1 Introduction
Myotendinous junctions (MTJs) transmit tension generated by muscle to tendon [1]. At the MTJ, the muscle fiber ends exhibit highly folded cell membrane that interdigitates with the extracellular matrix. This structural configuration, described in Rhesus monkeys in our previous study [2], is common to various vertebrates. It increases the muscle-tendon interface [3–5] and reduces stress on the sarcolemma during muscle contraction [6–9]. In this work, we examine the MTJ soleus morphology in the Rhesus monkey by analyzing serial ultrathin sections.
Changes in mechanical constraints alter MTJ morphology. Ultrastructural modifications have been described not only after increased muscle use [10] but also after reduced muscle loading [11–14], as that experienced during spaceflight [15]. We have previously investigated the effects of a 14-day spaceflight on MTJ ultrastructure of soleus muscle in rats [16] and Rhesus monkeys [2]. Both species showed a degenerative process in the muscle fiber end whereas the microtendon showed signs of anabolic activity.
Special hardware was required to fly the animals on the spacecraft. During the Bion 11 mission, two Rhesus monkeys were seated on a restraint chair aboard the satellite. This ground-based situation leads to structural and functional modifications of skeletal muscle fibers [17,18]. In our previous studies [2,16], we did not distinguish specific effects of microgravity from those induced by animal housing conditions. In the present study, we sought to determine which of the changes observed at the soleus MTJ could be ascribed to the reduction of gravity and which were related to the confinement of the animal induced by the safety chair during the Bion 11 mission.
2 Materials and methods
The Bion 11 program was an international project between NASA (National Aeronautics and Space Administration) and the Russian space agency (RKA). This program was developed to study the effect of spaceflight on primate physiology.
2.1 Flight procedures
The Bion 11 experiments included three sets of young male Rhesus Monkeys (Macacca mulatta). The first set consisted of two monkeys (Multik, 357 and Lapik, 484), flown aboard the Bion 11 satellite and weighing, at the time of the flight, 4.9 kg and 5.1 kg, respectively. The Bion 11 spacecraft was in orbit for 14 days (24 December 1996 to 7 January 1997) with an apogee of 401 km and perigee of 225 km. According to data received at the control center (behavioral activity, body temperature, heart rate, food and juice consumption), the animals were in good health during the flight. Aboard the satellite, the monkeys were housed in the Bios-Primate capsule, which was equipped with water and food delivery systems and a urine collection system. To ensure their safety, the animals were placed in body-conforming, shock-absorbing seats (safety seats). The monkeys were lightly secured with a chest harness attached to the back of the chair to limit leaning forward at the waist. The chair limited lateral movement of the legs in the transverse plane. Additionally, a lap-plate was positioned over the thighs of the monkeys to limit movement of the leg at the hip joint in the sagital plane. The lower legs were unsecured [19,20]. The monkeys were housed in capsules 3 days prior to launch [21].
When the spacecraft landed (at 08:00 a.m. on 7 January 1997) in the north of Kazakhstan, the two monkeys were placed in a transportation couch tilted back at an angle of 45 deg and shipped to Moscow. They arrived at the Institute of Biomedical Problems at 8:45 p.m. where they were left overnight in their transportation couches at 45 deg. The next morning, after physiological tests showing that both animals were healthy, they were removed from the transportation couch and were exposed to postflight experiments (e.g., body fluid measurement, neurosensory test, bone and muscle biopsies). On return to Earth, each monkey was found to have lost 0.6 kg body weight.
2.2 Ground control situations
The Bion 11 protocol included two Earth control situations. A baseline control group (vivarium control) consisted of two monkeys that had remained in vivarium cages. A second ground control group consisted of three additional animals (confinement group). In this group, two monkeys were housed in the flight-type capsule and placed in a flight-type safety seat during a period equivalent to the flight duration plus 3 days (like the flight animals, the confinement animals were housed in capsules 3 days prior to launch). The third monkey was placed in a flight-type safety seat for 17 days. The confinement group served to determine whether the reduced motor activity imposed by the safety seat induced changes in the musculoskeletal system. Thus, we could determine which changes could be ascribed to the effects of microgravity and which were related to the hardware required to fly the animals on the satellite. Simulated post-flight procedures were performed in strict adherence with the post-flight schedule. The three monkeys were placed in a transportation couch tilted back at an angle of 45 deg for 24 h after the flight simulation. After physiological tests showing the animals were healthy, they were exposed to postflight experiments.
