1 Introduction
The Manx shearwater Puffinus puffinus group historically included eight subspecies [1,2], now considered as separate species. All have reduced distributions and they are endemic, except the Manx shearwater. The most recent taxonomic revision concerned the two Mediterranean species [3–6], and using ecological, morphological and genetic traits, elevated the yelkouan shearwater P. yelkouan and the Balearic shearwater P. mauretanicus to the rank of distinct species. While the Manx shearwater is widely distributed throughout the Atlantic Ocean, the overall breeding area of the yelkouan and Balearic shearwaters is reduced to the Mediterranean Basin and, to a lesser extent, the Black Sea. While the Manx shearwater has been thoroughly studied for many years [1] and the Balearic shearwater has recently attracted considerable attention from conservation biologists [7,8], very few studies have dealt with the little known yelkouan shearwater. The yelkouan shearwater breeding range is thought to extend from Minorca (Balearic archipelago [8]) to the Bulgarian islands in the Black Sea; the breeding population is estimated at 15 000 pairs [9].
Due to its new species status and lack of studies, little is known about yelkouan shearwater ecology and conservation threats. Other related species are known to be negatively affected by introduced predators [10–12] or loss of suitable habitat [12,13]. Little is known about the impact of these factors on the yelkouan shearwater, but introduced mammals seem to be among the main onshore factors threatening this species [14–16]. Filling the knowledge gaps for this species is sorely needed as even basic parameters of its ecology are lacking, particularly habitat requirements. Breeding habitat selection, protecting both adults and young from environmental conditions and predation, appears to be a strong factor in bird survival and reproduction, particularly for Procellariiforms among those most species nest in cavities or in burrows [1,2,17].
This study was undertaken to examine, for the first time, the general pattern of nest-cavity selection by the yelkouan shearwater. Our particular aim was to characterize nest-cavities selected by this species and assess the influence of these characteristics on its breeding success. This study was conducted on the Hyères archipelago (2890 ha overall), off the southeastern coast of France, which houses 360–480 breeding pairs. This population is particularly interesting because mostly settled in a nature reserve area, but its breeding numbers are quite small in comparison with taxonomically close species (e.g., Manx shearwaters on Rhum Island: 120 000 pairs on 10 700 ha [17]; black-vented shearwaters Puffinus opisthomelas on Natividad Island: 76 500 pairs on 1000 ha [13]). Here, we tested two hypotheses for this limited population: there is not enough suitable breeding habitat available to allow a larger population, and/or the yelkouan shearwater suffers competition from the sympatric Cory's shearwater Calonectris diomedea, known to be a potential strong competitor for smaller burrowing Procellariiforms [18]. The analysis of the nest-cavity selection by yelkouan shearwaters allowed us to compare (1) occupied versus unoccupied nest cavities, (2) yelkouan versus Cory's shearwater nest cavities, and (3) successful versus unsuccessful nest cavities. These comparisons correspond to the following primary questions. (1) Is breeding habitat saturated and affected by intra-specific competition [1]? (2) Do yelkouan shearwaters compete for nest-cavities with Cory's shearwaters? (3) What is the vulnerability to external environment and predation at the breeding site [17]?
2 Materials and methods
2.1 Study area
This study was conducted on four shearwater colonies located on Port-Cros and Porquerolles, two adjacent Mediterranean islands in the Hyères archipelago (southeastern coast of France; Fig. 1). Port-Cros (640 ha) and Porquerolles (1250 ha) islands are nature reserves managed by the Port-Cros National Park. Two hundred and ten to 270 pairs of yelkouan shearwater and 130 to 160 pairs of Cory's shearwater nest along the coast of both islands, on indented cliffs and fallen boulders, with varying degrees of vegetation. Colonies are sparse, with low densities of birds compared with colonies of most Procellariiforms, and their access is very difficult due to dense vegetation, unstable, siliceous substrate, and steep slopes. Yelkouan shearwaters generally nest in natural rock cavities (e.g., among fallen boulders) or pre-existing crevices (our personal observation). Because soil depth is limited and less of 50% of nests are in burrows excavated by yelkouan shearwaters, we preferred the term ‘cavity’ to ‘burrow’ in this study. Black rats Rattus rattus and feral cats Felis catus were introduced several hundred years ago [15,19,20] and purportedly constitute an important direct and/or indirect threat to yelkouan shearwaters [14–16]. The yellow-legged gull Larus michahellis is a superabundant species in the Mediterranean [21] and as similarly sized gulls (the western gull Larus occidentalis) have been shown to negatively affect similarly-sized shearwaters (the black-vented shearwater; [22]), it can potentially have a negative impact on Procellariiforms [23,24]. This species has colonized the entire coast of the study islands since the early 20th century [25], and predation on yelkouan shearwaters has been observed [26].
