1 Introduction
The Bay of Algiers (Algeria, southwestern Mediterranean), with its 3.5 million inhabitants, is characterised by strong demographic expansion and coastal development and harbours the main Algerian port facilities. However, it lacks working sewage-treatment plants, and most sewage waters, both of domestic and industrial origin, are directly discharged into the sea via a main outflow (El Harrach) and a number of smaller outflows scattered along the coast. As a result, coastal waters can be considered as polluted.
Unfortunately, chemical data about this pollution are scarce and when available, date from the 1970s and 1980s [1–3].
Macrophyte benthic communities are considered as good biological indicators of marine pollution. Some species, such as Cystoseira spp., are very sensitive to urban pollution, while other, such as Ulva spp. [4–12], are enhanced. In addition, macrophyte benthic communities constitute one of the ecological quality elements required to implement the Water Framework Directive, the legislation targeting the sustainable management of the European Community aquatic environment [13–15].
In the Mediterranean Sea, a number of phytosociological samples of the subtidal fringe (i.e., 10 to 50 cm below the mean sea level) are available from a variety of sites localized in most parts of the western basin, ranging from highly polluted through pristine waters [12,16–19]. Sample size (), sorting method and species abundance estimation, through the Braun-Blanquet scale, are identical.
In this study, we sampled the subtidal fringe macrophyte communities of the Algiers (Algeria) region, using strictly the same methods as in the above-mentioned studies, at sites assumed to correspond to high-, middle- and low-pollution level, in order to place them within the context of other Mediterranean regions. This was an attempt to calibrate these proxy records of pollution level in the region of Algiers against well-known regions, especially that of Marseilles (Provence, France).
2 Material and methods
Samples were collected at four sites: Cherchell (36°37′N, 2°12′E), a small town of 45 000 inhabitants, 80 km west and upstream of Algiers, according to the dominant current that runs eastwards, Alger-Plage (36°45′N, 3°12′E), Bordj-El-Kiffan (36°46′N, 3°13′E), and Tamentfoust (36°48′N, 3°14′E), the three latter sites in the Bay of Algiers, east of the centre of the city. These sites are under exposed conditions: the vertical range of the mid-littoral zone (as defined by Pérès and Picard, 1964 [20]) is over 50 cm. Six to ten replicates per site were collected on subhorizontal hard substrate, 20–40 cm below the mean sea level (here the upper limit of Corallina elongata) by scrapping all macrophytes present within a frame. Samples were designated as follow: C1 to C10 (Cherchell), A1 to A10 (Alger-Plage), B1 to B6 (Bordj-El-Kiffan), and T1 to T6 (Tamentfoust). In the laboratory, the cover C of macrophytes belonging to Fucophyceae (Chromobionta), Rhodobionta, and Chlorobionta (Plantae) was rated according to the Braun-Blanquet scale [21]: (1), (2), (3), (4) and (5). Species whose cover was negligible (rated + in the Braun-Blanquet scale, and in cover data) were not taken into consideration. The taxonomical nomenclature used is that of [22] and [23–26].
Though samples were actually collected and sorted during four seasons (spring, summer, autumn and winter 2000), only spring samples will be presented here, the analysis of seasonal changes being not the target of the present study.
For comparison purposes, we used samples collected from similar biotopes (depth, slope, vertical range of the mid-littoral zone) at Banyuls-sur-Mer (French Catalonia; sample 282 in [27]), Marseilles (1AC1, 1AC2, 2AC1, 2AC2, 3AC1, 3AC2, 4AC1, 4AC2, 5AC1, 5AC2 in [12]), Giens (Var, France; samples 3 and 6, in [19]: 138–140; sample 1 in [19]: 144–145), Le Brusc (Var, France; sample 2 in [19]: 149–150), Syracuse (eastern Sicily; samples Da, Ea and Fa in [28]: 47–50), Tremiti Islands (Italy; samples on transects 5, 14, and 16 in [29]: 250). Samples were renamed as follow: BA (Banyuls-sur-Mer), M1 to M10 (Marseilles), V1 to V4 (Giens and Le Brusc), S1 to S3 (Sicily), and I1 to I3 (Tremiti Islands). Marseilles samples (M1 to M10) were collected along a pollution gradient, from the outfall of partially treated sewage from 1.5 million inhabitants (M1–M2) to pristine waters (M9–M10). All other samples correspond to more or less unpolluted localities according to water-quality data and/or the presence of species very sensitive to pollution [30].