Vivarium and confinement animals were the same age and provided with the same diet. The average weight was for vivarium animals and for confinement animals. On average, the body weight of the vivarium increased by 0.3 kg during the time of experiment whereas the body weight of the confined animals decreased by 0.3 kg.
2.3 Muscle biopsy procedure
Samples from vivarium, confinement, and flight animals were treated the same way. The animals were anesthetized and biopsies of the distal MTJs region from the right soleus muscles were surgically obtained. The biopsies were taken ∼24 h after landing. They were fixed by successive immersions in a graded series of glutaraldehyde solution (3, 4.5, and 6%) in 0.4 M cacodylate buffer at pH 7.4. They were subsequently post-fixed in a 2% osmium tetroxide solution for 2.5 h at 4 °C. After dehydration they were embedded in epoxy resin. Longitudinal ultra-thin sections (70 nm) were cut through the muscle-tendon interface and mounted on copper grids. For morphological study of control MTJ, we made serial ultrathin sections. Both uranyl and lead citrate were used to enhance the image contrast. The sections were examined with a JEOL EM 1220 electron microscope.
3 Results
3.1 MTJs of control animals
The myotendinous junction in Rhesus monkey was structurally complex. At the muscle fiber end, the cells were broken into major subdivisions by deep sarcolemmal invaginations. These subdivisions contained several sarcomeres and branched out into ramifications from which extended finger-like processes. Subdivisions, ramifications, and processes were arranged in different directions in three-dimensional space (Fig. 1). The structural complexity was enhanced because most of the ramifications and processes were incompletely separated and collapsed one on the other. They often had a wavy trajectory and were intermingled. These multidimensional trajectories displayed not only closed spaces of extracellular matrix trapped at the end of the muscle fiber but also sections of ramifications and finger-like processes isolated within the microtendon (Fig. 2).
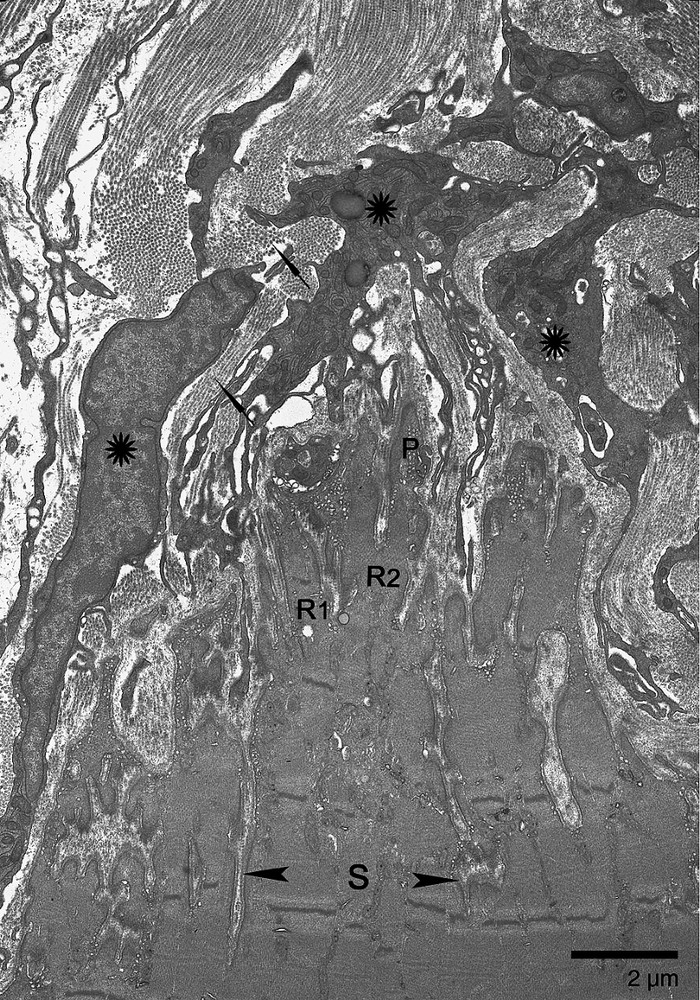
Electron micrograph of longitudinal section through the soleus MTJ from a control animal. The sarcolemmal invaginations of varying depth determine three levels of organization: subdivisions (delimited by arrowheads), ramifications (R1, R2), and finger-like processes (P). Orientation of sarcomeres in S and R shows that S and R can take different directions (R1, R2). R1 is parallel to the longitudinal axis of the muscle fiber. R2 appeared cut in oblique section. Numerous fibroblasts (asterisks) and multidirectional collagen fibers (arrows) make up the dense microtendon.