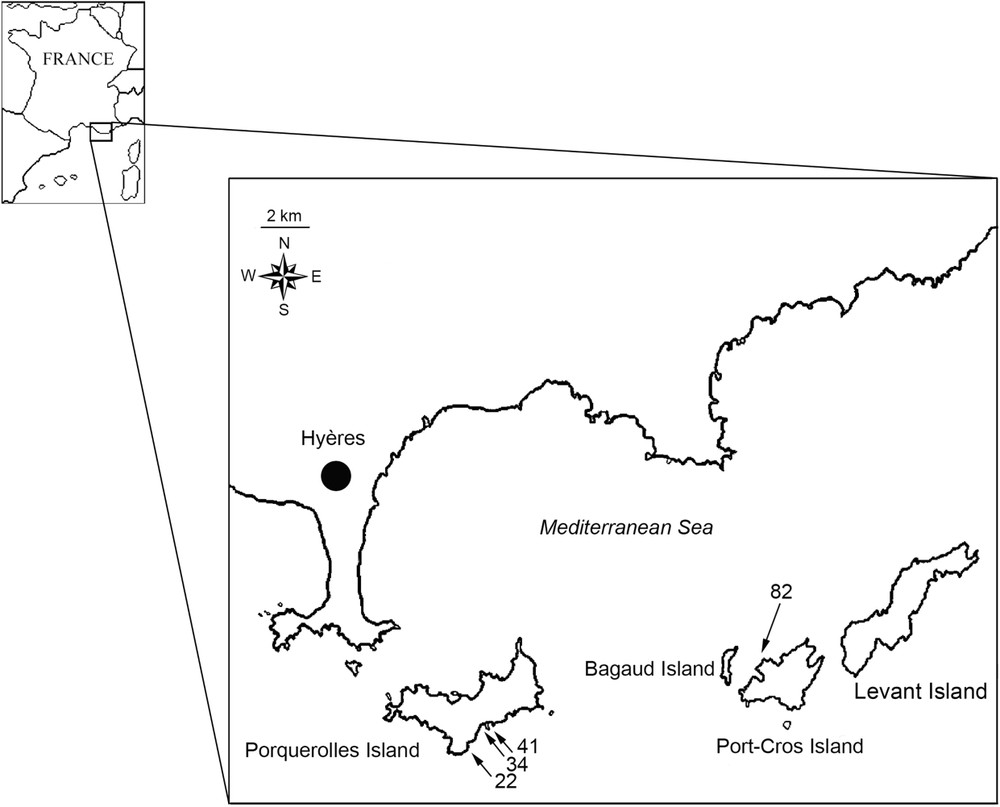
Study site map indicating study colonies with the number of cavities suitable for shearwater nesting.
2.2 Cavities suitable for shearwater nesting
The cavities studied (i.e., natural cavities, crevices, and burrows) were considered suitable for shearwater nesting when (1) cavity depth and (2) entrance height were sufficient to entirely house and allow the passage of the smaller of the two species, i.e., the yelkouan shearwater, which is 30–36-cm long [27] and 6–6.4-cm high ( on average, , unpublished data). (3) The maximum entrance dimension (height or width ) was also considered, to provide protection for the yelkouan shearwater according to its maximum length. One hundred and seventy-nine cavities found in the four colonies surveyed were thus considered as suitable for shearwater nesting and monitored for four entire breeding seasons (Fig. 1).
2.3 Cavity monitoring
The 179 study cavities were monitored throughout four breeding seasons from 2003 to 2006 (from mid-March to the end of July for the yelkouan shearwater [14] and from mid-May to mid-October for the Cory's shearwater [28]) to determine cavity selection and to measure the breeding success of the yelkouan shearwater.
