1 Introduction
Fluctuating asymmetry (FA) is defined as small, random deviations from symmetry in bilaterally symmetrical characters [1]. FA is thought to be the outcome of developmental instability, the impressive expression of design due to perturbations during development [2–4]. Early interest in FA has been centred on its potential as an indicator of environmental stress [5–9]. FA has considerable appeal as an indicator, because it is relatively easy to estimate and it can be used to measure the effect of multiple stressors in natural populations [10]. Indeed, Clarke [10] suggested that when an analysis of FA indicates no biological stress, it would be reasonable to assume that the population, and hence the ecosystem, is not being adversely affected. However, this assumption has been criticized [11–13], for example because it is difficult to separate genetic from environmental components in FA [5,9,10,14,15] or because measurement errors can lead to unsupported conclusions [16–18].
In aquatic ecosystems, studies concerning the influence of environmental stress on FA levels are scarce, generally concern aquatic insects [15,19–21], and have widely varying results. Hence, Mpho et al. [20] showed that exposure to an insecticide at varying temperatures induced significant FA in Culex quinquefasciatus (Insect: Diptera), while Hogg et al. [15] could not highlight an increase in FA level in Nemoura trispinosa (Insect: Plecoptera) subjected to temperature increases. Bonada and Williams [21] studied the impact of anthropogenic pollution on certain Ontarian rivers over a 48-year period and found a positive correlation between Hydropsyche morosa (Trichoptera: Hydropsychidae) FA and an increase in conductivity or nitrogen or chloride concentrations. However, only four of the twelve traits measured showed that responses were significantly and positively correlated with increasing incidence of FA. Among the possible causes of such varied results, one hypothesis suggests that the developmental mechanism that establishes symmetry or asymmetry is based on feedback from the left and right sides of symmetrical organs [22,23], but empirical data showing feedback regulation are inconclusive. Three studies measuring FA at successive stages of the same individuals pointed to a decrease in asymmetry over time, and thus suggested possible feedback regulation [23–25], while three other studies did not find similar patterns [26–28]. Concerning insects, Klingenberg and Nijhout [24] demonstrated the existence of different systems regulating the growth of symmetric structures or regions in Precis coenia (Lepidoptera: Nymphalidae) and Servia et al. [25] showed such a mechanism in Chironomus riparius (Diptera: Chironomidae).
The aim of our study was to characterise the evolution of FA during larval development in a natural population of Hydropsyche exocellata (Trichoptera: Hydropsychidae), a species belonging to one of the most cosmopolitan genera of aquatic insects, and to be able to highlight the effect of certain environmental stress [29]. Two main hypotheses to explain our results are discussed and advice about the use of FA to evaluate environmental stress on natural insect populations is given.
2 Material and methods
2.1 Hydropsyche exocellata sampling
We used three different larval instars and adult individuals of a natural population of H. exocellata sampled in 2003 in the Meurthe River, a sixth-order stream tributary of the Moselle River, in northeastern France. The study area is a perennial-flow section, located upstream from the city of Nancy (48°41′52″N, 6°11′14″E). A preliminary analysis based on 500 larvae collected in 2002 was done to determinate the five instars. The length and width of each individual's head capsule were measured to the nearest 0.01 mm using a stereomicroscope fitted with an eyepiece micrometer. The Bhattacharya method [30] was used to separate the length–frequency distribution into cohorts, with each cohort being defined as those individuals of the same larval instar.
To study the FA variation, larvae were collected in June and September 2003 using artificial substrates (six-litre plastic boxes filled with pebbles). H. exocellata is bivoltine (two generations per year) in this part of France [31] with adult emergence occurring from May to late July. According to the biology of the species, 5th-instar larvae were collected in June, while 1st- and 3rd-instar larvae were sampled in September. Adults were collected in July with a Jermy-type light trap installed on the bank. An additional sample was collected using a butterfly net in the lighted area around the trap. All individuals were preserved in 70% ethanol and taken to the laboratory for measurement.