Soleus MTJ from control animals. Electron micrographs (A and B) of two serial sections 0.3 μm apart. An enclosed space of extracellular matrix (A, asterisk) results from anastomosis of ramifications (B: R1, R2). A short finger-like process (A, arrowhead) has a wavy trajectory and appears longer in B (arrowhead). Sections of ramifications (B, triangle) and sections of intermingled processes (B, circle) are isolated into the microtendon.
The subsarcolemmal region of the muscle fiber extensions displayed numerous vacuoles. Concerning the contractile structures at the MTJ, myofilaments of the terminal sarcomeres inserted into the plasma membrane via an electron-dense subsarcolemmal layer (Fig. 3). The dense microtendon was made up of multidirectional collagen fibers. However, at the level of sarcolemma invaginations, the collagen fibrils remained in most cases oriented parallel to the long axis of the finger-like processes. Numerous mononucleated cells, resembling fibroblasts, lay near the muscle fiber end (Fig. 1). Their cytoplasm showed well-developed rough endoplasmic reticulum, a prominent Golgi complex, and numerous vesicles. Their cytoplasmic processes extended not only to the muscle cell but also to other adjacent mononucleated cells. At this level, the cytoplasmic processes seemed to be in close contact (Fig. 3).

Soleus MTJ from control animals. The myofilaments insert into an electron-dense subsarcolemmal layer (white arrowheads). Numerous vacuoles (white arrow) lie beneath the plasma membrane of the muscle cell. Fibroblasts with well-developed rough endoplasmic reticulum (asterisks), Golgi complex (black arrowhead), and numerous vesicles (black thin arrows) send cytoplasmic processes towards the muscle fiber (large black arrows). Note that adjacent fibroblast processes are close to one another (square).
3.2 MTJs of confinement animals
No difference was observed between the animal placed in a flight-type safety seat and the two chaired animals housed in the flight-type capsule at 1 g.
MTJs of confinement animals showed the structural complexity we observed in the controls. Some MTJs, however, were more shredded than control myotendinous interface (Fig. 4). In these remodeling MTJs, the finger-like processes displayed a discontiguity between the cell membrane and the basal lamina at their extremities. These loops of basal lamina, detached from the plasma membrane, contained granular and vesicular materials (Fig. 5). Caveolae and vacuoles were located both at the bottom of the plasma membrane invaginations and along the lateral side of finger-like processes (Fig. 6).

Electron micrograph of a soleus MTJ longitudinal section from confined animals. MTJ is more shredded than the control myotendinous interface as a result of deeper and longer invaginations of the sarcolemma (asterisks). The nucleus (N) is in a central position at the extremity of the cell. The microtendon (T) is less electron-dense than in control animals.