Because many cavities were deep, winding, and nesting chambers impossible to directly observe, the following steps were used to optimize bird detection (failure at one step justified continuing to the next): (1) direct chamber observation, (2) chamber observation using an infrared mini-camera when entrances were high enough and tunnels not too winding to allow the camera through, (3) shearwater response stimulation to pre-recorded call-playback (even if shearwater response to call-playback is not 100% [29]). The second step is non-traumatizing because infrared light makes the video system invisible [30]. Each cavity was monitored six to nine times during the breeding cycles (mid- and end-laying and hatching periods, 15 days before the beginning and at the end of fledging periods, in relation to yelkouan and Cory's shearwater breeding cycles). These repeated visits allowed us to observe new signs of presence and increased the chances of both response to call-playback and chick observation.
Cavities were considered as occupied by shearwaters when we observed (1) feathers, guano, footprints or species-specific odour at the entrance regularly throughout the breeding season, (2) at least one adult call-playback response or direct observation in daytime more than half-way through the laying period for each species (i.e., from early April for the yelkouan shearwater and from early June for the Cory's shearwater), and/or (3) signs of egg or chick presence (eggs or chicks themselves, shell remains, and chick calls or down [29,31]). Out of the 179 cavities monitored, 84 were unoccupied, 73 occupied by yelkouan shearwaters during at least one breeding season and 31 occupied by Cory's shearwaters during at least one breeding season. Although every effort was made to optimize monitoring, the breeding success of few yelkouan nest-cavities remained uncertain because cavity layouts did not permit incubating chamber observation and because there were no further signs of presence. The remaining yelkouan shearwater reproductive cavities were thus taken into account in the analysis of the influence of cavity selection on breeding success. An occupied nest-cavity was classified as successful or unsuccessful based on whether the egg led to fledging or not.
2.4 Cavity characterization
To characterize the 179 suitable nest-cavities, several topographical, physical, and biotic variables were measured (Table 1). All the percentage covers around the cavity entrance were visually estimated using 5%-intervals. Slope (s) at the cavity entrance was calculated with a trigonometric formula: , where the adjacent side (AS) was fixed at a 1-m value and the opposed side (OS) was measured with a plumb level, a plumbline, a metallic tape measure and a 1-m rigid stick, which allowed us to fix the AS-value at 1 m. Cavity dimensions (depth, maximum entrance height and width) were measured using a 3-m metallic tape measure. The types of protection taken into account were slabs or branches that concealed the cavity entrance, being just above or in front of it, or increased substrate stability. Colony edges were defined as the outermost cavities within a colony. Distances to nearest neighbours and colony edge were measured with a 20-m tape measure.
Variables recorded for each cavity
| Variables | Description |
| – Vegetation covers | Covers of herbaceous, shrub and tree within 1-m radius around cavity entrance (%) |
| – Substrate covers | Covers of blocks, stones, gravel and sand within 1-m radius around cavity entrance (%) |
| – Physical cavity characteristics | |
| Altitude | Altitude of cavity entrance (m) |
| Entrance width | Maximum width of cavity entrance (m) |
| Entrance height | Maximum height of cavity entrance (m) |
| Depth | Maximum length from entrance to back of cavity (m) |
| Slope | Minimum slope within 1-m radius around cavity entrance (°) |
| – Protection of breeders | |
| Tunnel presence | Absent (0), present (1) |
| Tunnel type | Straight (0), winding (1) |
| Protection presence | Absent (0), present (1) |
| – Neighbours | |
| Neighbour distances | Distances to the nearest unoccupied cavities, cavities occupied by conspecifics and cavities occupied by other species birds (m) |
| Neighbour numbers | Numbers of cavities occupied by conspecifics and cavities occupied by other species of birds within a 3-m radius around cavity entrance |
| Distance to colony edge | Minimum distance to the nearest colony edge (m) |
2.5 Nest-cavity selection analysis
To test species (yelkouan versus Cory's shearwaters) and occupation-success (shearwater-occupied versus unoccupied) differences in nest-cavity selection, we used Kruskal–Wallis tests with Bonferroni-adjusted significance levels to compare vegetation (herbaceous, shrub and tree) and substrate (block, stone, gravel and sand) cover data, and multivariate analysis of variance (Manova) to compare physical variables (altitude, cavity entrance width and height, cavity depth, and slope at entrance). Cavity entrance width and height and cavity depth were log-transformed [32]. In order to pinpoint differences detected among datasets, unplanned comparisons were performed using post-hoc Scheffé tests. Finally, cavity-entrance protection presence, tunnel presence, and type were analysed by -independence tests and G adjustment tests, using Williams' corrections to detect protection availability effects on nest-cavity selection in shearwaters [32].
