1 Introduction
Beside surgical resection, the treatment of human colorectal cancer (CRC) uses various chemotherapeutic agents. Much effort concentrates on the research of new anti-cancer drugs to target more specifically and efficiently malignant cells and/or to prevent neo-angiogenesis. The development of drugs generally uses mouse models of CRC, either genetically manipulated or treated with carcinogens [1] in order to carry out preclinical trials aimed at validating the activity of the putative therapeutic agents [2]. A critical limitation of these experiments is the number of animals needed to follow up progression of the cancers induced experimentally in order to assess anti-tumour activity of the therapeutic agents. Therefore, developing innovative in vivo imaging technology of the intestinal tumours grown in situ is a desirable to better survey progression in individual animals and reducing their total number in trials while improving the statistical value of the results.
Imaging (X-ray computed tomography – CT –, Magnetic Resonance Imaging – MRI –) is nowadays widely used for colonography in human. The quality of the images for diagnosis purposes depends on their spatial resolution and on the achievable contrast based on natural properties of the tissue or on its enhancement by contrast agents. This latter point is essential for CT of the gut because of the lack of X-ray natural contrast. Applying this imaging technology in laboratory animals encounters the same problem as in human, in addition to that related to the small size of the animals, like mice, which necessitates developing dedicated devices able to achieve the required high spatial resolution. Although μCT is the gold standard for bone and vasculature imaging in living mice [3,4], its use for the intestine is hampered by the small size of these animals even compared to other laboratory animals like rats, since mice are 10- to 15-fold smaller in volume compared to rats. This underlines the intrinsic difficulty to perform imaging in living mice. μCT imaging in the gut has recently been proposed in order to visualize intestinal tumours in mice [5], but, without clearly demarking the gut wall, possible confusion exists between polyps and regular intestinal folds or Payer's patches.
Here, we describe a simple non-invasive procedure based on multiple-contrast colonography, to trace morphological lesions and tumours developing in situ in the colon of living mice. This imaging method should greatly improve the management and follow up of animal series for future experimental research on CRC.
2 Material and methods
2.1 Animals
Wild-type and Cdx2+/– heterozygous mice in the 129sv/Bl6 background [6–8] were housed under standard laboratory conditions. A total of 31 mice were used for this study. Mice treatments and managements were approved by the Ethic Committee of the University Louis-Pasteur of Strasbourg. Colon carcinogenesis was induced in six two-month-old wild-type mice as described [7], by intraperitoneal injection of azoxymethane (AOM, Sigma, St. Louis, Missouri, USA) at 10 mg/kg once a week for five weeks. Analyses were performed 30 weeks later.
2.2 μCT imaging
Mice were anaesthetized using isoflurane in air (5% at induction, then 1.5–2% during examination), injected subcutaneously with morphine (1 mg/kg) to inhibit intestinal motility [9]. Then, several iodine-based X-ray contrast agents were administered: Iomeron 400 (Bracco, Milan, Italy, 1.5 mL, dilution 1:5) was injected intraperitoneally; Telebrix (Guerbet, Roissy CdG, France, dilution 1:10) was administered by rectal route with a cannula until reflux from the anal orifice. In studies with the chemical carcinogen, animals were fed ad libitum for 24 h with a mixture of standard chow containing BaSO4 powder (Micropaque, Guerbet, Roissy-CdG, France) diluted 1:10th in water to contrast the faecal matter. Dilutions of the contrast agents were chosen for differential attenuation between contrast agent inside the abdominal cavity and that inside the intestine.
Mice were placed in an isolation cell aimed at maintaining homeostatic conditions (Minerve, Esternay, France), kept anaesthetized via a mask and immobilized with a tooth bar. Imaging was performed using an X-ray μCT scanner (eXplore RS Locus, GE Healthcare, London, Canada). It produced 400 views over 360°, with three frames averaged for each view, at 80 kV, 450 μA. Data acquisition lasted about 10 min for the abdomen and about 35 min if whole-body imaging was required. After reconstruction using a Feldkamp algorithm of backprojection, the imaging volume was made of cubic voxels of 93 μm × 93 μm × 93 μm. Visualization and analysis were performed using MicroView (GE Healthcare, London, Canada).
