1 Introduction
Coral bleaching is defined as the loss of color in corals resulting from the partial or total loss of endosymbiotic algae, referred to as zooxanthellae, or the degradation of photosynthetic pigments within zooxanthellae [1]. Two environmental factors that can trigger bleaching are increased water temperature and high solar irradiance. Mass bleaching events are usually observed when both factors occur simultaneously [2–5]. However, coral bleaching has also been observed following prolonged exposure to darkness, heavy metal toxicity, pathogenic organisms, terrestrial runoffs, and reduced water temperature [6–8]. Symbiosis with zooxanthellae is essential for scleractinian corals, as zooxanthellae, via photosynthesis, can provide their host with up to 95% of their nutrients requirements [9,10] (but see [11]). Bleaching has immediate or short-term effects on coral growth, fertility, and reproduction [12–15], and can lead to mass mortalities resulting in long-term changes in coral population and community dynamics [16–20].
During the last three decades, mass bleaching events and associated mortalities of corals have been reported with increasing frequency, becoming one of the most important threats to coral reefs worldwide [6,21–26]. In particular, the global 1998 bleaching event was estimated to have eliminated 16% of the world's living corals [22]. The prevalence of bleaching among coral colonies, and the intensity of bleaching within individual colonies usually increases with sea-surface temperature and the duration of the temperature anomaly [5,27,28]. Taxonomic composition of coral assemblages also plays a critical role in bleaching events, because not all coral taxa respond similarly: some species are more susceptible than others, and some species recover at higher rates than other ones [29–32]. Species-specific response to temperature stress can modify the community composition of corals, and in some cases, local extinction of susceptible species can dramatically change the physical structure and biodiversity of a reef assemblage [12,30,32–34].
Bleaching severity also varies as a function of environmental conditions, including physical habitat structure [26,33,35,36], exposure to flow and other hydrodynamic conditions [37,38] (see also Lenihan and Adjeroud, unpublished data) and shading [39–41]. Recent research indicates that the history of disturbances influences bleaching severity [23,25,41–43]. For example, bleaching prevalence and intensity declines across a time series of heat stress events, because impacted coral assemblages and/or their zooxanthellae may acquire phenotypic (acclimation) and/or genotypic (adaptation) resistance to bleaching [7,26,44,45]. The adaptive bleaching hypothesis postulates that the loss of photosymbionts has the potential to allow some representatives of the host species to re-establish a symbiosis with a different dominant alga, resulting in a new association that is better suited to the altered environmental circumstances [46,47]. Yet, a recent study indicates that only a minority of species (23%) may be able to change their symbiont, indicating that the adaptive bleaching hypothesis would only be applicable to these species [48].
Mass coral bleaching events have been reported numerous times from a wide variety of locations worldwide. The majority of these studies provide data on the bleaching responses of entire coral assemblages (e.g., susceptibility, mortality, and decline in live coral cover) at regional (10–100 km) or local (1–10 km) spatial scales [49–52]. Examination of bleaching at both these scales has proved critical in understanding how mechanisms such as weather, circulation, upwelling, and land-based environmental stressors (e.g., sedimentation and anthropogenic pollutants) influence coral bleaching and recovery [6,53,54]. Processes that operate at smaller, within-reef spatial scales (0.01–1 km), including variation in hydrodynamic conditions, shading, and disease also influence bleaching responses within and among different coral taxa [38,40,55,56]. Rarely have species-specific bleaching responses been examined across different spatial and temporal scales as a means of quantifying the effects of different environmental drivers [29]. Such information would enable the predictions concerning specific ecological mechanisms that underlie coral bleaching and recovery to be more accurate. Here we report on the results of a sampling program designed to test whether bleaching responses vary as a function of coral genera, water depth, location of reefs relative to ocean currents, and their interaction. By comparing our data with those collected at previous bleaching events, we also explore whether taxonomic responses and spatial patterns in bleaching vary with time, and whether corals in our model system, in Moorea, French Polynesia, developed resilience to bleaching after experiencing three bleaching events over an 11-year period [33,35,57].
2 Methods
This study was conducted on the island of Moorea (, ), French Polynesia, located in the Society Archipelago. Moorea is volcanic in origin, 134 km2 in area, and has 49 km2 of lagoon and nearshore coral reef habitat (Fig. 1). The island experienced coral bleaching events in 1991, 1994, and 2002. The bleaching events in 1991 and 1994 are well documented [33,35,57]. Bleaching in 2002 occurred from late March to late July (see § Results). Moorea, like other French Polynesian islands in the Society, Gambier, and Austral Island chains, represents a good model system to examine small-scale spatial variation in coral responses to natural disturbances, including bleaching events. Reefs on these islands support abundant coral populations that inhabit narrow lagoons ( wide) and outer reef slopes ( wide), that, in turn, are characterized by steep environmental gradients in terms of exposure to ocean currents, land-based runoff and sedimentation, and water depth. These gradients cause, at least in part, species zonation within scleractinian coral communities [58]. We describe bleaching patterns observed on the reef slope located on the seaward side of lagoons. By sampling corals located on different reefs located at different water depths, we were able to examine through correlations the relationship between the bleaching responses of several coral taxa and a suite of potential environmental drivers.
