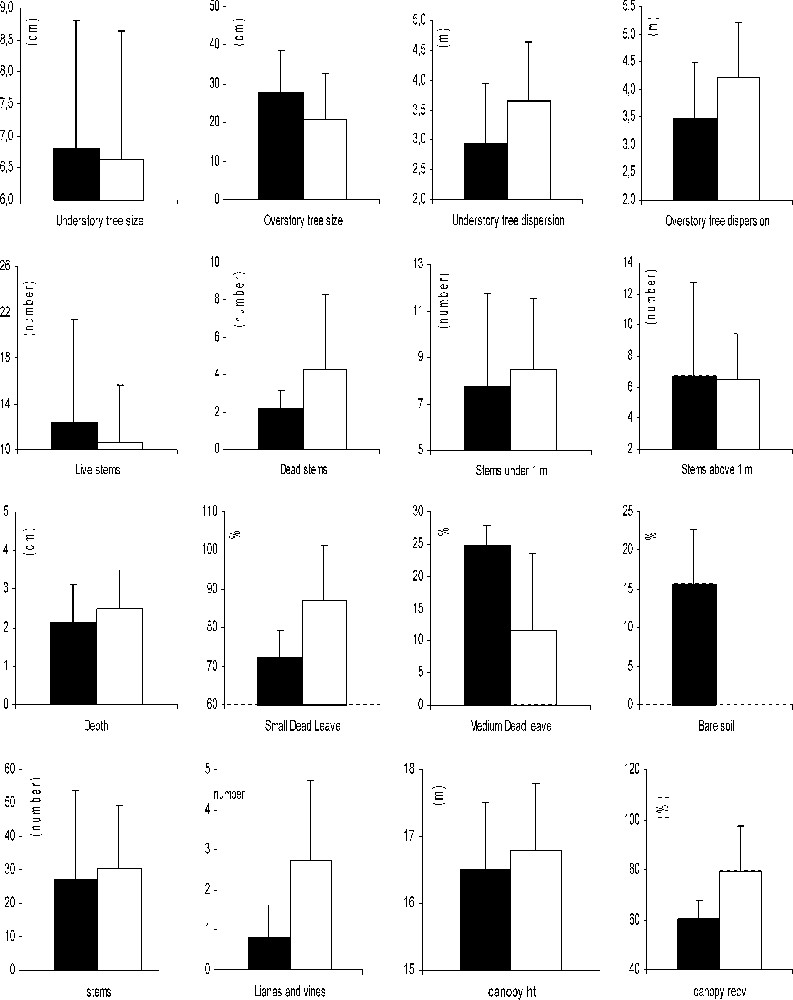
Comparisons of the values of different microhabitat variables used by the Coquerel's Coua in unlogged gallery forest (black) and in logged gallery forest (white) in Kirindy. Standard deviations are indicated in each habitat.
In the foraging sites used by the Coquerel's coua in logged gallery forest, broad-scale variables differed only for the canopy height, which was smaller than that of the control sites (Table 2). At fine scale, this species foraged in the sites with more stems than control sites (MANOVA, overall , for the nature of the stems; , for the distribution of the stems). The foraging sites had a smaller proportion of bare soil. Lianas were also more abundant in the microhabitats, where this species foraged (Table 2).
In the unlogged gallery forest, the giant coua used microhabitats, with no difference on the broad-scale variables, except for the canopy cover, which was smaller than that of the control sites (Table 2, see also Table 1 and Fig. 4). At fine scale, this species used sites with few live stems compared to the control sites (MANOVA, overall , for the nature of the stems) and also with few stems under 1-m height compared to the control sites (MANOVA, overall , for the distribution of the stems). The foraging sites were also characterised by few stems on the ground and by few lianas compared to the control sites (Table 2).