For data analysis, the five Braun-Blanquet cover classes were converted (when necessary) into the median (percentage cover) of each class, respectively, 2.5, 15, 37.5, 62.5, and 87.5. Ordination of data by a Correspondence Analysis (CA) [31] was used to describe community changes and to identify the possible parameters involved. For each study, three CA were performed, based upon presence–absence, Braun-Blanquet cover classes, and percentage cover, respectively. Finally, a hierarchical classification was carried out, taking as input the presence–absence data. The hierarchical classification was the agglomerative Ward's method based on Euclidean distance, computed from the factorial coordinates. All statistical analyses were carried out using the Statistica software (version 6.1, Statsolft®).
3 Results and discussion
Sixty-six taxa were recorded in the lowest Algerian sites. The diversity points (i.e., the mean number of species per sample) were 9.5 (Cherchell), 9.6 (Alger-Plage), 13.7 (Bordj-El-Kiffan), and 14.8 (Tamentfoust). They are not higher at Cherchell than at the three last localities, localized in the probably much polluted Bay of Algiers (Table 1). Either Cherchell is as polluted as the Bay of Algiers, or the paradigm of reduction of species diversity by pollution [32–34] does not prove correct here. The mean number of species per sample is similar to that recorded in the vicinity of the sewage outfall of Marseilles (France), 14.6, but lower than at a control site characterized by pristine waters, near Marseilles, 25.7 [12].
Macrophyte samples at Cherchell (C) (80 km east of Algiers), Alger-Plage (A), Bordj-El-Kiffan (B) and Tamentfoust (T) (Bay of Algiers). Figures (1 through 5) correspond to the Braun-Blanquet cover classes
| Sites | Cherchell (C) | Alger-Plage (A) | Bordj-El-Kiffan (B) | Tamentfoust (T) | ||||||||||||||||||||||||||||
| samples | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | B1 | B2 | B3 | B4 | B5 | B6 | T1 | T2 | 73 | T4 | T5 | T6 |
| Acrosorium venulosum | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 2 | 1 | 2 | – | 1 |
| Ahnfeltiopsis pusilla | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Apoglossum ruscifolium | – | 1 | 1 | – | – | – | 2 | 2 | – | 1 | – | – | – | – | – | – | – | – | – | – | 2 | 1 | 1 | 1 | – | 1 | – | – | – | – | – | – |
| Boergeseniella fruticulosa | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Bonnemaisonia clavata | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 | – |
| Bryopsis muscosa | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3 | – | 2 | 1 | 1 | – | 1 | 1 | 1 | 1 | 1 | – |
| Callithamnion granulatum | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | – | – |
| Ceramium ciliatum | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | – | – | – |
| Ceramium codii | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | 1 | 2 | – | – | – | – | – | – | – |
| Ceramium echionotum | – | – | – | – | – | – | – | – | 2 | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Ceramium flaccidum | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | 1 | 1 | 2 | – |
| Ceramium rubrum | – | – | – | – | – | – | – | – | – | – | 2 | – | – | – | – | – | – | 1 | 1 | 2 | – | 1 | 1 | – | 1 | – | – | – | – | – | – | – |
| Ceramium siliquosum | – | – | – | – | – | – | – | – | 3 | 2 | 1 | – | – | – | – | – | 1 | 1 | – | 1 | 2 | 1 | 1 | 2 | 1 | – | 1 | – | 2 | – | – | – |
| Ceramium tenerrimum | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 3 | 1 | 1 | – | 1 | 1 | – | – | – |
| Chaetomorpha aerea | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | 1 | 1 | – | – | – | – | – | – | – | – |
| Chaetomorpha mediterranea | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | 1 | – | 2 | – | – | – | – | – | – | – |
| Champia parvula | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | – | – |