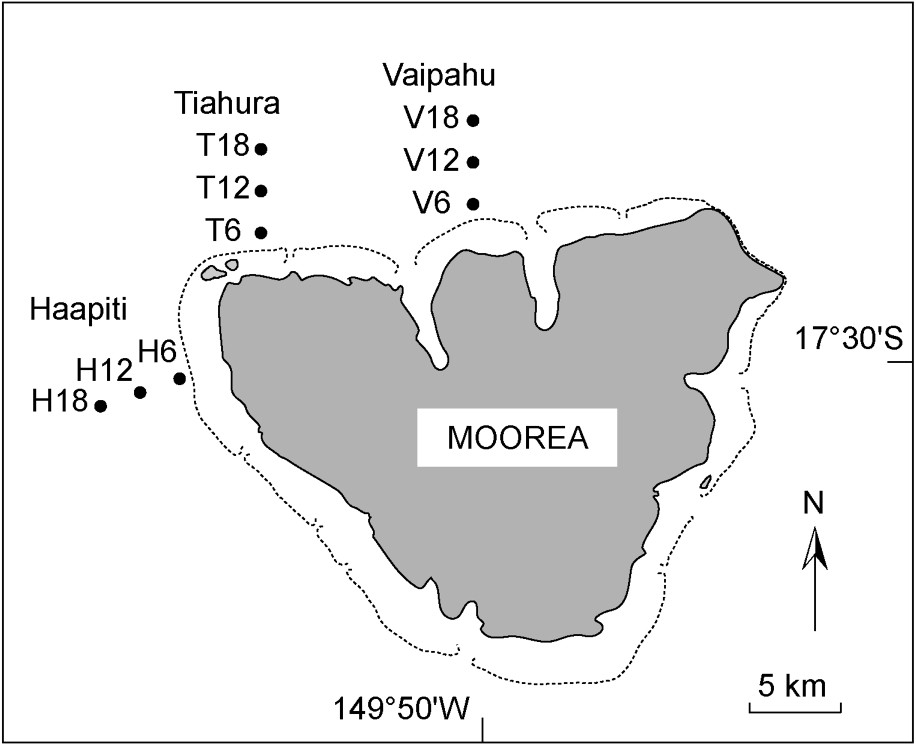
Map of Moorea, showing the position of the nine stations sampled on the outer reef slope, representing three locations (Vaipahu, Tiahura, Haapiti), and three depths (6, 12, and 18 m). Stations codes are abbreviated as follow: the first letter indicates the location (V: Vaipahu; T: Tiahura; H: Haapiti), and the associated number corresponds to the depth (6, 12, and 18 m). Dashed lines represent the approximate extent of the reef front surrounding Moorea.
In 2002, nine stations were sampled on the outer reef slope, including three locations (Vaipahu, Tiahura, Haapiti), and three water depths (6, 12, and 18 m; Fig. 1). Haapiti is located on the western shore of the island, while the other two sites are located on the northern shore. Despite similarities in reef structure (i.e. spur and groove formations from the reef crest to ∼30-m depth), the three locations vary greatly in their exposure to oceanic swell, which varies seasonally around the island. Haapiti, the most exposed of the three locations, is subjected to southwestern swells that prevail throughout much of the year. The direction and intensity of swells creates a fairly consistent and high-energy wave field. In contrast, the Vaipahu and Tiahura sites are sheltered from southwestern swells, but are directly exposed to the more low-energy northern swells that occur from November to April. Variations in seasonal flow fields are provided in Laurent et al. [59]. At each location, the distance between successive stations along the depth gradient was 50–70 m, depending on the reef slope morphology.
At each station, all colonies visible to divers were counted and identified to genera within three belt-transects of 10 m2 (10 m long × 1 m wide) located parallel to each other and to the coastline. Sampling was conducted over three weeks from 12 April 2002, about two weeks after the beginning of the bleaching phenomenon. Healthy colonies (i.e. with no visible sign of bleaching) were differentiated from bleached ones. Colonies were either classified as healthy (no apparent bleaching), or as partially bleached (only portions of colony were bleached), or as completely bleached (the whole colony was white). As our study occurred at the very beginning of the event, no recently dead colonies (i.e. covered with a thin turf layer) were observed during this survey.
ANOVA was used to test the influence of location and depth on the proportion of bleached colonies (partially + completely) for each of the six main genera, and for all genera combined. Student–Newman–Keuls (SNK) post-hoc tests were used to determine differences among specific treatments, and data were arcsine transformed and tested for normality and heterogeneity of variances before ANOVA using Levene's test. ANOVA could not be used to compare the proportion of either partially bleached or proportion of completely bleached colonies, because the assumption of normality was not satisfied, even after transformation.
Seawater temperature on the outer slope of Tiahura at 8-, 14-, and 25-m depth has been routinely surveyed since 1998 using permanent thermographs. These thermographs measure temperature every hour and have a precision of 0.01 °C.