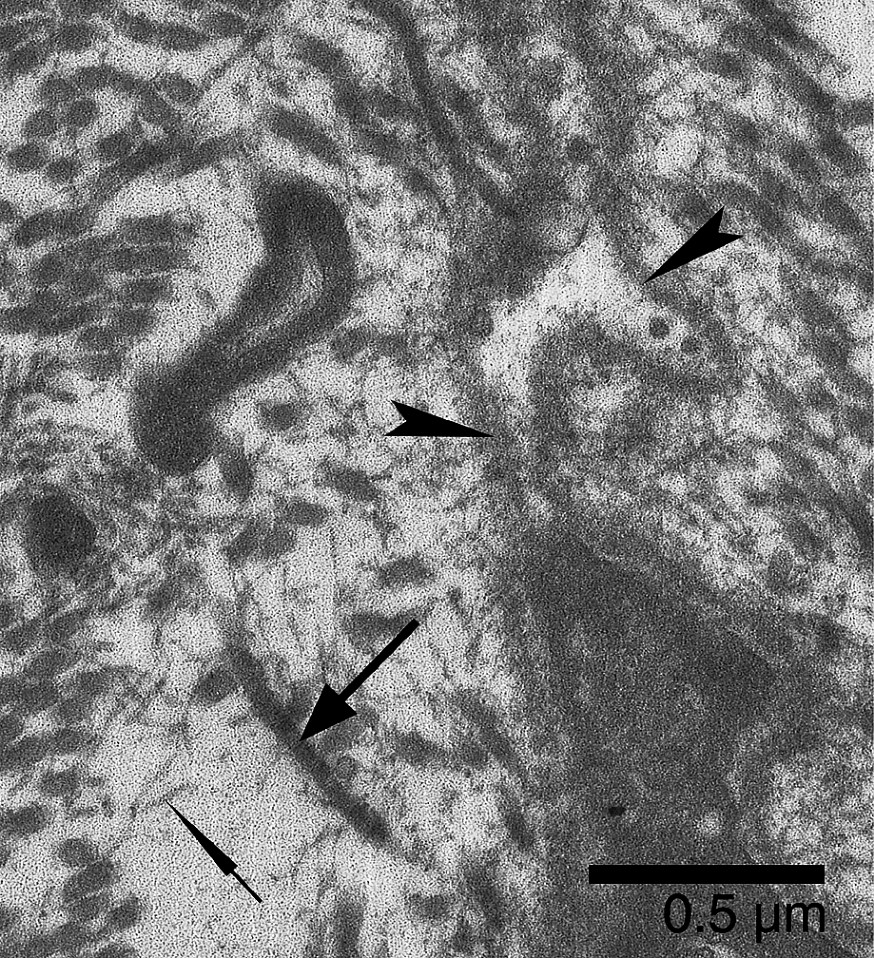
Soleus MTJ of confined animals. At the extremity of a remodeling finger-like process, the basal lamina (arrowheads) is detached from the plasma membrane. The envelope of basal lamina contains granular and vesicular materials. In the microtendon, scattered collagen fibrils (large arrow) are associated with fibrils of very small diameter (thin arrow).

Soleus MTJ of confined animals. A finger-like process presents numerous subsarcolemmal vacuoles (thin arrow). They are also located at the bottom of the sarcolemmal invaginations (large arrows). The contractile apparatus do not show significant alterations except for rare Z-disk streaming (arrowheads).
As compared to the control animals, the nuclei of the muscle fibers were frequently in a central position at the cell extremity (Fig. 4). Except for rare Z-disk streaming (Fig. 6), the contractile structure of the cells was not notably modified and myofilaments inserted into the dense subsarcolemmal densities. The fibroblasts showed no change in position, morphology, or number. The microtendon was less electron-dense than that of control animals (Fig. 4). The collagen fibrils were disorganized and were associated with fibrils of small diameter (Fig. 5).