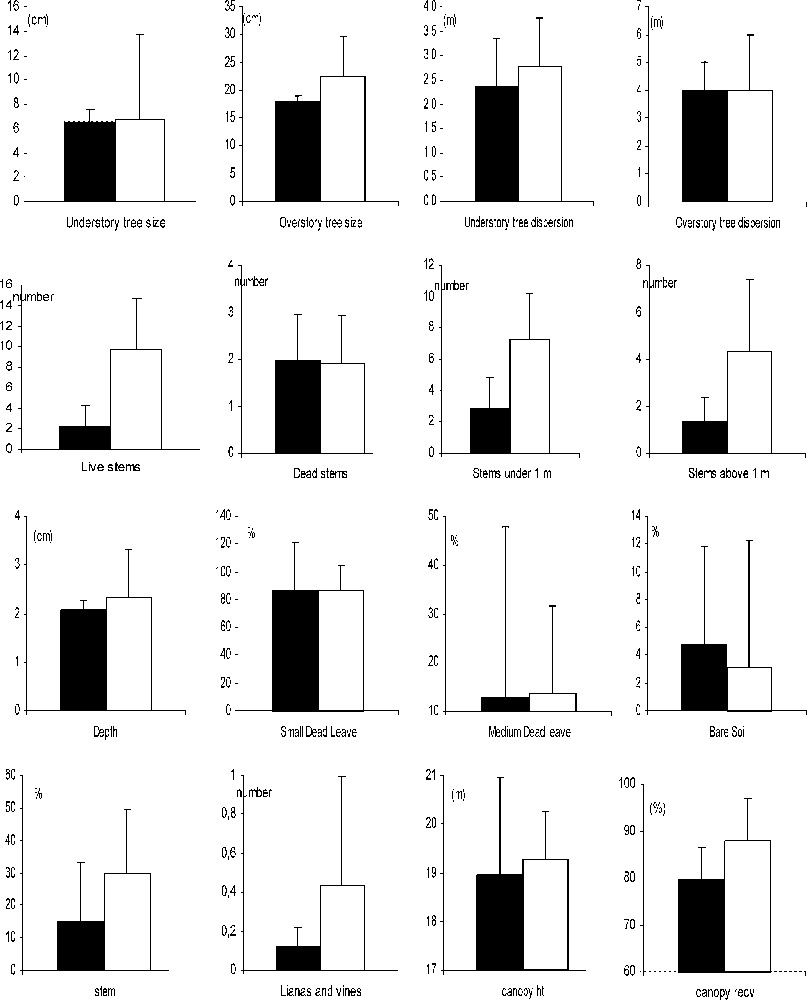
Comparisons of the values of different microhabitat variables used by the Giant Coua in unlogged gallery forest (black) and in logged gallery forest (white) in Kirindy. Standard deviations are indicated in each habitat.
In the logged gallery forest, the foraging sites used by the giant coua were also characterised by a higher canopy than that of the controls sites (Table 2). The other broad-scale variables did not differ between feeding sites and the control sites. At fine scale, the giant coua foraged at sites with more live stems than the control sites (MANOVA, overall , for the nature of the stems) and with more stems above 1-m height than the controls sites (MANOVA, overall , for the distribution of the stems).
3.3 Comparison of habitat
The Coquerel's coua selected feeding sites with greater overstorey tree dispersion, but smaller overstorey tree size in the logged forest compared to the unlogged forest (Table 3 and Fig. 3). In both unlogged and logged forest, feeding sites did not differ for the nature and for the repartition of the stems (MANOVA, overall , for the nature of the stems; , for the distribution of the stems). The Coquerel's coua did not use foraging sites with bare soil in the logged forest. The foraging sites used by this species in the two habitats differed also by the canopy cover, which was greater in the logged forest (Fig. 3). At last, the Coquerel's Coua used foraging sites with more lianas around compared to the sites used in the unlogged forest (Fig. 3).