3 Results
Out of the 6966 colonies examined around Moorea, (mean ±1 stdev) were healthy (unbleached), partially bleached, and completely bleached during April through May 2002. The proportion of healthy, partially bleached, and completely bleached colonies varied among locations and depths (Table 1). The percentage of bleached colonies (partially + completely) for all genera pooled varied among depths, and was significantly lower at 6 m than at 12 and 18 m (ANOVA; Table 2; SNKs, ), whereas it did not vary significantly between 12 and 18 m (SNK, ). Corals at Haapiti had fewer partially and completely bleached colonies (32.2 and 14.5%) than at Tiahura (42.6 and 15.1%) or Vaipahu (40.2 and 17.9%; Fig. 2; Table 1), and there was a non-significant trend for variation in the amount of partially + completely bleached colonies among sites (ANOVA, ; Table 2).
Mean abundance (number of colonies per 10 m2) and proportion (in %) of healthy, partially bleached and completely bleached colonies at each location (all depths pooled) and at each depth (all locations pooled). Standard deviation in brackets
| Haapiti | Tiahura | Vaipahu | ||||
| Healthy | 344.3 | 53.3% | 368.0 | 42.3% | 338.3 | 41.9% |
| (42.6) | (12.1) | (48.2) | (16.1) | (28.7) | (11.6) | |
| Partially bleached | 208.0 | 32.2% | 369.6 | 42.6% | 324.3 | 40.2% |
| (27.1) | (10.7) | (38.0) | (14.5) | (31.5) | (9.2) | |
| Completely bleached | 93.6 | 14.5% | 131.0 | 15.1% | 144.6 | 17.9% |
| (14.1) | (4.8) | (21.0) | (5.0) | (16.7) | (4.7) | |
| 6 m | 12 m | 18 m | ||||
| Healthy | 366.3 | 57.0% | 409.3 | 44.0% | 275.0 | 36.7% |
| (38.9) | (14.6) | (38.8) | (9.9) | (28.7) | (9.1) | |
| Partially bleached | 206.6 | 32.2% | 337.3 | 36.3% | 358.0 | 47.7% |
| (30.9) | (13.6) | (28.8) | (7.9) | (37.9) | (9.9) | |
| Completely bleached | 69.3 | 10.8% | 182.6 | 19.7% | 117.3 | 15.6% |
| (8.0) | (2.9) | (12.4) | (3.7) | (8.2) | (3.0) |
Results of ANOVA testing the influence of location and depth on the percentage of bleached colonies (i.e. partially + completely bleached colonies) for all genera pooled as well as for the six major genera (Acropora, Montastrea, Montipora, Pavona, Pocillopora, Porites). Data arcsine transformed. df: Degree of freedom
| df | Mean square | F-ratio | p | Wilk's lambda | |
| All genera pooled | |||||
| Location | 2 | 0.108 | 3.444 | 0.0541 | 6.888 |
| Depth | 2 | 0.261 | 8.342 | 0.0027 | 16.684 |
| Location × depth | 4 | 0.057 | 0.917 | 0.4757 | 3.666 |
| residual | 18 | 0.281 | |||
| Acropora | |||||
| Location | 2 | 0.429 | 22.908 | <0.0001 | 45.817 |
| Depth | 2 | 0.102 | 5.453 | 0.0141 | 10.906 |
| Location × depth | 4 | 1.440 | 38.456 | <0.0001 | 153.825 |
| residual | 18 | 0.169 | |||
| Montastrea | |||||
| Location | 2 | 0.115 | 2.465 | 0.1132 | 4.930 |
| Depth | 2 | 0.028 | 0.602 | 0.5585 | 1.204 |