2.2 Measurement of fluctuating asymmetry
To measure FA levels, three pairs of legs were cut off each specimen to the basal end under a stereomicroscope and placed between two microscope slides. Each slide was positioned on a stereomicroscope coupled with a high-resolution CCD analogical video camera (IAC 500 of i2S™). The image was displayed on a computer equipped with Scion Image software (Scion Corporation™) to measure the maximum right (R) and left (L) lengths of the six traits. Because the FA level is generally in the order of a few percent [17], it is essential to have an accurate estimate of the measurement error. In this study, three blind measurements were made to check the accuracy of the procedure.
2.3 Statistical analysis
The statistical analysis was consistent with the procedure initially proposed by Palmer [32] and improved by Palmer and Strobeck [33]. The data were inspected for robustness and statistically significant outliers were rejected before conducting later analyses. Two individuals presenting significant aberrant values for trait size or asymmetry were also rejected.
A first step to investigating FA variations in detail was to test the absence of anti-symmetry and directional symmetry. A Kolmogorov–Smirnov statistic was used to test the normality of the () values, traducing an anti-symmetry. The absence of directional asymmetry was tested with a T-test comparing the mean () to zero. The second step was to test the measurement error. To have any hope of detecting meaningful differences in FA among groups, the between-side differences due to FA must be shown to be significantly greater than the between-side differences due to measurement error (ME). To test this, a two-way ANOVA (sides × individuals) on untransformed replicate measurements for each trait and each site was conducted. ME was estimated using the ME3 descriptor and the measure repeatability with the ME4 descriptor of Palmer and Strobeck (2003), estimated from the previous ANOVA.
FA10b of Palmer and Strobeck (2003) , where the estimated underlying developmental instabilities of a given side of individual i, and and , M=number of replicate measurements per side, from a sides × individual ANOVA on log-transformed replicate measurements and .
Finally, an ANOVA was also performed to verify the heterogeneity of variance of relative FA . Changes in relative FA of each trait were investigated with a post-hoc analysis based on the Tukey's HSD test.
3 Results
A total of 120 larvae and 37 H. exocellata adults (17 males and 20 females) were collected. For each trait, results of the two-way ANOVAs on repeated measurements (sides × individual) confirmed that between-side variations were highly significant and greater than expected, due to measurement error (). Kolmogorov–Smirnov tests on signed () differences showed that trait asymmetries were normally distributed () and T-tests revealed that asymmetry means did not differ significantly from zero (). These analyses clearly showed the absence of both directional asymmetry and anti-symmetry, and the occurrence of FA in each trait for each developmental stage. No relationship was found between FA and trait lengths (Pearson's correlation, Spearman's correlation, ), except for the length of femur 1 in the fifth instar (Spearman's correlation, ) and the tibia in the first instar (Spearman's correlation, ). Femur FAs did not depend on femur size for either groups or traits. Similarly, tibia FAs did not depend on tibia size for either instar or other traits. After applying a sequential Bonferroni correction for multiple tests, no association remained significant.
Descriptor ME3 [33] expresses the average difference between replicate measurements as a percent of average difference between sides. This error variance differed between traits or groups and ranged only from 1.4 to 15.9% (Table 1), which represents a small fraction of the between-side variance. The ME4 descriptor [33] expresses FA variation as a proportion of the total between-side variation. This descriptor is simply a different way of measuring repeatability. The repeatability (ME4) of our results was very high with values from 65.9 to 99.3% (Table 1).