We modelled the characteristics of cavities used by yelkouan shearwaters for nesting using binary logistic regressions [33], following the modelling procedure described by Franco et al. [34]. To assess suitable cavity availability (i.e., cavities with characteristics similar to cavities used by nesting yelkouan shearwaters), we followed the procedure described by Kesler and Haig [35], comparing values of the predicted probability of cavity selection for nesting () between occupied and unoccupied cavities. Similar values for occupied and unoccupied cavities indicate unsaturated nesting habitat with unused, suitable cavities. Conversely, two distinct sets of , with unoccupied cavities receiving low values and occupied cavities receiving high values, would suggest that nesting habitat in the study area was limited in abundance [35]. In this analysis, we used five categories for covers (1 = 0–20%, 2 = 21–40%, 3 = 41–60%, 4 = 61–80%, 5 = 81–100%), seven categories for entrance dimensions (1 = 0–0.1 m, 2 = 0.11–0.2 m, 3 = 0.21–0.3 m, 4 = 0.31–0.4 m, 5 = 0.41–0.5 m, 6 = 0.51–0.6 m, 7 = 0.61–0.7 m), and six categories for cavity depth (1 = 0–0.5 m, 2 = 0.51–1 m, 3 = 1.01–1.5 m, 4 = 1.51–2 m, 5 = 2.01–2.5 m, 6 = 2.51–3 m). We grouped tunnel characteristics into one variable (0 = no tunnel, 1 = straight tunnel, 2 = winding tunnel).
2.6 Analysis of the influence of nest-cavity selection on breeding success of the yelkouan shearwater
To compare characteristics of successful and unsuccessful reproductive nest-cavities and to pinpoint characteristics affecting breeding success, a two-way (breeding success and season) Manova with post-hoc Scheffé tests was performed on altitude, minimum cavity entrance dimension chosen between height and width, cavity depth, slope at entrance, numbers of neighbours (conspecifics and Cory's shearwaters) and distance to colony edge. Altitude, minimum cavity entrance dimension and cavity depth were log-transformed [32]. Numbers of neighbours were square-root-transformed. Kruskal–Wallis tests were used to analyse heteroscedastic data (vegetation and substrate covers, tunnel characteristics).
2.7 Analysis of the influence of neighbours on cavity occupancy and on breeding success of the yelkouan shearwater
To investigate attractive and repulsive effects of intra- and inter-specific neighbours in nest-cavity selection, paired Wilcoxon T-tests were used to compare distances to the nearest unoccupied and occupied cavities. As Cory's shearwaters arrive in colonies later than yelkouan shearwaters, neighbouring cavities occupied by Cory's shearwaters were considered unoccupied for yelkouan shearwaters. Distances to the nearest conspecific neighbour were compared between the two species using an unpaired Mann–Whitney U-test. U and T statistics (adjusted for ties) are given with a Z-value (normal distribution variate value), and the respective P-value [36]. Cavity densities on colonies were estimated using 42 square plots (side = 10 m, area = 100 m2).
Throughout the four breeding seasons, we searched for the signs of intra- and inter-specific competition for cavities already observed in other Procellariiforms [1,18,37,38]: signs of fighting (eggs expelled from cavities, dead yelkouan shearwaters at or near the entrance of a cavity occupied by Cory's shearwaters or conspecifics), aberrant behaviour (eggs laid on the surface or two eggs laid in the same cavity), intense digging or eviction of yelkouan shearwater breeders by Cory's shearwaters (change in occupant species of a cavity).
Statistical analyses were performed using Statistica 6.0, except for - and G-tests. Significance levels were established at and we report means ± standard deviations.
3 Results
3.1 Nest-cavity selection
Shearwaters occupied in average of the cavities suitable monitored during the four study breeding seasons (yelkouan shearwaters: , Cory's shearwaters: ). We describe the nest-cavity characteristics of the yelkouan shearwater in Table 2.