| Location × depth | 4 | 0.026 | 0.276 | 0.8896 | 1.104 |
| residual | 18 | 0.420 | |||
| Montipora | |||||
| Location | 2 | 0.016 | 0.101 | 0.9044 | 0.202 |
| Depth | 2 | 0.318 | 2.056 | 0.1570 | 4.112 |
| Location × depth | 4 | 0.404 | 1.306 | 0.3051 | 5.226 |
| residual | 18 | 1.393 | |||
| Pavona | |||||
| Location | 2 | 0.023 | 0.866 | 0.4373 | 1.733 |
| Depth | 2 | 0.317 | 11.802 | 0.0005 | 26.605 |
| Location × depth | 4 | 0.133 | 2.482 | 0.0806 | 9.929 |
| residual | 18 | 0.241 | |||
| Pocillopora | |||||
| Location | 2 | 0.069 | 1.077 | 0.3615 | 2.154 |
| Depth | 2 | 0.152 | 2.371 | 0.1219 | 4.742 |
| Location × depth | 4 | 0.018 | 0.137 | 0.9664 | 0.549 |
| residual | 18 | 0.576 | |||
| Porites | |||||
| Location | 2 | 0.147 | 4.328 | 0.0292 | 8.656 |
| Depth | 2 | 0.681 | 20.063 | <0.0001 | 40.126 |
| Location × depth | 4 | 0.395 | 5.817 | 0.0035 | 23.269 |
| residual | 18 | 0.305 |
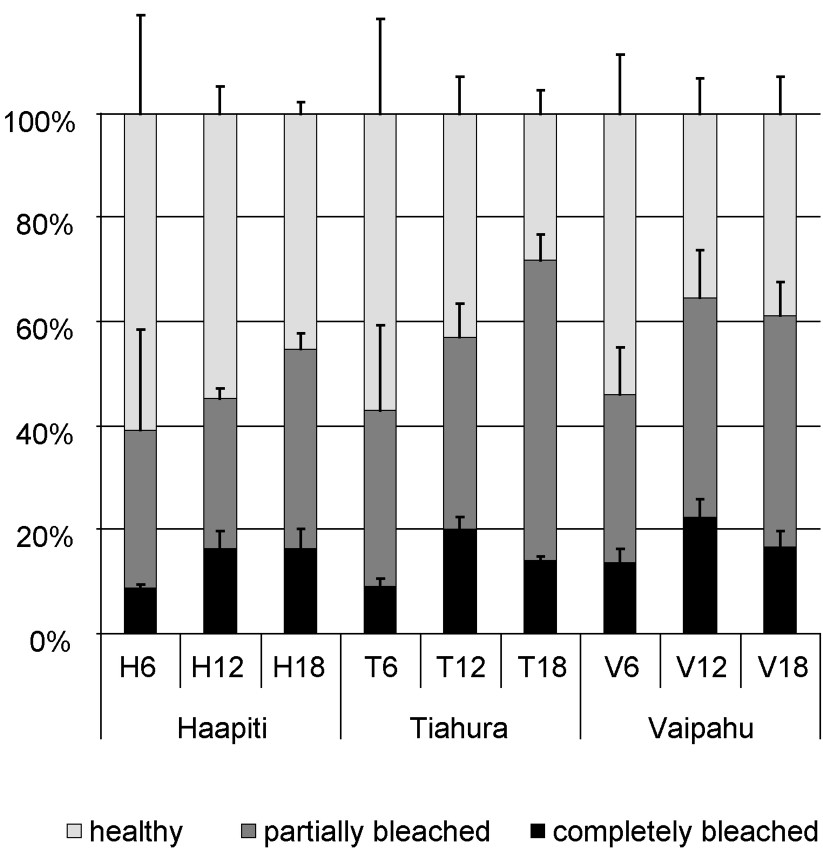
Proportion of completely bleached, partially bleached, and healthy colonies (all taxa pooled) recorded at the nine stations around Moorea. Error bars represent standard deviations.
Among the 18 genera encountered, all showed signs of bleaching (Fig. 3). However, substantial differences in susceptibility were found among coral taxa, and the proportion of healthy, partially bleached, and completely bleached colonies varied greatly among genera (Fig. 3). Fungiids (genera Fungia, Herpolitha and Sandalolitha), Montastrea and Acropora were particularly affected with at least 90% of colonies showing signs of bleaching. All recorded colonies of Favia, Lobophyllia and Gardineroseris were bleached, but these genera were rarely observed in our transects (a total of 9, 6, and 3 colonies, respectively, were found among all nine stations). In contrast, Acanthastrea, Pavona, Porites and Psammocora showed lower rates of bleaching, with less than 25% of colonies bleached, including less than 5% of colonies completely bleached. In addition, 36–66% of the colonies of Cyphastrea, Pocillopora, Astreopora, Leptastrea, Montipora and Leptoseris bleached. For some genera, such as Psammocora, Acanthastrea and Cyphastrea, no completely bleached colonies were found, and all affected colonies were only partially bleached. For Porites, Pavona, Leptastrea, Leptoseris and Fungia, less than 5% of colonies that bleached did so completely. Nevertheless, 90% of Fungia spp. partially bleached. Finally, a relatively high proportion of Montipora, Herpolitha, Sandalolitha, Acropora, Montastrea and Favia colonies bleached completely (Fig. 3).
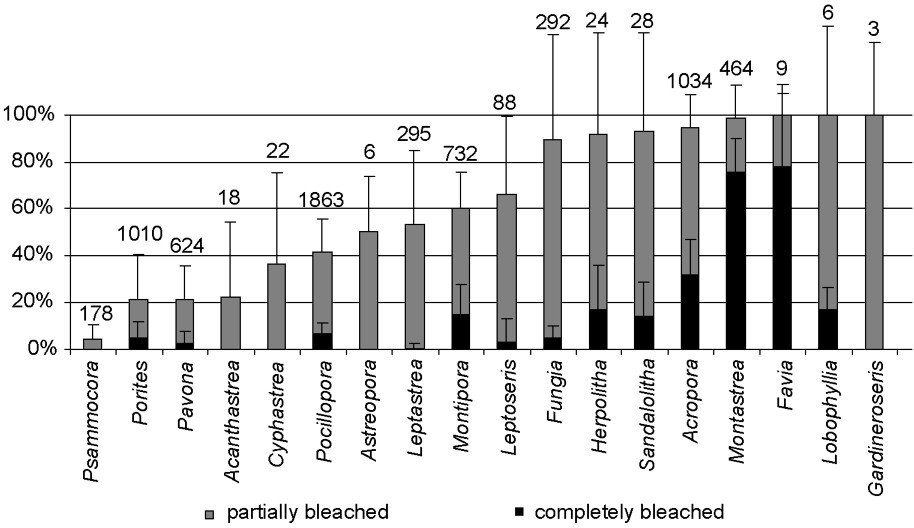
Proportion of completely bleached and partially bleached colonies for each of the 18 coral genera encountered (all stations pooled). Error bars represent standard deviations. Numbers above error bars represent total number of colonies recorded.