3 Results
3.1 Setup of the μCT procedure to visualize the gut wall and gastric-type heterotopia in the caecum of living mice
To set up the procedure of X-ray micro-computed tomography (μCT) in living mice, we used wild-type mice and heterozygous animals for the homeobox gene Cdx2. Cdx2+/– mice exhibit gross developmental malformations in the skeleton, on the one hand, and in the gut in the form of non-cancerous polyp-like heterotopia made of gastric-type mucosa replacing the normal intestinal mucosa in the caecum region [6], on the other hand. Fig. 1A shows the apparatus for data acquisition in living mice, including the scanner for X-ray μCT and the isolation cell aimed at maintaining the mice anaesthetized and immobilized in homeostatic conditions. By μCT, calcified structures were easily observed and 3D reconstruction of the vertebral axis allowed visualizing the small homeotic bone malformations typical of Cdx2+/– mice, exemplified by anterior shifts of cervical and upper thoracic vertebrae and by supernumerary ribs (Fig. 2). Without X-ray imaging, these analyses require sacrifice of the animal and time-consuming skeletal preparations [6].
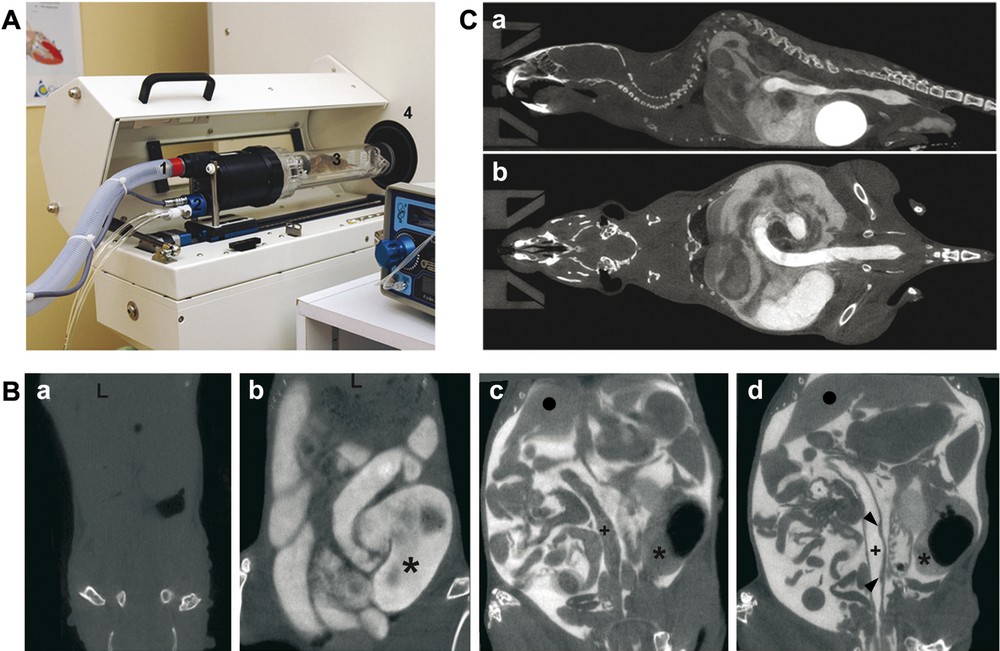
Setup of the procedure of multiple-contrast X-ray μCT in mice. A. The device combines an X-ray μCT scanner (4) and an isolation cell (3) connected to a gas system for anaesthesia with isoflurane (1) and to a temperature controller via air flow (2). B. Improvement of the μCT visualization of soft intestinal tissue by the multiple-contrast procedure. μCT data were obtained without contrast material (a), with Telebrix administrated via rectal route (b), with Iomeron 400 injected intraperitoneally (c), and with both Telebrix and Iomeron 400 contrast agents (d). Pictures correspond to coronal slices with cranial direction at the top. (+) and (∗) indicate the colon lumen and the caecum lumen, respectively; (●) shows the liver; arrowheads in (d) indicate Payer's patches. C. Whole-body imaging. Sagittal (a) and mid coronal sections (b) of a mouse following intraperitoneal injection of Iomeron 400 and Telebrix administration via intrarectal route clearly identify the abdominal cavity and the lumen of the distal colon. High amount of contrast material accumulates in the bladder, due to renal elimination of contrast agent. Masquer