Comparison of the microhabitats used by each coua species in the disturbed and the undisturbed gallery forest. See Table 1 for statistical analysis
| Microhabitat variables | Coua coquereli | Coua gigas |
| Broad scale | ||
| Understorey tree size | 4.00 ns | 1.50 ns |
| Understorey tree dispersion | 2.09 ns | 2.59 ns |
| Overstorey tree size | 8.88** | 3.80 ns |
| Overstorey tree dispersion | 10.20** | 0.10 ns |
| Canopy height | 1.23 ns | 1.14 ns |
| Canopy cover | 7.99** | 8.38* |
| Fine scale | ||
| Number of Live stems | 0.31 ns | 44.71*** |
| Number of dead stems | 2.89 ns | 0.62 ns |
| Number of stems under 1-m height | 0.38 ns | 30.71*** |
| Number of stems above 1-m height | 0.01 ns | 12.59*** |
| Depth of the litter | 1.18 ns | 1.12 ns |
| Small dead leaves on the ground | 3.46 ns | 0.18 ns |
| Medium dead leaves on the ground | 2.95 ns | 0.13 ns |
| Proportion of bare soil | Not calculated | 0.90 ns |
| Stem cover on the ground | 1.03 ns | 8.78** |
| Number of lianas | 11.36*** | 4.14* |
* ;
** ;
*** .
Giant coua used microhabitats in the logged forest with a greater canopy cover (Table 3 and Fig. 4). At fine scale, the foraging sites used in the logged forest differed by a greater density of live stems (MANOVA, overall , ) and a greater density of stems under 1-m height and above 1-m height (, ). Lianas were also more abundant in the foraging sites of the logged forest.
4 Discussion
4.1 Effect of vegetation structure on the microhabitat
The heterogeneity depends of the scale at which the habitat is studied. Many abiotic factors, as climate and the nature of the soil [29,47,48], could explain the differences between the two habitats (logged and unlogged forests), which influenced the vegetation composition and probably the habitat structure. The dry forest was characterised by a lack of homogeneity at small scale, which can be important in terms of distribution of the food availability in the home range of different individuals of a given species [7,31].
I obtained some results significantly different from those obtained by other habitat structure studies conducted by Hawkins in Kirindy in 1993 [44]. Hawkins did not compare microhabitats used between logged and unlogged forests in Kirindy, because this kind of studies was not based on before-and-after controls. I agree with him about the conclusions drawn by Ganzhorn's study [7] from the study of different parcels far from each other in this forest. However, I tried to study the logging effects by working in two contiguous parcels: the parcel CS7 (the gallery forest parcel close to the camp and left unlogged according to the CFPF annual reports) and the contiguous one CS6 (logged in 1980). At last, I avoided to study microhabitat variables in the forest on shallow calcareous soil described by Hawkins [44] encountered in the parcel CS7 and which was really different from the contiguous gallery forest.
At broad scale, my results indicated little variation for microhabitat variables between the logged and the unlogged areas, except the canopy cover, which was smaller in the logged forest, as described by Hawkins [44]. These results can be explained by the nature of selective logging, which altered vegetation structure only locally, although it is not excluded that I measured microhabitat sites in the remaining unlogged forest, far from the site of logging. Severe logging would have effects at broad scale as modified overstorey tree size, and there is a strong presumption toward an effect on tree dispersion by increasing it [39,44].
At fine scale, my results were similar to those obtained by Hawkins, particularly about the density of stems. It is usually accepted than a disturbed dry forest can recover only after several decades [3,49]. Previous work indicated that density of live stems was increased by logging [50] and it is possible that there is always a positive effect on the growth of the live stems since the time of the logging of parcel CS6. The fact that the size of the dead leaves differed between logged and unlogged habitats could indicate a difference in the flora and the vegetation.
Logging was considered as not disturbing in Kirindy (at least at the human scale), and probably altered vegetation only locally. In this case, there would be no difference (at the microhabitat scale) between sites randomly measured in ‘logged’ and ‘unlogged’ areas. If it was impossible to use a before-and-after control and a follow along many years, a better comparison would be if microhabitat variables were measured at selected places near the logging sites (with evidence as stumps) and compared to sites randomly chosen.