3.3 MTJs of flight animals
In comparison with confinement animals, the soleus MTJ showed deeper sarcolemmal invaginations; longer and thinner finger-like processes enhanced the shredded aspect of the MTJ. Moreover, signs of basal lamina remodeling were frequent (Fig. 7). As in confinement animals, the finger-like processes of the muscle cell contained a myriad of caveolae and vacuoles, which often merged with one another. Clusters of vacuoles were also present at the bottom of the sarcolemmal invaginations. Contrary to confined animals, the contractile apparatus displayed significant changes after spaceflight. Terminal sarcomeres were not disorganized, but Z band and myofilaments appeared less electron-dense, suggesting a loss of myofibrillar protein. Furthermore, the subsarcolemmal layer in which terminal filaments insert was less thick than that of confinement animals (Fig. 8). We also observed changes in sarcoplasm at the muscle fiber end, where there were multivesicular bodies, probably associated with mitochondrial alterations and T-tubule dilation (Fig. 9). With regard to the microtendon, no difference was noted between confinement and flight animals.

Electron micrograph of a soleus MTJ longitudinal section from spaceflight animals. The finger-like processes are longer and thinner (large arrows) than those of confined animals. The shredded aspect is greater than in confined animals. Loops of basal lamina (thin arrows), containing granular and vesicular materials, are always found at the extremity of the finger-like processes after spaceflight.
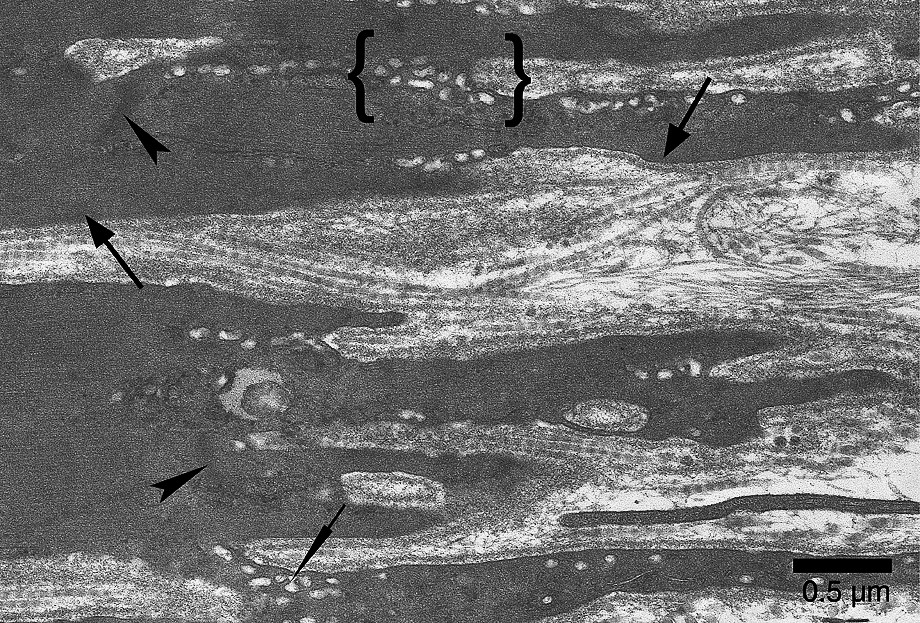
Soleus MTJ after 14-day spaceflight. The Z bands appear diffuse and not very electron-dense (arrowheads). Terminal sarcomeres display hardly distinguishable M lines and a clear loss of myofilaments in comparison with confined and control animals. The subsarcolemmal layer is less dense and less thick (large arrows) than those of confined animals. As in confined animals, caveoles and vacuoles (thin arrow), which merged with one another, are numerous. Note the clusters of vacuoles (between brackets) at the bottom of the sarcolemmal invaginations.