Setup of the procedure of multiple-contrast X-ray μCT in mice. A. The device combines an X-ray μCT scanner (4) and an isolation cell (3) connected to a gas system for anaesthesia with isoflurane (1) and to a temperature controller via ... Lire la suite
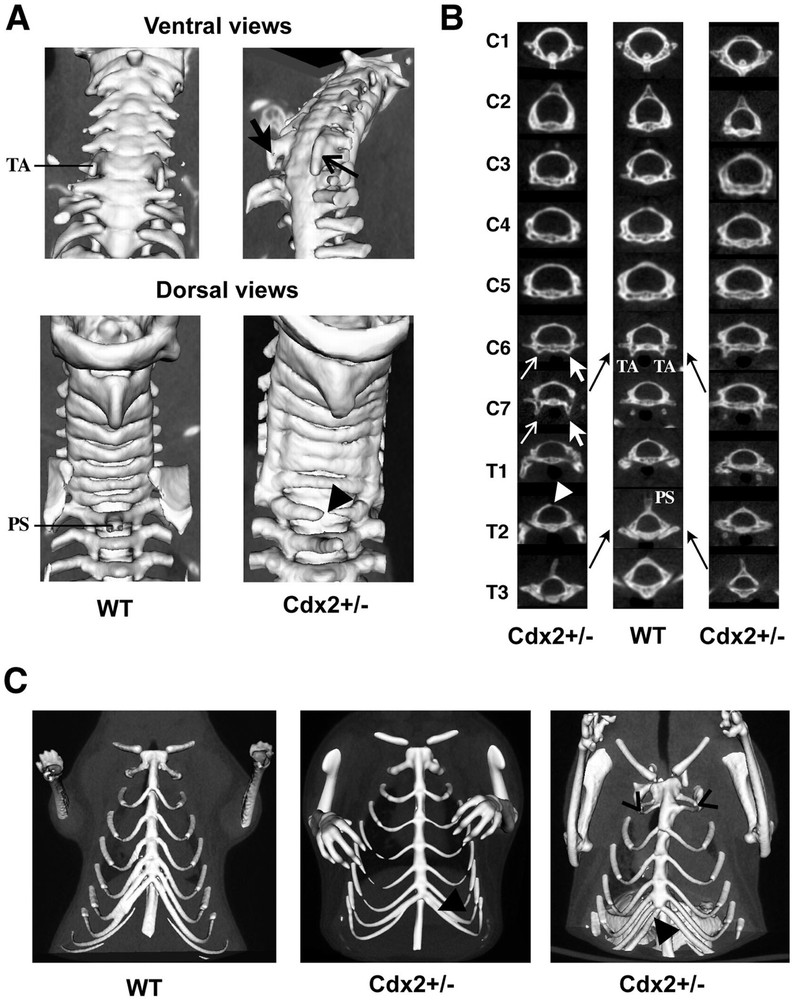
μCT detection of bone malformations in Cdx2+/– mice A. 3D reconstruction from μCT data of the upper vertebral axis. The ventral view shows the anterior tubercles (TA) present in the cervical vertebra C6 in wild-type mice and their extension to C7 in a Cdx2+/– mouse (arrows). The dorsal view shows the spinous process (PS) of the thoracic vertebra T2 in wild-type mice, which is absent in Cdx2+/– mice and occasionally associated with the incomplete fusion of the dorsal arch (arrowhead). B. Virtual sections through each vertebra in the cervical and upper thoracic region of Cdx2+/– (left and right columns) and wild-type mice (central column) illustrate the anterior homeotic shift observed in Cdx2+/– heterozygotes (black arrows). The left column corresponds to the Cdx2+/– specimen shown in (A). Anterior tubercles (TA) characterizing C6 in wild types are present in C6 and C7 in Cdx2+/– mice (white arrows). The spinous process (PS) located on T2 in wild types is relocated onto T3 in Cdx2+/– mice. The white arrowhead shows the incomplete fusion of the dorsal arch on T2. C. 3D reconstruction of the rib cage in wild-type (left) and Cdx2+/– mice (centre and right). Heterozygotes show additional ribs (black arrowheads) and/or fusion of the first pairs of ribs (open arrowheads). Masquer
μCT detection of bone malformations in Cdx2+/– mice A. 3D reconstruction from μCT data of the upper vertebral axis. The ventral view shows the anterior tubercles (TA) present in the cervical vertebra C6 in wild-type mice and their extension to ... Lire la suite