In addition, I realized my measures 19 years after the logging, a time important enough for the vegetation to have grown in the logged sites and masked many differences between the habitat structure and the unlogged forest. I suggest that vegetation dynamic can explain the important difference between our two studies since Hawkins realised his. The natural variability was increased enough during the six years separating our two studies, but more studies incorporating other microhabitat variables, will have to be realised for more conclusions about these differences and to explore better the aspects of habitat selection by terrestrial insectivorous birds, allowing to understand what are the effects of forest management.
4.2 Criteria for habitat selection
In the unlogged gallery forest, at broad scale, the Coquerel's coua selected its foraging sites at broad scale on the base of the overstorey trees size and dispersion, with a canopy cover and a canopy height smaller than in the control sites. These habitat selection factors could be related to aerial-predator escape behaviour. Actually, the Coquerel's couas select habitats wherefrom the raptor's threat can be more easily anticipated, and where these predators have difficulty in flying through. I did not observed a direct attack of raptors against the Coquerel's coua, but I recorded this species' fear when raptors flied around it. In addition, a smaller canopy cover could assure a habitat more luminous, making the preys easier to see and capture.
At fine scale, the two main criteria used by the Coquerel's coua to select its foraging sites were the stem distribution and their nature: this species searched for sites with more live stems and stems under 1-m height. This can be partly explained by the fact this species fed much on caterpillars, which are found mainly on leaves. Caterpillars were interesting prey, because they did not escape quickly, they were easily and rapidly captured, and they provided more energy than small prey. Coquerel's coua was also fond of the sweet secretion produced by the larvae of Phromnia rosea (Flatidae), a heteroptera living only on a vine Elachyptera minimiflora [31]. I saw mainly vines in disturbed places, and logging could increase the density of them too. Probably this particular vine was more abundant in these disturbed places. Shrub density could also contribute to provide a protection against predation during the foraging events for this species.
Another criterion used to forage was the litter structure: the Coquerel's coua forage on the ground with a great proportion of medium-sized dead leaves, which can be used by arthropods to be hidden, particularly when the dead leaves are rolled up. I observed often couas foraging by turning down the dead leaves and capturing the prey. Small dead leaves could not be used as much efficiently as larger ones by arthropods to be hidden. In addition, greater proportion of bare soil makes capture of arthropods easier when they try to escape, making them more visible and providing no other place to be dissimulated.
I mapped the territory of the Coquerel's coua and the giant coua in the unlogged gallery forest [51]. The Coquerel's coua was never recorded in a 1-ha area in the unlogged forest, where there was no understorey vegetation, but where the giant coua was often encountered. This area was probably not favourable to foraging, and in addition did not provide a protection against predators for the Coquerel's coua. This species experimented a neophoby to explore this non-suitable habitat.
The giant coua selected foraging sites at broad scale on the base of understorey tree dispersion (greater than the control sites) and canopy cover (smaller than the control sites). As for the Coquerel's coua, small canopy cover could assure an environment more luminous. I did not record any attack of raptors against this species, which did not seem scared whenever when a raptor flew around it.
At fine scale, the giant coua selected its foraging sites with low shrub density, probably because this species, twice bigger than the Coquerel's coua, cannot move easily in dense shrubs.
The density of shrubs appeared as an important criterion to choosing foraging sites. This suggests this criterion could help to prevent the competition for food between the two couas species in the unlogged forest in Kirindy, but a study of the diet of each coua species would be necessary to considerate this hypothesis.
4.3 Scale of habitat selection for the couas
The two coua species selected their foraging sites at the different scales. There is an advantage in this [21]. At fine scale, on the base of information such as shrubs density and/or structure of the litter, couas can chose a location to make a single foraging event. At broad scale, the area chosen presumably provides information about the possibility to forage successfully over a large number of foraging events [21].
In the logged forest, both coua species did not select the foraging sites at broad scale, because the habitat structure is like this searched by the Coquerel's coua.
