1 Introduction
In many gregarious ruminants, adult males and females tend to live in separate groups outside the rutting season. This sexual segregation is especially widespread among polygynous species exhibiting a sexual dimorphism in body size, i.e. most cervids and mountain-dwelling bovids [1]. Nevertheless, while the adults of the two sexes are found in single-sex groups more frequently than is expected by chance (the social component of segregation), they also very often differ in their use of habitats (the habitat component of segregation) [2]. As a consequence, several non-mutually exclusive hypotheses have been proposed to explain sexual segregation, some focusing on the habitat component of segregation, the others on the social component.
Some authors have suggested that sexual segregation primarily results from sex-related habitat choices. According to the ‘predation-risk’ hypothesis [3,4], these sex-related differences occur because males enhance their reproductive success by maximising their body condition and thus their competitive ability, while females increase their fitness by improving the security of their offspring. Therefore, males invest in body growth by using areas with high-quality food, whereas females select safe habitat at the expense of the better forage. In contrast, under the ‘sexual dimorphism-body size’ hypothesis [5,6], the sexes differ in their use of habitats or vegetation patches due to allometry of the digestive system. Indeed, large-bodied males are able to tolerate poorer quality forage whereas small-bodied females need to feed on higher-quality food. The ‘scramble competition’ hypothesis [7] states that in areas with short grass, females are thought to outcompete males because their smaller incisor breath enhance their food intake, so that males are forced to use areas with abundant but low-quality forage.
However, while sex-related differences in habitat use have been found regularly, several recent studies have provided evidence that this feature explains only partially the sexual segregation recorded: indeed, at least in mouflon sheep (Ovis gmelini), Alpine ibex (Capra ibex), soay sheep (Ovis aries) and red deer (Cervus elaphus) [8–11], adult males and females tend to consistently make up separate groups when in the same habitat. Subsequently, two hypotheses putting forward a behavioural incompatibility between the sexes have been proposed and have received increasing attention over the last decade. According to the ‘social affinities’ hypothesis [12,13], males and females primarily differ in their motivation and style of interacting with conspecifics: males frequently perform agonistic and pseudo-sexual interactions since early stages of life, while females avoid or are indifferent to males' interactions, hence the formation of mainly single-sex groups. According to the ‘activity budget’ hypothesis [14–17], the main behavioural incompatibility between the two sexes concerns activity rhythm: females should spend more time in grazing and walking activities and less time ruminating or lying down than males because they are less efficient at digesting forage than males. Consequently, activity asynchrony between group members should be higher in mixed-sex than in single-sex groups, hence a shorter lifetime of mixed-sex groups.
Our aim in the present paper was to test predictions deriving from the ‘activity budget’ hypothesis, by investigating group instability in a population of fallow deer (Dama dama) living in a wooded park. While this hypothesis has been widely investigated over the last few years in bovids exhibiting sexual segregation (see [17] for a review), it has been more rarely tested in sexually-segregating cervids (but see [18,19]). Fallow deer is a typical woodland-dwelling cervid exhibiting high sexual dimorphism in body-size [20]. Several authors have reported that sexual segregation in this species is influenced by ecological factors [4,21–23]. However, fallow deer are also known to make up groups that frequently split up and merge [24–26], and the adults of the two sexes are sometimes reported to make intensive use of the same habitat if not the same habitat patches [22–24]. So, we can wonder to what extend sexual segregation in fallow deer depends on behavioural mechanisms such as differences in activity rhythm between females and males. With this aim, we firstly used Conradt's segregation coefficient [27], a suitable method for determining whether sexual segregation is mainly based on sex-related differences in habitat and area use or based on behavioural incompatibilities. Thereafter, we tested four predictions deriving from the ‘activity budget’ hypothesis and explicitly stated by [14]. We expected (i) activity asynchrony to be more frequent in mixed-sex than in single-sex groups, (ii) activity asynchrony to enhance the probability for a group to split up, especially in mixed-sex groups since in their case asynchrony is assumed to be a persistent disagreement between individuals of opposite sexes and not a transient state; (iii) mixed-sex groups to exhibit a higher fission rate than single-sex groups; and (iv) mixed-sex groups to split up into all-female and all-male parts more often than expected by chance.