Nest-cavity characteristics selected by the yelkouan shearwater and comparison with the Cory's shearwater and unoccupied nest-cavity
| Values | Statistical tests | |||||
| Variables | Yelkouan shearwater () | Cory's shearwater () | Unoccupied () | Y vs. C | Y vs. U | C vs. U |
| – Vegetation covers (%) | ||||||
| Herbaceous | 20.4±24.8 | 31.5±34.7 | 23.8±25.4 | ns | ns | ns |
| [0–90] | [0–95] | [0–90] | ||||
| Shrub | 17.9±27.3 | 19.5±31.4 | 26.2±30.5 | ns | ns | ns |
| [0–100] | [0–100] | [0–100] | ||||
| Tree | 16.8±33.3 | 15.8±30.6 | 11.1±27.3 | ns | ns | ns |
| [0–100] | [0–100] | [0–100] | ||||
| – Substrate covers (%) | ||||||
| Blocks | 57.5±24.3 | 48.7±27.3 | 58.9±19.6 | ns | ns | ns |
| [5–100] | [0–95] | [0–90] | ||||
| Stones | 10.3±7.5 | 11.7±9.1 | 10.5±7.4 | ns | ns | ns |
| [0–40] | [0–30] | [0–30] | ||||
| Gravel | 11.9±10.2 | 15.0±10.6 | 9.6±7.3 | ns | ns | ** |
| [0–40] | [3–45] | [0–45] | ||||
| Sand | 20.3±17.1 | 24.2±19.1 | 20.0±16.2 | ns | ns | ns |
| [0–85] | [0–85] | [0–70] | ||||
| – Physical cavity characteristics | ||||||
| Altitude (m) | 10.3±6.3 | 15.0±6.2 | 11.6±6.4 | ** | ns | * |
| [1.5–33] | [4–30] | [2–31] | ||||
| Entrance width (m) | 0.21±0.09 | 0.22±0.08 | 0.22±0.13 | ns | ns | ns |
| [0.07–0.43] | [0.1–0.38] | [0.08–0.7] | ||||
| Entrance height (m) | 0.16±0.07 | 0.19±0.10 | 0.15±0.07 | ns | ns | * |
| [0.075–0.37] | [0.09–0.49] | [0.075–0.54] | ||||
| Depth (m) | 1.08±0.50 | 1.00±0.37 | 0.74±0.28 | ns | *** | *** |
| [0.4–3] | [0.65–1.9] | [0.34–3] | ||||
| Slope (°) | 24.2±12.3 | 22.3±10.6 | 24.4±13.6 | ns | ns | ns |
| [0–50.2] | [0–50.2] | [0–50.2] |
The physical characteristics of cavities occupied by yelkouan shearwaters significantly differed from those of cavities occupied by Cory's shearwaters and unoccupied cavities (, , Table 2). Yelkouan shearwaters selected the deepest cavities and their nest-cavities were located at a lower altitude than nest-cavities of Cory's shearwaters.
Yelkouan shearwater cavity occupancy was significantly associated with tunnel presence and type (, ), but not with entrance protection (, ). Moreover, tunnel occurrence in yelkouan shearwater-occupied cavities was higher than expected when compared with overall tunnel occurrence (93.2% vs. 76.6%, , ). Winding tunnels were more frequent than expected in cavities occupied by yelkouan shearwaters (35.3% vs. 22.9%, , ).
3.2 Nest-cavity availability for the yelkouan shearwater
The variables that significantly influenced cavity occupancy in the univariate logistic regression analyses were tunnel characteristics (Wald = 19.0, ) and cavity depth (Wald = 6.4, ). The Pearson goodness-of-fit test provided no evidence of model inadequacy (, ). Both backward and forward procedures yielded the following model: cavity depth + 1.86 tunnel characteristic. Thus, there is a positive relationship between cavity depth and tunnel winding, and the chance of selection as a nest-site. Data for all cavities were entered into the inferential model, and resulting distributions for occupied and unoccupied cavities overlapped substantially (Fig. 2).