Several genera exhibited spatial variation in bleaching intensity (Table 2; Fig. 4). For Porites, results of ANOVA comparing percentage of bleached colonies (partially + completely) showed a significant location × depth interaction (2-way ANOVA; Table 2). This interaction was caused by a significant increase in the percentage of bleaching with depth at Tiahura (18 m > 12 m > 6 m; SNK, ), but generally low levels were observed at all other stations, except 18 m at Haapiti (Fig. 4). Percentage of bleached Porites colonies at 18 m at Tiahura and Haapiti did not differ (SNK, ), but were higher at these two stations than at all other stations (SNKs, ). There were no significant differences among all other stations (SNKs, ), except for the increase with depth at Tiahura, described already. For Acropora, the percentage of bleached colonies (completely + partially) also varied with location and depth (2-way ANOVA, location × depth interaction; Table 2). At Tiahura, percentage of bleached colonies for Acropora was lower at 6 m than at 12 and 18 m (SNKs, ; Fig. 4). There were also no significant differences between 12 and 18 m at Tiahura, or between these two stations and any other location–depth combination (SNKs, ). For Pavona, the percentage of bleached colonies varied significantly with depth, but not with location (ANOVA; Table 2), and bleaching increased with depth (SNKs, ). For Montipora, Pocillopora and Montastrea, the percentage of bleached colonies (partially + completely) did not vary significantly with depth, location, or their interaction (ANOVA; Table 2).
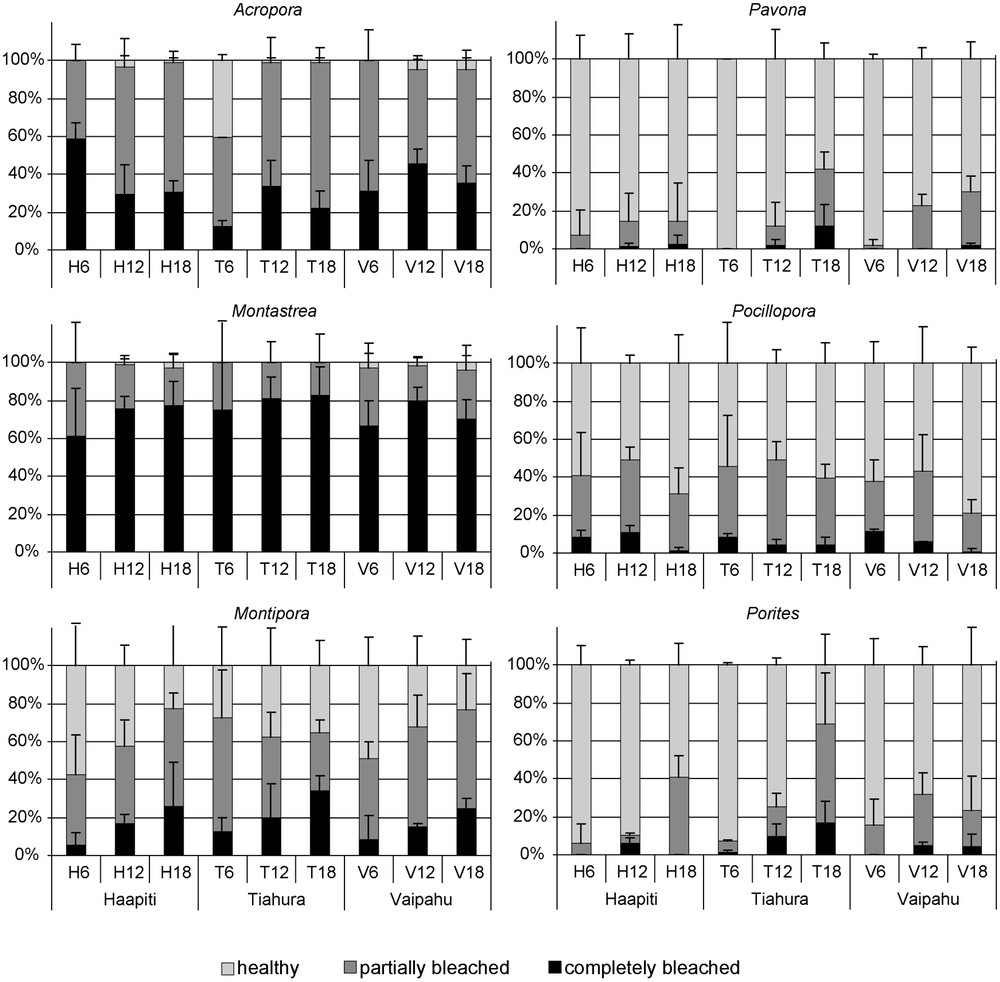
Proportion of completely bleached, partially bleached and healthy colonies recorded at the nine stations for the six dominant genera. Errors bars represent standard deviations.