| Chondracanthus acicularis | – | 2 | – | – | – | 2 | – | – | 3 | – | – | – | 1 | – | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | 2 | 1 | 1 | – | 2 |
| Cladophora hutchinsiae | 2 | 2 | 2 | 2 | – | 1 | – | – | 1 | 3 | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | 1 | – | – | – | – | – | – | – | – |
| Cladophora laetevirens | 1 | 2 | – | 2 | 2 | – | – | – | 1 | 2 | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | – | 1 | – | 2 | – | 2 | 1 | 1 | 1 |
| Cladophora prolifera | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 2 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Cladophora rupestris | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Codium fragile | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | 2 | 2 | – | – | – | – | – | – |
| Codium vermilara | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 2 | – | – | – | – | – | – |
| Colpomenia sinuosa | – | – | – | – | – | – | – | – | – | – | 2 | 2 | 1 | – | – | 2 | 1 | 5 | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Corallina elongata | 3 | – | 2 | 2 | 2 | 5 | – | – | 2 | 2 | 2 | 2 | 2 | 1 | 3 | 3 | 2 | 3 | 2 | 1 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 2 | 2 | – | – | 2 |
| Cystoseira amentacea stricta | – | 2 | – | – | – | 2 | 5 | 5 | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 | 2 |
| Cystoseira compressa | – | – | – | 3 | – | – | 4 | – | – | 5 | 3 | 2 | – | – | – | 2 | 2 | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – |
| Dictyopteris polypodioides | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | 3 | – |
| Dictyota fasciola | 3 | 3 | – | 3 | 1 | – | 2 | – | 2 | 3 | 2 | 2 | 1 | – | – | 1 | – | – | – | 2 | – | – | – | – | – | – | – | 3 | 2 | 2 | – | – |
| Dictyota spiralis | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – |
| Dipterosiphonia rigens | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | 1 | – | – | – | |
| Enteromorpha compressa | 2 | – | – | – | – | – | – | – | – | 1 | – | 1 | 1 | – | – | 1 | 1 | – | – | – | 2 | – | 1 | – | 2 | 3 | – | – | 1 | – | 3 | – |
| Enteromorpha intestinalis | – | – | 2 | 2 | – | – | 5 | – | – | 3 | 1 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Falkenbergia rufolanosa ⁎ | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | – | 1 | – | – | – | – | – | – | – |
| Feldmannophycus rayssiae | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Gelidium crinale | – | – | – | – | – | – | – | – | – | – | – | 1 | 2 | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Gelidium spinosum | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 | – | – | – |
| Gracilaria bursa-pastoris | – | – | – | – | – | – | – | – | – | – | 2 | – | 3 | – | 1 | – | 2 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Gracilaria dura | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Grateloupia lanceola | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | – | 3 | – | – | – | – | – | – | – |
| Griffithsia opuntioides | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | 1 | – | – |
| Haliptilon virgatum | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | 1 | – |
| Halopteris scoparia | 3 | 5 | 3 | – | 5 | 4 | 3 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Herposiphonia secunda | – | – | – | – | – | 1 | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | – | – | 1 | 1 | – | – | – | – | – |
| Herposiphonia tenella | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | – | 1 | 1 | – | – |
| Heterosiphonia crispella | 1 | 1 | 1 | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Jania adhaerens | 1 | 1 | – | 2 | – | – | – | 1 | 2 | – | – | – | – | 1 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Jania rubens var. corniculata | – | 1 | – | – | 1 | – | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Jania rubens var. rubens | – | – | 1 | 1 | – | 1 | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Lithophyllum byssoides | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 2 | 3 | 2 |