Measurement error (ME3) and repeatability (ME4) calculated from results of the two-way mixed model ANOVAs (sides × individuals) on untransformed repeated measurements for six traits: femur length and tibia length of the first leg (FL1, TL1), the second leg (FL2 and TL2) and the third leg (FL3, TL3) for each group of Hydropsyche exocellata (see [33], for a complete explanation of the statistical approach)
| Index | Group | n | FL1 | TL1 | FL2 | TL2 | FL3 | TL3 |
| ME3 (%) | 1st-instar larvae | 40 | 8.3 | 9.8 | 4.8 | 8.2 | 12 | 7.2 |
| 3rd-instar larvae | 40 | 7.3 | 3.8 | 6.7 | 9.3 | 6.5 | 5.2 | |
| 5th-instar larvae | 40 | 3.0 | 7 | 3.2 | 3.1 | 1.4 | 6.0 | |
| Adults | 37 | 1.7 | 8.6 | 7.3 | 15.9 | 2.2 | 2.1 | |
| ME4 (%) | 1st-instar larvae | 40 | 93.2 | 84.8 | 92.4 | 87.3 | 90.1 | 87.3 |
| 3rd-instar larvae | 40 | 81.5 | 67.3 | 89.3 | 65.9 | 87.7 | 73.0 | |
| 5th-instar larvae | 40 | 98.1 | 92.0 | 97.1 | 95.0 | 98.3 | 94.4 | |
| Adults | 37 | 99.1 | 91.9 | 95.4 | 90.6 | 99.3 | 98.3 |
FA values are presented in Fig. 1; the highest FA was observed in the first-instar larvae and the lowest values in the fifth-instar and the adults. We found a significant difference between the FA values of each group (Table 2, ANOVA, , ). Furthermore, individual FA decreased significantly after the third instar (t test for paired samples, ).
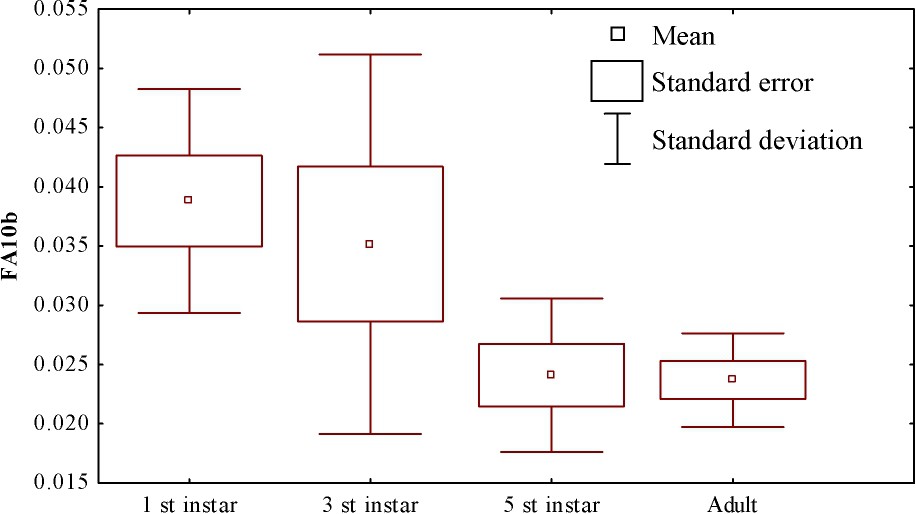
Fluctuating asymmetry level (mean ± SE) obtained with the FA10b index of each group of Hydropsyche exocellata collected in the Meurthe River in 2003.
Relative FA ([|R−L|/(R+L)/2]×100) (mean ± SD) calculated for six traits: femur length and tibia length of the first leg (FL1, TL1), the second leg (FL2 and TL2) and the third leg (FL3, TL3) for each group of Hydropsyche exocellata
| TL1 | FL1 | TL2 | FL2 | TL3 | FL3 | |
| 1st-instar larvae (%) | 2.7 ± 2.0 | 4.4 ± 3.3 | 3.5 ± 2.9 | 5.3 ± 3.9 | 4.8 ± 4.0 | 4.6 ± 4.5 |
| 3rd-instar larvae (%) | 3.0 ± 2.2 | 2.1 ± 1.7 | 2.1 ± 2.0 | 6.2 ± 5.0 | 3.7 ± 3.2 | 3.9 ± 3.8 |
| 5th-instar larvae (%) | 1.5 ± 1.8 | 2.1 ± 2.7 | 2.1 ± 2.3 | 3.4 ± 3.4 | 2.7 ± 2.9 | 2.9 ± 2.3 |
| Adults (%) | 2.2 ± 1.9 | 3.9 ± 3.0 | 2.4 ± 2.4 | 2.7 ± 1.8 | 1.9 ± 1.7 | 2.3 ± 1.7 |
Results of the second ANOVAs on the values of relative FA confirm that most of the FA of each trait differed significantly between groups (Table 3). However, the FA level between the first and third instars and between the last instar and the adults were not significantly different (Table 3). The highest FA values were obtained for the first- or third-instar larvae, while the lowest values were observed in the fifth-instar larvae and the adults, except for the length of the first-leg's tibia and the second-leg's femur (Table 2).