Coral genera that exhibited a mean of 90% or more bleached colonies were considered as highly susceptible genera (Acropora, Favia, Fungia, Gardineroseris, Herpolitha, Lobophyllia, Montastrea and Sandalolitha). We compared the relative abundance of these highly susceptible genera in total assemblage among locations and depths and found a significant interaction (ANOVA, flocation × depth = 5.242, ). At all stations, the percentage of susceptible genera significantly increased with depth (SNKs, ). At each of the three depths, stations located at Haapiti presented less relative abundance of susceptible genera than stations located at Tiahura and Vaipahu (SNKs, ), whereas no differences were found between Tiahura and Vaipahu stations (SNKs, ). The percentage of highly affected genera was positively and significantly correlated with the percentage of bleached colonies at the nine stations (Pearson's , ).
In Moorea, bleaching events caused by thermal stress were reported in 1984, 1987, 1991 and 1994, and corresponded to periods when sea-surface temperature rose above 29.2 °C for at least one month (Fig. 5). No bleaching event was observed in 1998, despite thermal conditions exceeding this theoretical threshold [7]. During the bleaching event in 2002, sea temperature did not vary significantly among depths (8, 14, and 25 m) at Tiahura prior and during the sampling period (ANOVA, , ; Fig. 6). Differences among depths were nearly always lower than 0.1 °C (Fig. 6).
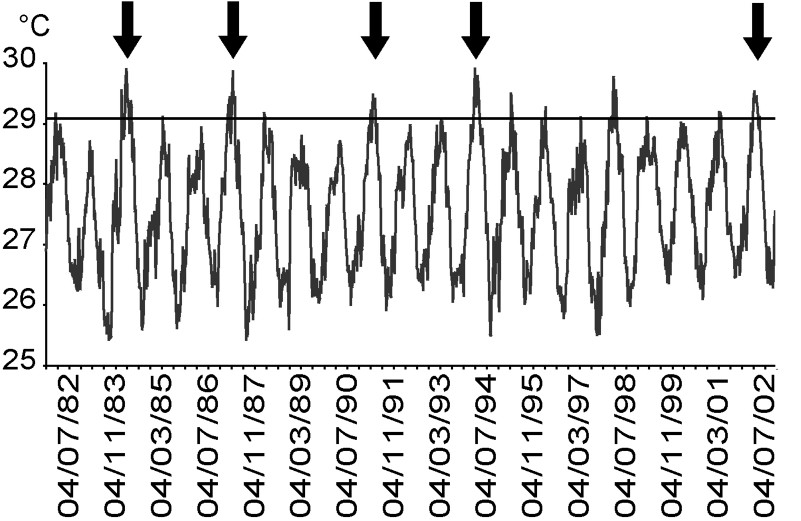
Weekly sea-surface temperature data for Moorea and Tahiti (two Society Islands separated by less than 20 km). Dates given as dd/mm/yy. IGOSS-nmc data by courtesy of the Lamont-Doherty Climate Center at Columbia University (http://rainbow.ldgo.columbia.edu). Arrows indicate major bleaching events caused by thermal stress reported in Moorea (1984, 1987, 1991, 1994, and 2002). Horizontal dotted line indicates the theoretical thermal threshold (29.2 °C; [7]).
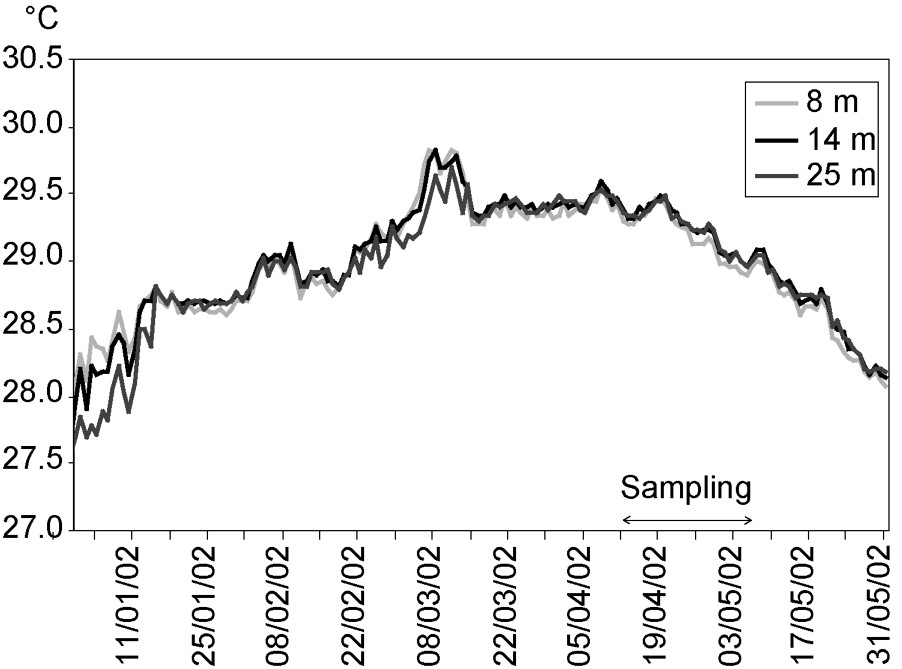
Mean daily sea-water temperature on the outer slope of Tiahura, at 8, 14, and 25 m from January 1st to May 31st, 2002. Data collected with thermographs measuring temperature every hour with a precision of 0.01 °C.