2 Materials and methods
2.1 Study area
The study was carried out in an enclosed park located in the Buzet Forest near Toulouse in south-western France (43°46′N, 1°35′E). The park had a surface area of 130 hectares, and was situated at low elevation (125–168 m above the sea level). During 1994, i.e. the study year, average minimum and maximum temperature were and in January (coldest month), and and in July (warmest month). Total annual precipitation was 793.4 mm, with almost no snowfall in winter.
More than 90% of the park was wooded, predominantly by deciduous-leafed oaks Quercus pubescens and Q. petrea (86% of the tree community). Overall, seven habitat types were distinguished inside the study area. Three categories of oak habitat were defined on the basis of tree density, namely pole forest (on average: 845 trunks/ha), intermediate forest (1442 trunks/ha) and coppice (2824 trunks/ha; see [28]). The park further supported plantations of robinia (Robinia pseudacacia), one humid stand of black poplar (Populus nigra), some small meadows, and some small clearings of grasses and bracken (Pteridium aquilinum).
2.2 Study population
The population was founded in 1980 with five fallow deer coming from a neighbouring region, then supplemented in 1986 with two additional animals originating from Scotland. Since 1992, deer have been captured by darting and fitted with coloured collars. Following an extensive cull in late 1993, with non-marked animals preferentially removed, the population was composed during the study period of 39 deer, all individually recognisable [29]: 21 adult females, 12 adult males and six fawns. Population density was thus 30 deer/100 ha and the sex-ratio among adult animals was 0.57 males for one female. The population had not been supplemented with maize since the extensive cull in late 1993.
The fawning and rutting seasons occurred in June and November, respectively. Because deer were used to human activities, they were little disturbed by the presence of the observer [28].
2.3 Data collection
The study was conducted from late January to late May 1994, i.e. outside the rutting and the fawning seasons. Because trees prevented the continuous monitoring of fallow deer groups, all the data, including those concerning group splitting-up probability, were collected along four fixed routes (totalling 19.1 km in length). This set of routes allowed 87% of the whole park to be sampled, and was walked once a day. One of the four routes was walked in the early morning, another in the late morning, a third in the early afternoon and the last in the late afternoon. The order of the four routes was randomly permuted from one day of data collection to the next.
In the course of a route, the observer recorded the location of each sighted group (solitary deer included), the habitat in which the group was spotted, the time of the observation, as well as the identity and activity of the group members. Deer were considered to belong to the same group when they were at a distance [24]. Following [30], animals were further classified as active (when grazing, moving, standing or interacting) or inactive (when lying down). On this basis, each sighted group was characterised by its size, its composition (classified as mixed-sex, all-male or all-female according to the sex of the adults that composed it), and by the activity synchrony of its members (simply classified as ‘synchrony’ when all the deer were active or inactive, or ‘asynchrony’ when some deer were active while others were inactive). Furthermore, when individuals were re-observed in the course of the same route, we recorded whether the group in which they were first sighted had split up or not, and the time elapsed between the two observations.