Results of the Tukey's HSD tests following the ANOVA II (traits × instars) on the relative FA ([|R−L|/(R+L/2)]) values. These analyses were applied for six traits: femur length and tibia length of the first leg (FL1, TL1), the second leg (FL2 and TL2) and the third leg (FL3, TL3) for each group of Hydropsyche exocellata
| 1st-instar larvae | 3rd-instar larvae | 5th-instar larvae | Adults | |
| 1st-instar larvae | – | |||
| 3rd-instar larvae | N.S. | – | ||
| 5th-instar larvae | <0.0001 | 0.0136 | – | |
| Adults | <0.0001 | 0.0289 | N.S. | – |
4 Discussion
Fluctuating asymmetry could potentially offer advantages as a bioindicator of environmental stress because it is relatively easy to estimate and it can be used to measure the effects of multiple stressors in natural populations [10]. Over the past decade, many studies have been concerned with the improvement of FA analysis for use as a biomonitoring tool for environmental stress or perturbation. Numerous studies emphasized the measurement errors [14,16–18,34] and we adopted a rigorous and approved statistical method [32,33]. We found a very low contribution of measurement error to FA variation and a high repeatability of our measurements. In FA analysis, the choice of traits is an essential step that has been widely studied [32,33,35–38]. Several studies have shown that multiple traits provide a better approach for assessing stress effects [6,32,39–41]. However, the actual FA evolution process during development has received relatively little attention.
In our study, we found differences in FA according to the larval and adult stages, indicating that asymmetry interacts with the larval development of H. exocellata. We observed a clear decrease in FA during larval development and a stabilization of FA level between the last larval instar and the adult stages. We suggest two hypotheses to explain this observation. The first hypothesis is that a feedback mechanism of compensational growth between left and right sides of characters [22–25] exists. This mechanism of compensational growth could restore symmetry as traits reach their final growth stages. Our results are not fully in accordance with this hypothesis, considering that we observed a decrease in asymmetry only between the first two larval instars and not between the last two. Furthermore, the adult FA level is relatively important. H. exocellata is a holometabolous insect, as are all Trichoptera, and thus presents a real nymph stage before adult emergence. During this stage, the development of adult organs causes a profound reorganization of the insect's morphology. The presence of a compensational growth mechanism could correct asymmetry during this step and adults would be less asymmetric than larvae. We could not exclude the presence of compensational growth, but it seemed to play a marginal role in the asymmetry of our natural Hydropsyche population.
The second hypothesis is that there is natural selection of larvae that exhibit an important level of asymmetry. We may suppose that some larvae presenting extreme asymmetry did not survive and only larvae with moderate asymmetry could achieve their development. It is well known that an important link exists between FA and survival (see [8] for a review). For hydropsychid larvae, several causes could explain this selection, such as larval drift downstream or increased predation on these larvae. Hydropsyche larvae have a particular diet consisting of suspended organic matter and tiny organisms captured in a net. Asymmetric larvae may have some problems with net-spinning. Several authors observed that hydropsychid larvae nets are modified when larvae have been subjected to environmental stress [42]. However, no study could link the FA of larvae and the net spinning. The absence of change in FA level between the last larvae instar and adults may corroborate this hypothesis. In fact, the nymph is characterized by a stationary, non-feeding stage when larvae are enclosed in a cocoon fixed to the substrate. During this period, natural selection is reduced and the FA level is thus stabilized. Furthermore, larvae do not feed during the nymph instar. As a consequence, we postulate that, in natural populations, the hypothesis of natural selection is more likely to influence the change of asymmetry throughout larval development. Considering our results, we recommend that researchers use the younger instars first, rather than adults or older instars that tend to underestimate the FA level in a natural population.
Acknowledgements
We thank Richard Palmer for his helpful comments on an earlier version of this paper. This study was supported by the French Ministry of Ecology and Sustainable Development (‘Ministère de l’Écologie et du Développement durable') as part of the 2001–2003 Biodiversity program.