4 Discussion
Moorea reefs, like many worldwide, have been subjected to several large-scale disturbances over the last few decades [10,17,45,60–64]. In particular, major coral bleaching events occurred in Moorea and other Society islands in 1984, 1987, 1991, and 1994 [33,35,57,62,65]. Most of these bleaching events corresponded to periods when sea-surface temperatures (SST) rose above 29.2 °C [35] for at least one month (Fig. 5). However, coral bleaching was absent at Moorea during the 1998 event that affected coral assemblages on some islands of the Tuamotu Archipelago [24]. This absence has been attributed to a variation in the cloud cover that could have reduced solar-radiation stress [39].
Results indicate that the 2002 bleaching event was one of the most severe ever recorded in Moorea. All genera encountered showed signs of bleaching, and the proportion of bleached colonies varied between 39–72% among the nine stations sampled, with a mean of 55%, including 39% partially bleached and 16% completely bleached colonies. However, no recently dead colonies were observed during our survey, as it was conducted only a few weeks after the onset of the bleaching event. When it occurs, mortality generally occurs several weeks after the first signs of bleaching are observed [14,57].
Different coral genera bleached at different levels, a pattern that has been reported frequently [30,32,66] (see also Lenihan and Adjeroud, unpublished data). Genera specific differences in the susceptibility to bleaching have generated the terms ‘loser’ and ‘winner’ coral species [29,30]. Among major constituents of coral assemblages at Moorea, Acropora, Montastrea and Fungiids can be classified as losers, that is, highly susceptible genera ( of bleached colonies). These genera were also recorded as highly susceptible corals during the 1991 and 1994 bleaching events in Moorea [33,35,57]. In contrast, Acanthastrea, Pavona, Porites and Psammocora were more resistant to bleaching, with less than 25% bleached colonies, and less than 5% completely bleached colonies. Pavona and Porites are commonly described as less susceptible or even unaffected genera [24,33,35,57]. Other genera, including Leptoseris, Montipora, Leptastrea and Pocillopora bleached at intermediate levels. It is interesting to note that bleaching resistance capabilities of coral genera do not seem to be linked with phylogeny, the four most resistant genera in Moorea belonging to four different families and three different suborders [67]. Resistant genera or ‘winners’ in Moorea have massive or encrusting growth forms, which is similar to other patterns observed by Loya et al. [30]. Thick-tissued species are thought to be more resistant to bleaching because of the photo-protective capacity of the tissue [30]. However, highly susceptible genera in Moorea include massive (Montastrea spp.) as well as other growth form species (Acropora spp., Fungiids), a pattern that differs from that of Loya et al. [30], who found that ‘losers’ were all branched or plate-like species. However, our survey focused on susceptibility during the bleaching episode and did not address mortality at the end of the event.
The proportion of partially vs. completely bleached colonies varied among genera. Genera such as Montastrea showed a high level of completely bleached colonies, whereas others, such as Psammocora or Cyphastrea, did not completely bleach, although many coral of these two genera did partially bleach. These differences should be taken with caution, as our study was carried out at the beginning of the event, and it is therefore possible that some coral genera may delay their responses to bleaching, which probably reflects different ‘bleaching dynamics’ among coral taxa [57].
Our results indicate that overall bleaching is highly variable across locations and water depths, which supports results found elsewhere [21,29,68]. We found that bleaching rate (all genera pooled) varied with water depth with higher levels of bleaching at deeper (12 and 18 m) rather than at shallower depth (6 m; Table 1; Fig. 2). This depth gradient in bleaching had been observed at some northern locations around Moorea, including Vaipahu, during the 1994 event [35]. We also found differences among locations, with corals at the western location (Haapiti) slightly less affected than the ones at the northern locations (Vaipahu and Tiahura).