2.4 Data analysis
2.4.1 Conradt's segregation coefficient
In order to test whether, in the studied population, sexual segregation was mainly due to sex-related differences in habitat use or to behavioural mechanisms relative to group cohesion and/or formation, we computed Conradt's segregation coefficient (SC) on the recorded numbers of adult males and females per group (SCgroups), per quadrat of one hectare (SCquadrats), and per habitat type (SChabitats). These computations were performed excluding the groups, quadrats, or habitats that included a single adult, and applying the formula [27]:
Following [9], we computed SCgroups, SCquadrats and SChabitats, pooling consecutive routes into (i) eight blocks of one fortnight, (ii) four blocks of one month, (iii) two blocks of two months, and (iv) one block of four months. As recommended by [27], the total number of observed individuals exceeded 30 for both adult males and females even when we considered eight blocks of one fortnight (min number of males and females: 38 and 42, respectively). If sexual segregation was a by-product of sex-related differences in habitat use, then we should have , whatever the number of routes per block. In contrast, if sexual segregation was mainly the outcome of behavioural mechanisms relative to group cohesion and/or formation, then , and SCquadrats should have an intermediate value that declined when the number of routes per block was increased. The rationale for the latter statement is the following: in the course of a single route, 1-ha quadrats rarely included more than one group of fallow deer, which implied that ; however, if segregation by groups was responsible for the segregation by quadrats measured during any route, then a quadrat supporting an all-female group during a given route might support an all-male group during a subsequent one; as a consequence, SCquadrats should decrease when blocks included a higher number of routes [9]. Differences between SCgroups, SCquadrats and SChabitats were tested for eight blocks of routes using Wilcoxon's paired-sample test as recommended by [27].
2.4.2 Group activity synchrony and splitting-up
In order to know which factors influenced the probability for a group to be composed of deer engaged in the same activity, we fitted a generalised linear model (dependent variable distribution: binary; link function: logit) in which activity synchrony was a function of the size and composition of the group, and of the interaction between these two factors. The same type of generalised linear model was used to identify the factors that influenced the probability for a group re-observed in the course of a route to split up. In this case, the model initially included the effects of the size, composition and activity synchrony of the group when first observed, all the possible interactions between these three factors, and in addition the time elapsed between the first and second observations of the group, and the day slot during which the group was observed (early morning, late morning, early afternoon and late afternoon). In both cases, a backward selection procedure was applied: the model was simplified by removing, at each step of the procedure, the component having the weakest effect among those of highest degree exhibiting a P value >0.05. For the two generalised linear model cited above, data were checked to exhibit correct dispersion using the goodness-of-fit test for binary data proposed by [31].
We tested whether mixed-sex groups tended to split up into single-sex parts, using simulations and a Monte Carlo test [32]. Under the null hypothesis that a group resulting from the splitting-up of a mixed-sex group is a random sample of the latter, the numbers of males and females in the resulting group should follow a hypergeometric law with parameters n, m and f, where n is the size of the resulting group, and m and f are the numbers of males and females in the mother-group, respectively. On this basis, we performed 10 000 simulations of the composition of the observed groups that resulted from the splitting-up of a mixed-sex group previously sighted in the course of the same route. Each simulation gave us a number of single-sex groups that might have been observed under the null hypothesis. Finally, the observed value was compared to the simulated distribution, and the P value of the Monte Carlo test was given by the ith percentile of the observed value.
3 Results
3.1 Sexual segregation and grouping pattern
During the study, a total of 350 groups of two or more adult fallow deer were sighted, at least two adults were spotted in 121 quadrats of one hectare, and the minimum number of adults sighted per habitat was 17 (in the only stand of black poplars present inside the park).
Fig. 1 shows the values of SCgroups, SCquadrats, and SChabitats that were obtained when computed for each duration of the blocks that pool consecutive routes. For blocks lasting one fortnight ( blocks), Wilcoxon's paired-sample test showed that SCgroups was significantly higher than SCquadrats (; ; ), and that SCquadrats was significantly higher than SChabitats (; ; ). In addition, while SCgroups was constant whatever the number of blocks, both SCquadrats and SChabitats declined as the number of blocks decreased. The values of the three coefficients thus reached , , and when all the routes were pooled into a single block. These results strongly suggest that behavioural mechanisms relative to group cohesion and/or formation were the main causes of the sexual segregation recorded.