| Lihophyllum incrustans | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – | – | 1 | – | 1 | – | 1 | – | 1 | 1 | – | – | – | – | – | – |
| Osmundea truncata | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | 2 | 1 | – | – | – | – | 1 | – | – | – | – | 1 | – | – | – | – | – | – |
| Padina pavonica | 2 | – | 2 | 1 | 2 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 | – | – | – | – |
| Peyssonnelia squamaria | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 | 1 | 2 | 1 | – | – |
| Plocamium cartilagineum | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | 1 | – | – |
| Polysiphonia sertularoides | – | – | – | – | – | – | – | – | – | – | 1 | 2 | – | – | 1 | – | 1 | 1 | 2 | 2 | – | – | – | 1 | 2 | – | – | – | 2 | – | 1 | – |
| Pterocladiella capillacea | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – |
| Pterosiphonia parasitica | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – |
| Pterosiphonia pennata | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | 1 | – | 1 | – | 1 | – | – | 1 | 1 | – |
| Sargassum vulgare | – | – | – | – | – | – | – | – | – | – | 5 | – | – | 1 | – | – | 4 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Sphacelaria cirrosa | – | – | 1 | – | – | – | – | 2 | 2 | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Sphaeroccocus coronopifolius | – | – | – | – | – | – | 2 | – | – | 2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Sphondylothamnium multifidum | – | – | – | – | – | – | – | – | – | – | 1 | – | 3 | – | – | – | – | 1 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Ulva lactuca | 2 | – | 4 | 2 | – | – | 3 | – | 2 | 2 | – | – | – | – | – | – | – | – | – | – | 1 | 1 | 1 | – | – | 1 | – | – | – | – | – | – |
| Ulva rigida | – | – | – | – | – | – | – | – | – | – | 3 | – | 1 | 3 | 2 | 2 | 2 | 2 | 3 | 1 | 1 | 1 | 1 | 2 | 2 | – | 1 | – | – | 1 | 2 | 2 |
| Taxa number | 10 | 10 | 10 | 10 | 6 | 9 | 8 | 5 | 12 | 15 | 14 | 10 | 9 | 7 | 10 | 9 | 11 | 10 | 9 | 7 | 19 | 8 | 19 | 10 | 16 | 10 | 17 | 15 | 19 | 19 | 12 | 7 |
⁎ Falkenbergia rufolanosa is a phase in the Asparagopsis cycle.
The structures observed along axes 1 and 2 of the Correspondence Analysis (CA) of the Algerian sites, based upon presence–absence, Braun-Blanquet cover classes, and percentage cover of species, were similar. However, presence–absence provided a slightly sharper separation of the four sites and we therefore present only the latter results hereafter. The structure observed along axes 1 and 2 represents 31.2% total inertia (eigenvalue of 5.65 and 4.32, respectively). Samples are distributed into three groups, Cherchell, Tamentfoust, and Bordj-El-Kiffan + Alger-Plage (Fig. 1). Taking into consideration taxa with the greatest contribution to the inertia explained by the two first axes (results not presented here), Cystoseira amentacea var. stricta, Dictyota fasciola, Halopteris scoparia, Padina pavonica, Sphacelaria cirrosa (Fucophyceae), Apoglossum ruscifolium, Heterosiphonia crispella, Jania adhaerens, J. rubens var. corniculata, and J. rubens var. rubens (Rhodobionta) are found on the negative side of axis 1. They are opposed to Enteromorpha compressa, Ulva rigida (Chlorobionta), Colpomenia sinuosa (Fucophyceae), Ceramium siliquosum, C. rubrum, Corallina elongata, and Polysiphonia sertularioides (Rhodobionta), which are found on the positive side of the axis. Most of the latter species are indicators of polluted habitats [9] or opportunistic species (Corallina elongata), suggesting that axis 1 could correspond to the degree of disturbance. As far as axis 2 is concerned, no obvious relationship with any possible environmental factor is apparent.