Coral taxa had a major effect on the spatial pattern of bleaching in our study (Fig. 4). For Pavona, the bleaching rate was 9.5 times more important at deep stations than at shallow ones. The genus Porites bleached 12 times more frequently at the Tiahura deep station (North coast) than at the Haapiti shallow station (West coast). For Acropora, the bleaching rate ranged from 95 to 100% at all stations, except at the Tiahura shallow station (6 m), where it did not exceed 59%. Spatial variability in the proportion of bleached colonies did not depend on previously reported susceptibility among taxa or growth form, as the three genera with the highest levels of spatial variability in bleaching, Acropora, Porites and Pavona, have been labeled as both susceptible (Acropora) and resistant (Porites and Pavona). Similarly, the three genera showing no spatial variation have also been labeled both susceptible and resistant. Spatial variability of bleaching is not driven by growth form either, as both spatially variable and stable genera have very different growth forms.
Several other factors may explain the spatial variability of bleaching impacts at the spatial scales examined in our study. Spatial variations of local assemblage composition has been proposed to explain the variation in bleaching response [29,30,35]. The proportion of highly affected genera in the local assemblage (i.e. percentage of colonies belonging to genera which exhibited a mean of 90% or more bleached colonies in our stations) was significantly and positively correlated to bleaching intensity among the nine sampling stations. Spatial variability in bleaching impacts may also reflect differences in local environmental conditions, such as hydrodynamic regime. We found that bleaching levels were lower at shallow station compared to deeper ones for the total assemblage, and for most of the genera. Shallow stations were located close to the reef front where oceanic waves break. Bleaching was also relatively lower at Haapiti than the other sites. This pattern may have been driven by the fact that Haapiti is exposed to higher wave intensity and frequency, and higher mean flow speeds than the other sites [59]. These patterns support previous studies that have demonstrated that water movement is a factor reducing stress during bleaching events [38,40,69] (see also Lenihan and Adjeroud, unpublished data, but see [70]). It is hypothesized that exposure to water motion may influence the concentration of peptides that play a role in protection from UV irradiance [37]. Exposure to swells may also be responsible for increasing the elimination of toxic oxygen radicals produced during bleaching and which are accountable for oxidative stress [10,38]. Variations in bleaching levels with depth could also be a result of seawater temperature differences. However, during the weeks prior to our study, seawater temperature at Tiahura did not vary significantly with depth (8, 14, and 25 m) and any difference was always lower than 0.1 °C (Fig. 6). As a consequence, variations in bleaching level with depth could not be successfully explained by variations in water temperature at this scale.
It has also been suggested that spatial variability in bleaching impact may reflect differences in the coral/algae association both within and among coral genera, as suggested by several authors [29,44,45]. Variability in algal assemblages could explain differences in response to stress observed among conspecific corals living at variable depths [2,71]. Coral/algae associations displaying more resistant genotypes (adaptation) and/or phenotypes (acclimation) may be more numerous in shallow waters, which are subjected to higher solar irradiances, thus decreasing the severity of bleaching [23,25,43,72] (but see [48]).
In terms of the proportion of affected colonies, the overall situation during this 2002 bleaching event was comparable with the last two previous bleaching events that have affected Moorea in 1991 and 1994 [33,35,57]. The amount of bleaching (all taxa pooled) at intermediate depths (12–13 m) at Tiahura was 55% in 1991, 47% in 1994, and 58% in 2002. At this location, Acropora was among the most susceptible genera during all three bleaching events, with more than 95% of colonies bleached. The amount of bleaching of Pocillopora colonies was slightly higher in 2002 (49%), compared with 1991 (35%) and 1994 (32%). For Porites, bleaching was highest in 1991 (45%), compared with 2002 (25%), and to 1994, where bleaching was considerably lower (9%). Thus, we found no clear temporal decrease in bleaching susceptibility among the last three bleaching events and, therefore, the pattern at Tiahura provides no support for the hypothesis that coral in the genera Acropora, Pocillopora and Porites located in the depths and sites that we examined acclimate or adapt to successive disturbances.
In conclusion, our results clearly demonstrate that bleaching intensity may be highly variable at small spatial scales (i.e. local or within-reef scale). This may be partly explained by the interactive effects of extrinsic factors such as hydrodynamic conditions (i.e. exposure to swells), and by intrinsic factors such as differential adaptation and/or acclimation of the coral/algal association. In terms of sampling strategy, our results demonstrate the importance of this small-scale variability, including variable locations, habitats, and depths, when accurately describing the extent of a bleaching event and comparing the impacts of successive episodes. The marked differences in susceptibility among coral taxa reported here, and the species-specific mortality associated with bleaching events and other large-scale disturbances, such as cyclones and Acanthaster planci outbreaks in French Polynesia [33,35,57,62], also imply that coral assemblages may change over successive disturbances, and emphasize the need for monitoring programs to examine these changes in coral community structure and to estimate their resistance and resilience [41].
Acknowledgements
We thank James Algret and Yannick Chancerelle from the ‘Centre de recherches insulaires et observatoire de l'environnement’ (CRIOBE) for logistic support and diving assistance, and Bernard Salvat, Claude Combes, and anonymous reviewers for their constructive remarks. We would like to acknowledge the financial support of the Total Foundation and the French ‘Ministère de l'Outre-Mer’.