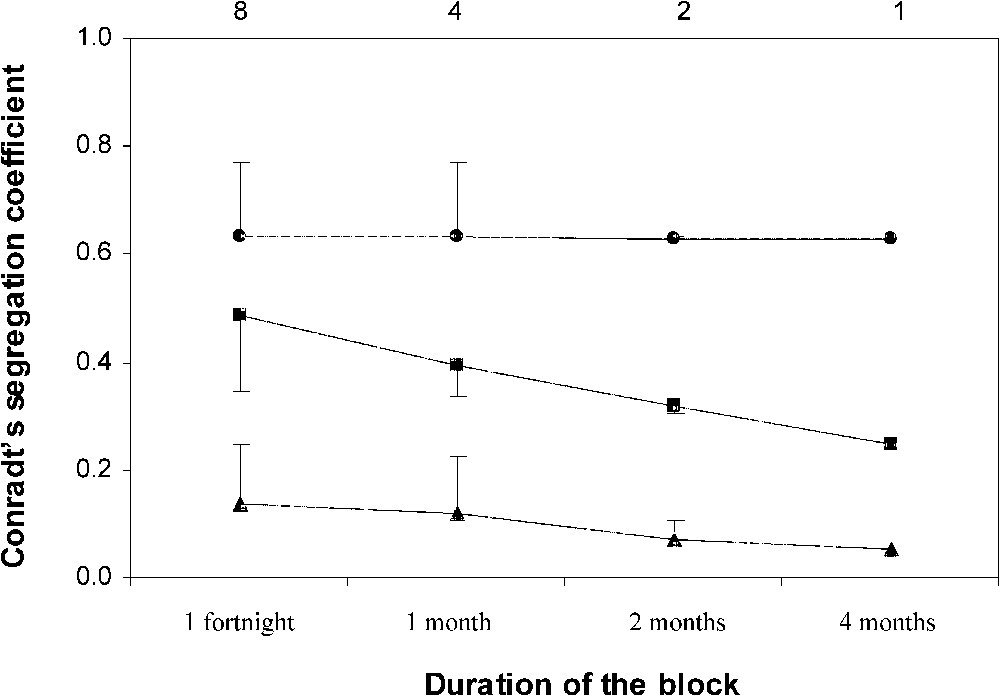
Mean values ± SD of Conradt's segregation coefficients SCgroups (●), SCquadrats (■), and SChabitats (▴) as a function of the duration of the blocks that pool consecutive routes. The number of blocks supporting the computation of the mean value of Conradt's segregation coefficients is indicated on the top of the diagram.
With respect to the grouping pattern, while the adult sex-ratio in the population was 0.57 males for one female, all-male groups were of approximately the same size as all-female groups. Indeed, the mean number of adults per was for the 181 all-male groups sighted during the study, and for the 155 all-female groups (solitary deer included). For the 106 mixed-sex groups sighted during the study, it was , with a mean number of adult males of and a mean number of adult females of .
3.2 Within-group activity synchrony
In most instances, all the members of a group were engaged in the same activity (i.e. active or inactive), since 75.6% of the mixed-sex groups, 89.5% of the all-male groups and 93.1% of the all-female groups were composed of synchronised members. Nevertheless, these percentages differed substantially, and the difference between group types was confirmed by the generalised linear model used for modelling the probability of activity synchrony as a function of group size and composition. Indeed, the backward selection procedure performed on the components of the model revealed no interaction between group size and composition (likelihood-ratio test: , , ), but a significant, negative effect of group size (, , ) and a significant effect of group composition (, , ). Actually, the probability of activity synchrony did not differ between all-female and all-male groups (, , ), nor between mixed-sex groups and all-male groups (, , ), but was significantly lower in mixed-sex groups than in all-female groups (, , ).
3.3 Splitting-up probability
Overall, 119 groups of two adults or more – among which 40 were mixed-sex groups, 42 all-male groups and 37 all-female groups – were re-observed in the course of a given route. After a maximum time elapsed of three hours (: minutes), 62.5% of those mixed-sex groups, 40.5% of those all-male groups and 24.3% of those all-female groups had split up when re-observed.