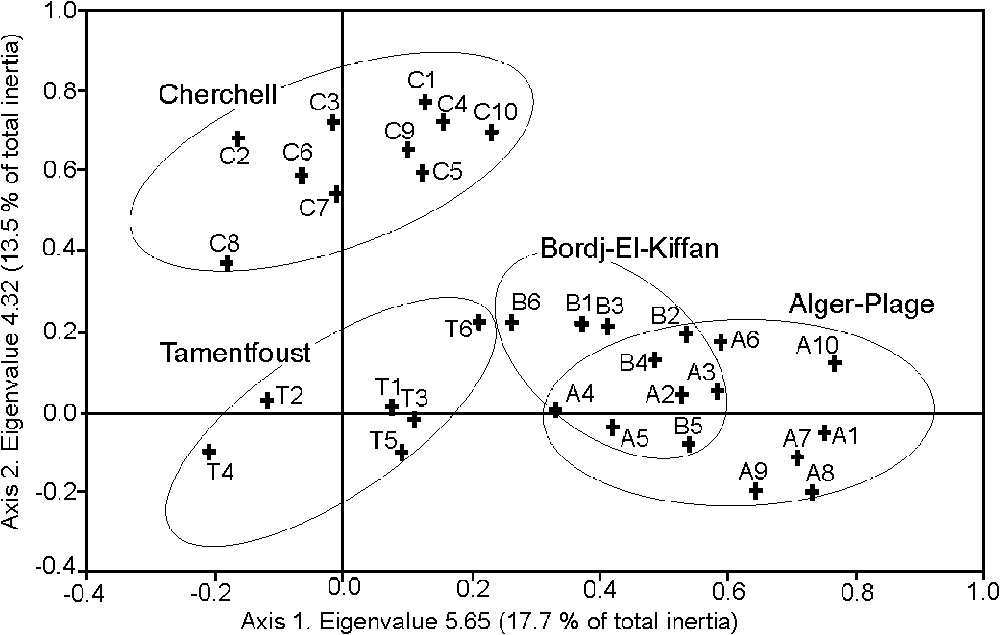
Ordination plot for the two first axes of correspondence analysis of the Algerian samples: Cherchell (C), Alger-Plage (A), Bordj-El-Kiffan (B) and Tamentfoust (T).
In order to place the Algerian samples within the context of other Mediterranean communities thriving in similar habitat conditions, we only retained two well-separated localities (Fig. 1), namely Cherchell and Alger-Plage, and included them within a new table (not presented here) together with samples from Banyuls-sur-Mer, Marseilles, Var (France), Syracuse, and Tremiti Islands (Italy). As for Algeria, CA for presence-absence, Braun-Blanquet cover classes and percentage cover data provided similar results. The structure observed along axes 1 and 2 of the CA (from presence–absence) represents 27.9% of total inertia (eigenvalue of 6.51 and 4.91, respectively) (Fig. 2). Marseilles (both highly polluted and pristine water samples), Cherchell (the supposedly most pristine Algerian site), and Alger-Plage (the Algerian site most different from Cherchell) form distinct clusters. The positions of the other samples (Banyuls-sur-Mer, Tremiti Islands, Syracuse, and Var), collected in pristine waters, appear dispersed, without a logical grouping with samples from pristine (Marseilles) or supposed pristine (Cherchell) sites. Ordination plot for axes 1 and 3 (9.8% of total inertia) (not presented here) gives the same picture. Axis 2 may represent a temperature gradient (see [35]), for spring and summer sea-surface temperature interannual variability of the western Mediterranean Sea, northern and central Adriatic Sea.