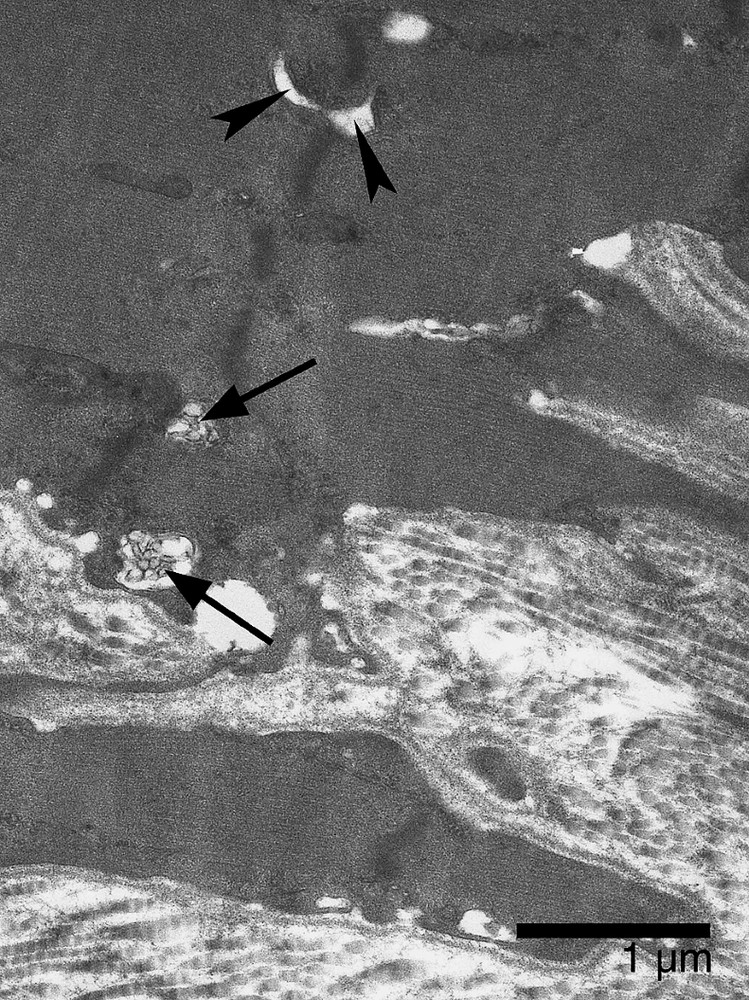
Soleus MTJ after 14-day spaceflight. Electron micrograph showing dilation of T-tubules (arrowheads) and small multivesicular bodies (arrows) lying near the extracellular matrix.
4 Discussion
Skeletal muscle fibers attach to the tendon at the myotendinous junction. This specialized region of the muscle fiber extremity constitutes one of the sites of force transmission from muscle to tendon [22]. Plasma membrane foldings and the structural chain linking myofilaments to tendon collagen fibers through the sarcolemma are found in all vertebrate MTJs [9]. Although we have described these characteristics in the Rhesus monkey MTJ [2] and suggested that force transmission in this animal does not differ from that in other species, our ultrastructural analysis of serial sections revealed morphological features specific of this animal. The soleus MTJ of the monkey shows a structural complexity which has never been described, and which results from random spatial organization of subdivisions, ramifications, and processes of the muscle fiber extending three dimensionally. Further complexity is added by intermingling of ramifications and processes from different cells and their wavy trajectories. One can suppose there is a relation between the structural complexity we observed in the Rhesus monkey MTJ and the functional requirements of the soleus muscle. Previous studies support this hypothesis. Dunbar [23] investigated the mode of locomotion of free and captive Rhesus monkeys. He observed that monkeys in the wild move around on the ground and climb over long-diameter branches and vines. In captivity, they employ similar behaviors on comparable supports (floor, suspended poles, and ropes). The locomotor activity of these animals is very different from the terrestrial locomotion of rats and from the bipedal movement of humans. One can suppose that morphological adaptations occurred to adapt the soleus muscle to constraints imposed by the terrestrial and arboreal environments. Previous studies have suggested that the environment can induce morphological adaptations in primate hindlimbs. Burr et al. [24] correlated the nature of locomotor activity of three monkey species with the structural rigidity of the humerus and femur. In primates that spend much time in a terrestrial environment, the structural rigidity is greater than in primates that are more restricted to climbing in an arboreal environment, suggesting an adaptation of bone to different biomechanical environments. More recently, Anapol and Barry [25] showed differences in the architecture of triceps surae muscles of semiterrestrial and arboreal guenons.