The backward selection procedure performed on the components of the generalised linear model used to analyse the splitting-up probability of the groups, did not reveal any significant effect of the interactions between factors involved in the initial model, including the interaction between the group's initial composition and activity synchrony (likelihood-ratio test: , , ). So, contrary to what could be expected under the ‘activity budget’ hypothesis, an asynchrony in activity between the members of a group did not particularly seem to enhance splitting-up when the group was mixed-sex. Furthermore, the backward selection procedure did not reveal any effect of activity synchrony (, , ), of the time elapsed between the two observations (, , ) and of the day slot (, , ). In contrast, as shown in Fig. 2, splitting-up probability increased with group size (, , ), and differed according to group composition (, , ). While mixed-sex groups were clearly more unstable than all-female groups (, , ), all-male groups exhibited an intermediate probability of splitting-up that did not differ significantly from that of the mixed-sex groups (, , ) or from that of the all-female groups (, , ).
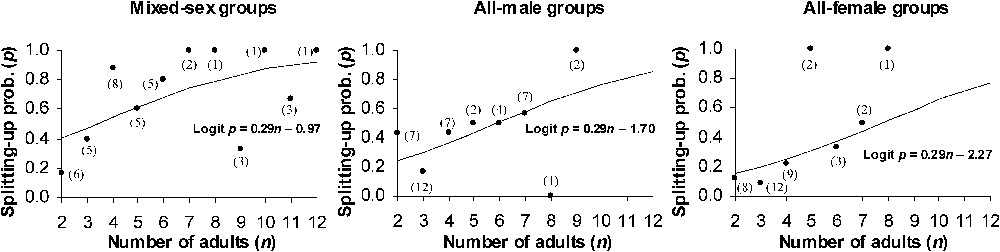
Probability of splitting-up as a function of the number of adults in the group, in each of the three group types. For each group type, the equation of the model is given below the fitted logistic curve. In brackets: number of groups over which the frequency is computed.
3.4 Splitting-up of mixed-sex groups
Over the 25 sighted groups that resulted from the splitting-up of a mixed-sex mother-group, 17 were composed of same-sex deer. The number of single-sex groups obtained by simulating the composition of the 25 sighted groups was higher or equal to 17 in only 129 simulations out of the 10 000 (min = 9; median = 13; max = 19). Thus, mixed-sex mother-groups significantly tended to split into single-sex parts (Monte Carlo test: ).
4 Discussion
4.1 Factors influencing group splitting-up
As in a number of other ruminant species (e.g., mule deer Odocoileus hemionus [19], red deer [33], bighorn sheep Ovis canadensis [34], Alpine ibex [35]), fallow deer groups have a rather short lifetime whatever their composition (see [24], this paper). Our findings further show that the probability for fallow deer groups to split up progressively increases with their size. Such a relationship is compatible with the assumption made in several dynamic models of group formation that any individual can initiate splitting-up of the group it is in with a certain probability per unit time which is independent of group size; in this case, the larger the group, the higher the probability for the group to split up during a given time interval [36–39]. Note that [26] found that in fallow deer, the trajectories of the members of a group become more dissimilar as group size increases and the authors suspected that this enhances the probability of splitting-up. Be this as it may, an increase of the probability for groups to split up when their size increases undoubtedly influences not only the exact shape of the group size distribution [38], but also the frequencies of single-sex and mixed-sex groups in a population [14,18].
As pointed out by [14], under the ‘activity budget’ hypothesis, activity asynchrony between the sexes is expected to lead to a higher splitting-up rate in mixed-sex groups than in single-sex groups. In the present study, prediction (i) was satisfied: as expected on the basis of the ‘activity budget’ hypothesis, activity synchrony was less frequent in mixed-sex groups than in single-sex groups or at least all-female groups. While this kind of pattern is certainly not universal in sexually-dimorphic ruminants [19,40], a lower synchrony in mixed-sex than in single-sex groups has also been found in red deer [11,14], bighorn sheep [30] and Alpine ibex [41]. However, such a finding is not sufficient for the ‘activity budget’ hypothesis to be considered as correct. The validity of this hypothesis crucially depends on prediction (ii), i.e. that activity asynchrony is a major cause of group splitting-up. The results obtained in the present study do not support it in fallow deer, be this for the mixed-sex groups or the groups of two adults or more taken as a whole. This suggests that in a group whose members are not synchronised, the active deer tend to stay in the neighbourhood of the inactive animals and/or the inactive deer tend to stand up and go back to the active animals before the latter leave their field of vision. The existence of such behaviour patterns should of course be checked. Note that in red deer and in domestic sheep (Ovis aries), respectively, [18] and [42] reported that activity asynchrony was not sufficient by its own to cause group splitting-up. However, while prediction (ii) was not supported by our results, predictions (iii) and (iv) were satisfied in the study population and for the four-month period considered: mixed-sex groups had a higher probability of splitting-up than single-sex groups or at least than all-female groups, and split up into single-sex groups more often than expected by chance. These two processes undoubtedly contribute to the high frequency of single-sex groups and the low frequency of mixed-sex groups in the population.