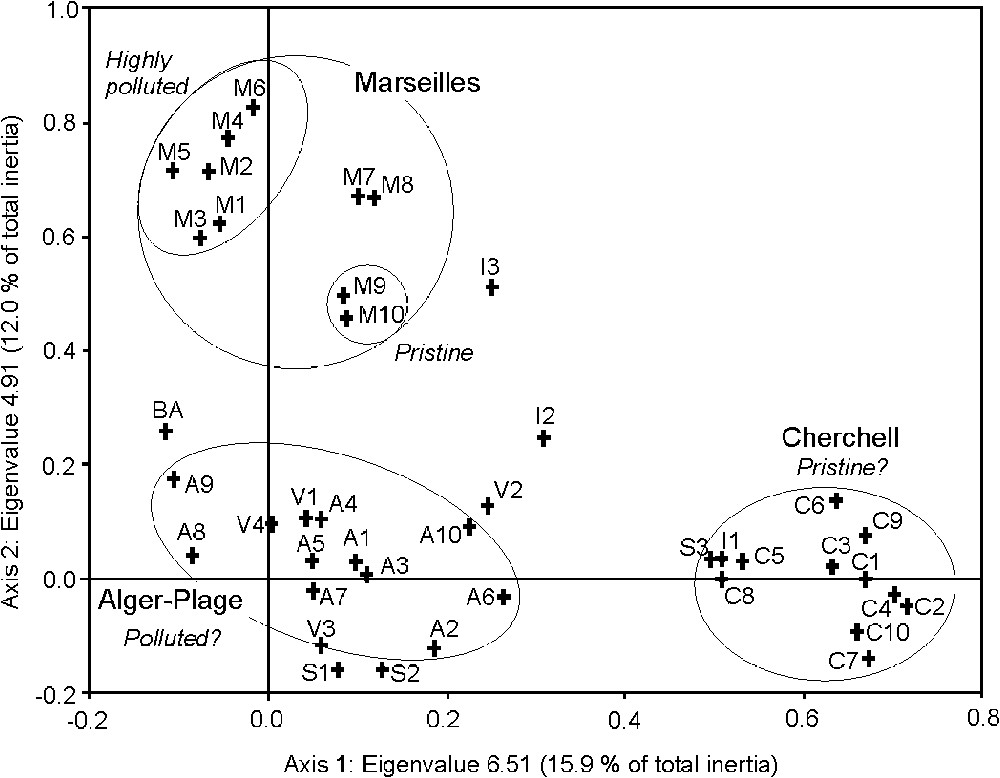
Ordination plot for the two first axes of correspondence analysis of Algerian and other Mediterranean samples: Alger-Plage (A), Cherchell (C), Tamentfoust (T), Banyuls-sur-Mer (B), Marseilles (M), Var (V), Syracuse, Sicily (S), and Tremiti Islands (I). For Marseilles samples, M1–M6 originates from a much-polluted area, M7–M8 from near the limit of the polluted area, and M9–M10 from a control area.
In fact, samples cluster primarily according to sites (e.g., Marseilles, Cherchell, Alger-Plage), irrespective of the known (Marseilles; [36]) or hypothesized degree of pollution. This is confirmed by the hierarchical analysis of the distance matrix (Fig. 3). Alger-Plage samples cluster with Cherchell ones, not with Marseilles samples, whatever the position of the latter along the pollution gradient, from M1–M2 (sites close to the outfall of partially treated sewage from 1.5 million inhabitants) to M9–M10 (control sites) [12,36].

Hierarchical classification of some Algerian and Marseilles samples: Alger-Plage (A), Cherchell (C), Marseilles (M). Cluster analysis uses Ward's method and Euclidean distance.