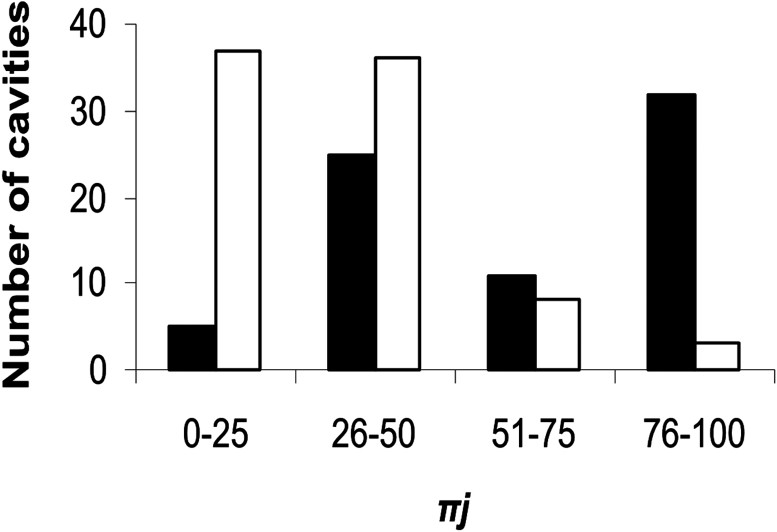
Distributions of predicted probability of cavity selection for nesting by the yelkouan shearwater () for occupied (black) and unoccupied (white) cavities, based on values for the logistic regression model.
3.3 Influence of nest-cavity characteristics on breeding success of the yelkouan shearwater
Breeding success for yelkouan shearwater cavities was in average for the four study breeding seasons (hatching success and fledging success).
Significant success-related differences were found among yelkouan shearwater nest-cavities (, ). The Manova did not reveal breeding season (, ) or breeding success-season interaction (, ) effects. Successful nest-cavities () were deeper ( vs. , ) and had higher minimum entrance dimensions ( vs. , ) than unsuccessful nest-cavities (). Successful nest-cavities exhibited higher block covers ( vs. , , ), lower sand covers ( vs. , , ) and more winding tunnels ( vs. , , ).
3.4 Influence of neighbours on cavity occupancy and on breeding success of the yelkouan shearwater
For yelkouan shearwaters, distances to the nearest conspecific were significantly higher than distances to the nearest cavities remaining unoccupied (Table 3). Cory's shearwater cavities were nearer to conspecific cavities than yelkouan shearwater cavities. Yelkouan shearwaters nested closer than Cory's shearwaters (, , [], ).
Comparison of distances (m) to the nearest unoccupied, conspecific and other species cavities for the two shearwaters
| Neighbour burrow occupancy | Statistical tests | |||||
| Unoccupied | Conspecific | Other species | U vs. C | O vs. C | O vs. U | |
| Yelkouan shearwater | 2.2±2.8 | 3.9±3.2 | – | ** () | – | – |
| Cory's shearwater | 4.7±5.7 | 4.9±4.8 | 10.6±18.1 | ns () | ns () | * () |
Cavity density appeared to be at the lower range recorded for other Puffinus species (Table 4). Yelkouan shearwater cavity occupancy rate was remarkably low, being about half that of other Puffinus species. No signs of fighting or aberrant behaviour were observed during the study. Only eleven new cavities due to digging were observed during the four breeding seasons. Three possible cases of eviction of yelkouan shearwater breeders by Cory's shearwaters were observed during the 2005 and 2006 breeding seasons. Finally, successful and unsuccessful yelkouan shearwater nest-cavities did not significantly differ in the number of neighbours.
Burrow (or cavity) density and occupancy for the yelkouan shearwater and other Puffinus species
| Species | Burrow density (burrow m−2) | Occupancy (%) | References |
| P. yelkouan | 0.082 ± 0.042 | 33±3.7 | This study |
| P. opisthomelas | <0.083 ± 0.07 | 66.9 | [13] |
| P. huttoni | 0.57 ± 0.42 | 70±12 | [39,40] |
| P. puffinus | 0.28 ± 0.21 | 58±22 | [41] |
| P. pacificus | 0.21 ± 0.03 | 48±3 | Modified from [42] |
4 Discussion
4.1 Nest-cavity selection by shearwaters
Several environmental parameters are known to influence greatly nest-site selection and reproductive success in seabirds. Vegetation and substrate cover, height and type are generally of importance because they provide protection from predators and from inclement weather conditions (e.g., [43–45]). They also mitigate neighbour interactions by reducing visibility between individuals [46,47]. Substrate type and slope also affect breeding success by influencing egg breaking or rolling and chick falling risks [48]. This study reveals that yelkouan shearwaters select cavities providing a high degree of protection and of concealment, occupying the deepest cavities available, preferably with winding tunnels. These characteristics probably reduce light penetration, stabilize temperature and humidity level, and increase protection from rain, wind, and predators (increased concealment and reduced accessibility) [17,18,43,45]. Cory's shearwaters select cavities located at a higher altitude and with a higher gravel cover. High-altitude selection seems to be difficult to explain, but this may facilitate take-off, as this species has a relatively large wingspan (more than 1 m). Cory's shearwaters often place stones or gravel at the entrance of their nest-cavity [18], which can explain the increased gravel cover. These results suggest that nest-cavity selection differs between yelkouan and Cory's shearwaters, as regards both physical nest characteristics and the degree of nest entrance protection.