4.2 Possible alternative causes of the recorded segregation
Although we focused on predictions derived from the ‘activity budget’ hypothesis, it should be noted that, in the general framework of sexual segregation, predictions (iii) and (iv) are not specific to the ‘activity budget’ hypothesis, but also fit three other hypotheses frequently found in the literature and summarised in the Introduction. Indeed, in the ‘social affinities’ hypothesis, females are expected to enhance the fission rate of mixed-sex groups because they tend to avoid the males and the interactions performed by the latter. Moreover, in the ‘predation-risk’ and the ‘sexual dimorphism-body size’ hypotheses, adult males and females are assumed to exhibit different habitat-use patterns; as a consequence, a group arriving at the edge of two habitat patches can be expected to remain more often cohesive when composed of same-sex conspecifics than in the opposite case.
Now, the results obtained using Conradt's segregation coefficient strongly suggest that, in the study population and for the four-month period considered, sexual segregation was poorly explained by sex-related differences in habitat use. Since the high splitting-up probability of mixed-sex groups did not seem to find its origin in the asynchrony between the sexes, the segregation recorded might be mainly due to sex-dependent affinities between individuals. Interestingly, [24] outlined that in mixed-sex groups of fallow deer, males and females tend to have same-sex conspecifics as nearest neighbours. The same tendency has been found in mouflon sheep [43] and domestic sheep [13,44,45]. Furthermore, [46] reported that mouflon rams often exchange ‘symmetrical’ interactions (during which the two animals involved behave in the same way), while ewes avoid the adult males directing interactions at them. Such reports suggest that the mechanisms put forward by the ‘social affinities’ hypothesis could also be at work.
Nevertheless, only detailed analysis of the dynamics of group formation in conditions allowing the continuous monitoring of groups will constitute a decisive estimation of the respective contribution of the ‘activity budget’ and ‘social affinities’ hypotheses in wild ruminants. According to the ‘social affinities’ hypothesis, we can expect that at least some group splitting-ups are triggered by interactions initiated by adult males inside the group. This should be the case in mixed-sex groups, in which case the females should be the individuals that actively leave the group, but also possibly in all-male groups. In our population, all-male groups seemed to be characterised by a higher fission rate than all-female groups, and this could be a consequence of the agonistic interactions that the males frequently exchanged. In addition, according to the ‘social affinities’ hypothesis, we can expect that two groups whose members perceive one another should have a higher probability of merging when they are composed of same-sex individuals. In our population, the probability for two all-male groups to merge was probably very high, since the mean group sizes of all-male and all-female groups were similar despite a sex-ratio biased in favour of females and a rather high fusion rate for all-male groups. Whether these predictions deriving from the ‘social affinities’ hypothesis are correct or not, and beyond the comparison of this hypothesis with the ‘activity budget’ hypothesis, an improved knowledge of the processes of fusion and fission involved in group dynamics will certainly lead to a better understanding the phenomena involved in sexual segregation.
Acknowledgements
We gratefully thank J.-P. Vincent for walking the routes and M. Bos for permission to work in his enclosure. We acknowledge M. Goulard for his insight into statistical procedures, P. Winterton for checking the English language and C. Bonenfant for useful comments. N. Villerette was financially supported by the ‘Conseil Régional de Champagne-Ardenne’ and the ‘Communauté de Communes de l'Argonne Ardennaise’.