How can these rather unexpected results be explained? (i) The isolation of Cherchell samples from most other samples, including those considered as more or less pristine, could be due to the fact that the site is located 80 km upstream (according to the prevailing current) from Algiers and to the absence of any major source of pollution for tens of kilometres eastward and westward. Cherchell would thus constitute the most pristine of the Mediterranean sites studied here. The presence of Cystoseira amentacea var. stricta, together with that of other species very sensitive to pollution in Cherchell samples, supports this assumption. In fact, in the absence of sewerage and of any sewage treatment plant, the wastewaters of this region are either directly discharged into the sea through a multiplicity of outfall ranging from tiny (isolated houses) to middle-sized (a neighbourhood or a village), or indirectly discharged through coastal wadis (non-permanent rivers). Though data on water quality are not available, it is unlikely that Cherchell would harbour the most pristine macrophyte community of the studied sample set. This assumption is supported by the presence, in some samples, of Enteromorpha intestinalis, E. compressa, and Ulva lactuca. (ii) The fact that Alger-Plage samples do not form a group with those from Marseilles polluted habitats (M1 through M6) and cluster with Var samples from pristine habitats (Fig. 2) could mean that Alger-Plage is not as polluted as we hypothesised. However, this is hardly credible. Algiers sewage is directly discharged into the Bay without efficient treatment (a treatment plant exists, but does not work). As far as Var samples (V1 through V4) are concerned, the area is today considered as far from polluted [30], but we must keep in mind that they were collected three decades ago, at a time when most cities lacked treatment plants, especially the main town of the area, Toulon. (iii) All Algerian samples could correspond to more or less polluted habitats. This is supported by the fact that both Cherchell and Alger-Plage samplings are closer to the very to slightly polluted Marseilles ones (M1–M8) than to the pristine Marseilles ones (M9–M10) (Fig. 3). This possibility cannot either be ruled out or proved by our result. (iv) Regional characteristics of the macrophyte community could be prevalent over the pollution forcing. This would have been an expected feature between localities from remote seas and oceans, but it constitutes a less expected one within a rather small basin (the western Mediterranean). Though sea-surface temperature is slightly higher in Algeria than along the continental coasts of France [35], both regions share 68% of their macrophyte flora (Fucophyceae, Ceramiales, and Chlorobionta) calculated from [22,37,38]. Furthermore, this percentage is largely underestimated due to the poor knowledge of the Algerian marine flora (but see [39]). This last hypothesis, i.e., the prevalence of regional characteristics over the pollution forcing, seems to be most probable.
By favouring opportunistic [40] and cosmopolitan species (that is frequently introduced species, i.e., species spread worldwide by human activity), pollution would logically lead to the uniformisation of marine communities over large geographical areas. As a result, communities from remote areas would be more similar when subjected to pollution than in pristine waters. However, such a feature is not apparent from the present results. The reason could be that the habitat studies are not localized in semi-enclosed and calm water areas, but on the contrary face the open sea under exposed conditions.
4 Conclusions
Data on water quality are available in many parts of the Mediterranean Sea. Of course, they are not meaningful by themselves but by their effect on species and communities. Along the region of coasts of Algiers, however, such data are lacking, at least for recent years. Individual species and communities as a whole are indicators of water quality and so were expected to provide an alternative way to estimate water quality.
We analysed macrophyte samples from the Algerian coast (Bay of Algiers and Cherchell), together with a set of samples from the region of Marseilles (France), ranging from the vicinity of a sewage outfall to pristine waters, i.e. along a pollution gradient. Other samples from a variety of Mediterranean regions were added, in order to take into account possible geographical factors. The analysis of this data set failed to calibrate the pollution level of the Algerian localities by inserting Algerian samples within the Marseille pollution gradient. In contrast, regional characteristics of the macrophyte communities appear to be largely prevalent. This means that water-quality biological indicators and indices based upon marine macrophytes [5–7,14,15,41], at least for the open waters and the exposed shallow Mediterranean habitats studies here, could be reliable within a given region, but may require validation and/or adjustment, perhaps considerable, for other regions, as already suggested by [15], and targeted by the European Union [13] in the framework of the Water Framework Directive.
Acknowledgements
The authors are indebted to Michael Paul for improving the English text.