VanderWerf [21] studied ‘Elepaio’ (Chasiempis sandwichensis) and investigated an intermediate scale I did not realise in my study. He found no habitat selection at broad scale, but ‘Elepaio’ is a small flying bird. Couas, because of their large size and ability to cover rapidly their territory, can use this scale to get more foraging opportunities in a short time.
4.4 Comparison of habitat selection between habitats
Comparison of patterns of selection between unlogged and logged forests allowed determining if the couas are restricted in their use of disturbed areas. In the unlogged forest, Coquerel's coua was selective at broad scale for overstorey tree size smaller and overstorey tree dispersion greater. These sites appeared uncommon in the unlogged forest. Logging increased their availability and consequently the Coquerel's coua no longer selected the foraging sites based on broad-scale selection in this logged habitat. However, these increases in the availability due to logging did not appear in my results comparing the habitat structure between unlogged and logged forest. This could indicate that the Coquerel's coua was more selective towards the same foraging sites in the unlogged forest, resulting in an apparent increase in their use.
At fine scale, the Coquerel's coua selected foraging sites with more stems around. These sites were uncommon in the unlogged forest, but their availability increased after logging, making an easier selection for this species. In addition, the Coquerel's coua took advantage of the increase of lianas to capture more preys, as Flatidae. As the size of dead leaves varied between habitats, the Coquerel's coua no longer used this variable to select its foraging sites.
At broad scale, the giant coua selected for great canopy cover, which was the most restricting variable, particularly in logged forest. At fine scale, this species selected foraging sites with few stems around and few lianas. In logged forest, this species would avoid the unfavourable foraging sites with more stems around. However, it used these foraging sites. This suggests that they could use them to capture preys different from those found in the unlogged forest. Live stems could harbour a greater supply of large prey, as caterpillars, which were abundant in some places. In logged area, at the scale of the birds, there are few microhabitats to forage efficiently, because of the increase of shrub density, increasing also the difficulty to move in this habitat. However, the high density of live stems compensated these difficulties, because the giant coua could find more preys on them. This hypothesis could be true, because I followed an individual in the logged area, which came back several times at the same place (probably because of the limitation of favourable microhabitats to forage efficiently), to capture each time a great quantity of caterpillars. Others large preys for this species, like chameleons and orthopteras, could also be found more often at these places, because these preys are often encountered on shrubs near the ground.
4.5 Implications for conservation
Different closely related species can respond differently to forest management and this result can be used in a conservation perspective. The Coquerel's coua could be favoured by the increase of optimal foraging sites by logging. Giant coua is sensitive to selective logging, even if it was supposed to have no important effect on vegetation structure. This practice reduces the availability of favourable foraging sites for the giant coua. Even if the plasticity of this species allowed it to be adapted to the new environment by exploiting the new foraging sites, the limitation can be manifested by larger territory sizes or lower population density [21].
In a previous work, I studied the density of each species in the gallery forest in Kirindy [43]. My results were compatible with this hypothesis. The Coquerel's coua was nearly twice more abundant in the logged gallery forest (24.2 ind/km2 vs 13.3 ind/km2 in the unlogged forest), but the giant coua was less abundant in the logged forest (3.7 ind/km2 vs 5.6 ind/km2 in the unlogged forest).
Hawkins [39,52] studied the microhabitat used by the endangered white-breasted mesite Mesitornis variegata [53]. He found that this bird foraged in microhabitat with dense shrubs above 1 m, but shrubs under 1 m, as for the Coquerel's coua, which selected foraging sites with dense shrub layer. Hawkins suggested that these sites would have been preferred because their denser structure facilitated searching and capturing preys, because they harboured more preys, and/or because they provided more cover from predators. This conclusion could be applied to the Coquerel's coua, which uses the same microhabitat than the mesite. In addition, the densities of both species varied in the same way in each habitat [43,44]. This suggests that the Coquerel's coua could be used as an umbrella species to manage the habitat for the white-breasted mesite. The Coquerel's coua is a conspicuous, more often seen bird than the discreet Mesite. In this sense, the Coquerel's coua could be used as the tool for habitat management, because the management of this species (e.g., observations, census) would be easier than for mesite and habitat management for this coua would benefit to the mesite.