4.2 Evaluation of breeding habitat saturation
The studied yelkouan shearwater breeding population has been stable for 20 years [49], with a low density of breeders. Though breeding habitat saturation is known to limit populations, our results indicate a low overall rate of cavity occupancy (33%) and an abundance of nest-like cavities in the study area, suggesting that cavity availability is not limiting for yelkouan shearwaters. Moreover, yelkouan shearwaters can excavate burrows if necessary, but we found only few signs of digging during four years of study. Together, these results suggest that although nest-sites may not be lacking, yelkouan shearwater populations and reproduction may be limited by other external factors on shore and/or at sea.
4.3 Relationship between cavity characteristics and breeding success
Yelkouan shearwater breeding success varies with nest-cavity characteristics: breeding success is increased in cavities that are deeper, with higher entrances, block covers, more winding tunnels and lower sand covers. Deeper cavities with winding tunnels allow increased protection by decreasing bird detection and nest accessibility [2,50]. They may also be chosen for increased insulation and better thermoregulation, protection from inclement weather conditions, and decreased light penetration [17,45,51]. The higher block and lower sand covers may induce a more stable substrate and a better thermoregulation [43]. The higher minimum entrance dimension of successful cavities may lead to an easier passage of birds and thus to decreased effort and more rapid entry. Finally, several other factors can affect breeding success in Procellariiforms, such as age, body size, and experience of breeders [52–54].
4.4 Evaluation of competition for nest-cavities
Yelkouan shearwaters often breed in mixed-species colonies with Cory's shearwaters, a larger and more widely distributed species, which is known to be a potential strong competitor for small burrowing Procellariiforms, particularly in large colonies [18]. However, we did not find any evidence of strong competition for cavities, either between yelkouan conspecifics or between yelkouan and Cory's shearwaters. First, cavity densities are quite small, but in the range of values observed for Puffinus species (Table 4). However, the occupancy rate of cavities by the yelkouan shearwater is approximately half that recorded for close species. As a result, the density of breeders is remarkably low, probably decreasing competition. Second, yelkouan shearwaters choose to nest at a high distance from conspecifics, although there are empty cavities available in closer proximity to them; it has been suggested that burrowing Procellariiforms prefer to nest in crowded situations [55]. Third, Cory's shearwaters occupy cavities farther from yelkouan shearwaters than from unoccupied cavities. These two data imply reduced interactions between yelkouan shearwaters and both conspecifics and Cory's shearwaters. Fourth, the yelkouan shearwater and the Cory's shearwater select different types of nest-cavities, thus limiting the risk of competition. Fifth, no effect of the neighbour number is observed on the breeding success of yelkouan shearwaters, whereas this is observed in dense and large colonies of Procellariiforms [18]. Sixth, we do not observe strong unusual behaviours suggesting intra- and inter-specific competition for cavities, as has already been observed in other Procellariiform species [1,18,38].
5 Conclusion
Yelkouan shearwater breeding habitat selection is described for the first time in this study and we found a surprisingly low rate of cavity occupancy. Since we demonstrated that cavities with nest-like characteristics are available and that yelkouan shearwaters little compete with Cory's shearwaters for these cavities, habitat saturation and competition are unlikely to be underlying factors in the population size and dynamics of the yelkouan shearwater. Other factors likely to affect population size of this species are predator pressure [16,56], mortality at sea and food resource availability [7]. These alternative explanations for the limited population of yelkouan shearwaters are worthy of additional research in order to design adequate management strategies.
Acknowledgements
Funds and support were provided by a Life Nature EU project (LPO–PACA, IMEP–CNRS, Port-Cros National Park [PCNP]), the PCNP, the ‘Conseil régional PACA’ and DIREN PACA. We are very grateful to all those from PCNP and IMEP–CNRS who helped us during fieldwork. We thank C.M. Suehs for improving the English and M. Brooke for valuable comments on the manuscript.