The present study corroborates the results of our previous work on the ultrastructure of the microtendon [2]. We observed numerous fibroblasts near the MTJ, with signs of highly anabolic activity and apparent membrane contacts between tendon cells. These ultrastructural features have previously been reported in young and growing human MTJ [26,27]. This finding suggests that the cellular aspect of the microtendon was more related to the age of the animals than to the species. This hypothesis is also supported by the work of Wells and Turnquist [28], who observed that infant and juvenile Rhesus monkeys had greater motor and locomotor activity than sedentary adults.
In other respects the present work contributes to our understanding of MTJ plasticity. MTJ morphology is sensitive to chronic changes in mechanical stress imposed upon this interface [11–16]. In our previous study [2], we showed that a 14-day spaceflight led to a morphological remodeling of the soleus muscle-tendon interface in the Rhesus monkey. The present study corroborates our previous ultrastructural observations and sheds light on the stimuli at the origin of these structural modifications. We studied the effects of confinement induced by the safety chair in which Rhesus monkeys were placed during the spaceflight and thus we could determine which changes could be ascribed to the reduction of gravity.
After confined animals had spent 17 days in a sitting position, the soleus MTJs displayed a structural remodeling characterized by an increase in the length and depth of sarcolemmal invaginations, and alterations in the plasma membrane and basal lamina. We have already described these morphological changes after 14-day spaceflight in soleus MTJs both of rats [16] and Rhesus monkeys [2] and suggested that granular and vesicular globules were due to muscle fiber degenerative material. Basal lamina and plasma membrane remodeling have also been reported by Abou Salem et al. [11] after 2 weeks of tenotomy and at degenerating post-synaptic foldings of the neuromuscular junction in subjects affected by myopathy [29].
Control experimental procedures were designed so that three animals (confinement animals) stayed in a safety chair for 17 days. In addition, two of the animals were housed in flight-type capsules. Results showed no difference between the two populations of animals staying in chairs. For this reason, we referred only to one group of animals made up of three subjects: i.e. the confinement group. The structural changes we observed at the MTJs of confined animals result from hypokinesia imposed by the safety seat. Although the chair allows animals to move their ankles, the sitting position reduces hindlimb mobility and suppresses locomotor activity. Previous studies have reported that the contractile apparatus was slightly affected by restraint in the non-junctional region of the muscle fiber, as we observed at the myotendinous interface. It has been demonstrated that this ground-based situation leads to a slight atrophy of soleus type I fibers [17], but to no significant shift in fiber type distribution [30] or total protein content [31]. Moreover, Fitts et al. [17] found only minor atrophy after 18-day ESOP (Experimental System for the Orbiting Primate) sit, an experiment-support primate facility developed by NASA that reduces Rhesus mobility.
The morphological modifications described at the MTJs from confined animals were also found in the flight MTJs. However, in comparison with confined animals, flight monkeys had a greater shredded aspect of the myotendinous interface and more extensive signs of sarcolemmal remodeling. These results suggest that, in addition to hypokinesia induced by the safety chair, weightlessness is also responsible for changes. The other signs of MTJ remodeling such as alterations of contractile apparatus and myofilaments anchoring structures, T-tubule dilation, and autophagic vacuoles can be ascribed to the reduction of gravity.
The morphological modifications we observed in Rhesus monkey MTJs were partly similar to those reported in rat soleus MTJ after the SLS-2 mission [16]. However, the degenerative processes of the muscle fiber end were less advanced in the monkey than in the rat after the same period of microgravity, and contractile structure were less affected. Roy et al. [30] also reported that changes in soleus muscle were smaller than those in rats after the same duration of flight. Although the intensity of soleus MTJ response to microgravity appears less extensive than in the rat, our observations suggest that events structuring MTJ remodeling are identical. These ultrastructural modifications affecting the structural chain of force transmission probably weaken the muscle-tendon interface after return on Earth.
Acknowledgements
The authors thank A. Bernardé and J. Jahan for muscle biopsies, J.P. Blanquie for collecting the data during the tests, and M. Viso. We also thank the IMBP and NASA. The present study was financially supported by grants from CNES.