5 Conclusion
Foraging site selection in couas is based on a diversity of microhabitat variables (e.g. tree size and dispersion, density of shrubs, structure of the litter), which can be linked to the diversity of techniques and substrates used by these birds to forage (Chouteau, unpublished manuscript). Limitations in the use of microhabitats can also appear in other forms, such as difference in time and energy budgets in disturbed habitat [21].
Other kinds of sites within microhabitats, such as nesting places, or singing sites [24], used in association with the foraging sites, would have given more information about the ecological requirement for the couas. But data about nests were scarce, because of the high rate of nest predation, which prevents identifying the best nesting place where predation was avoided [51].
At last, it would be necessary to study the diet of both species and the foraging techniques they use in each habitat.
Acknowledgments
I thank the ‘Commission tripartite’ of the Malagasy Government, the ‘Ministère pour la Production animale et des eaux et forêts’ for the permission to work in Madagascar. The staff of WWF Madagascar and Steven M. Goodman provided logistic support and very pleasant hospitality in Antananarivo. O. Langrand initiated the study and advised me on the methods. Jean-Marc Thiollay, Christian Erard, Frank Hawkins, and Pablo Inchausti made some comments on the manuscript. I thank the Deutsch Primaten Zentrum of Göttingen, especially Jorg Ganzhorn and Peter Kappeler, for the permission to use their station in the Kirindy forest and to take advantage of the logistic; I thank the ‘Centre de formation professionnelle en foresterie’ in Morondava, for having facilitated my access to the Kirindy concession. I thank all the people from the Kirindy forest and the German students for having facilitated my accommodation during my stay in the forest.
Annexe A Variables used to describe the microhabitat structure
‘Broad-scale’ variables
Overstory tree size:
average diameter (cm) of the four nearest overstorey trees ( DBH) around the site.
Overstory tree dispersion:average distance (m) of the four nearest overstorey trees around the site.
Understory tree size:average diameter (cm) of the four nearest understorey trees (5–10 cm DBH) around thesite.
Understorey tree dispersion:average distance (m) of the four nearest understorey trees around the site.
Canopy height:height (m) of the canopy over the site.
Canopy cover:average measure of the canopy cover (%) at four directions over the site measured with an angle of 60°.
‘Fine-scale’ variables
0L, 0.5L, 1.0L, 1.5L, 2.0L:
number of live woody stems and branches, within a 2-m2 circle around the feeding site at ground level and at 0.5, 1.0, 1.5, and 2.0 m above the ground.
0D, 0.5D, 1.0D, 1.5D, 2.0D:number of dead woody stems and branches, within 2-m2 circle around the feeding site at ground level and at 0.5, 1.0, 1.5, and 2.0 m above the ground.
From these measures, I calculated four new variables:
- – total number of live stems and branches above the ground;
- – total number of dead stems and branches above the ground;
- – total number of stems and branches (dead and live) from the ground to 1.0-m height;
- – total number of stems and branches (dead and live) above 1.0-m height.
mean of four samples of litter layer depth, one in each quarter around feeding site.
Small dead leaves:percentage cover of small dead leaves () on forest floor in a 2-m2 circle around the feeding site.
Medium dead leaves:percentage cover of medium dead leaves (5–10 cm) on the forest floor in a 2-m2 circle around the feeding site.
Large dead leaves:percentage cover of large dead leaves () on the forest floor in a 2-m2 circle around the feeding site.
Bare soil:total percentage of exposed litter in a 2-m2 circle around the feeding site.
Stem cover on the ground:total percentage of dead stems and branches on the forest floor in a 2-m2 circle around the site.
Lianas and vines:number of lianas and vines within 4 m2 around the site, until 2-m height.