As already reported [10], the absence of natural X-ray contrast prevents any detection of soft tissues within the abdominal cavity, including the gut, without the use of exogenous contrast agent (Fig. 1Ba). Adding a single contrast agent either in the intestinal lumen (Fig. 1Bb) or in the peritoneal cavity showed the fate of the gut (Fig. 1Bc). However, even when using a single contrast agent, it is not easy to distinguish when a constriction in the gut lumen is due to a regular fold or to the presence of an abnormal structure. This problem could be overcome by demarcating both internal and external limits of the gut wall to visualize its thickness. For this purpose, we developed a multiple-contrast procedure where contrast material was administered in the peritoneal cavity and in the colonic lumen. Dilutions of contrast agents were chosen for differential attenuation between contrast agent inside the abdominal cavity and that inside the intestine, as shown by whole-body imaging (Fig. 1C). At higher magnification, this procedure allowed visualizing the colonic wall as a continuous dark line with regular thickness of about 200 μm, delineating the inside of the colonic lumen from the outside peritoneal cavity (Fig. 1Bd). The thickness is in line with previous estimations [11]. Payer's patches are small and local thickenings of the gut wall, typically located near the blunt end of the caecum and in the distal colon. By μCT, they appeared as flat elevated structures compared to the adjacent gut wall, indicating that thickenings as small as 400 μm (about twice the measurement of the normal gut wall) can be unambiguously detected by this approach (Figs. 1Bd and 3Aa, b).
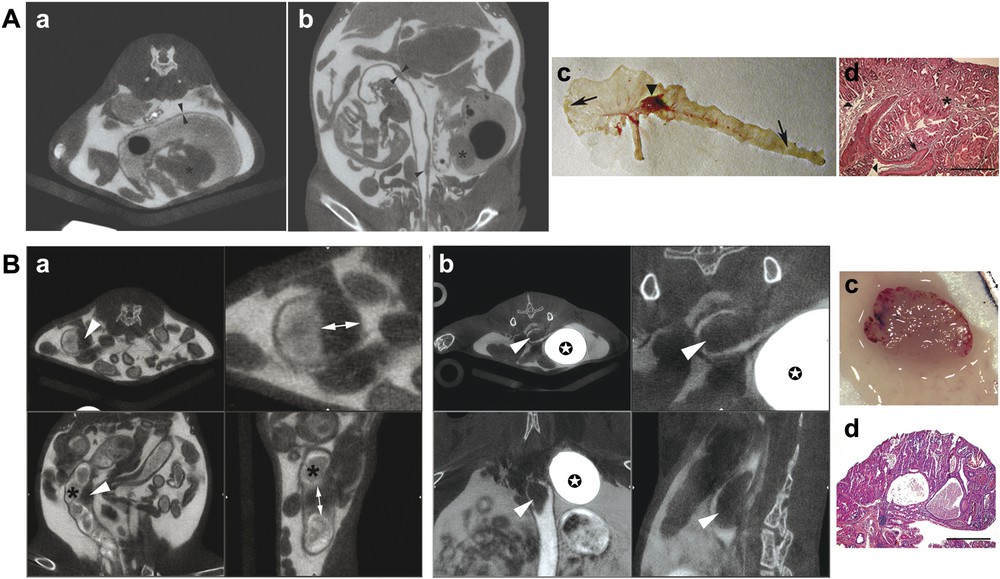
Visualization of non-cancerous heteroplasia in the caecum and of tumours in the colon by multiple-contrast X-ray μCT in mice. A. Heteroplasic lesions in the caecum of Cdx2+/– mice injected intraperitoneally with Iomeron 400 and intra-rectally with Telebrix. (a,b) Two examples of multiple-contrast μCT imaging show the colonic wall as a dark line that separates the gut lumen from the abdominal cavity (thickness of the colonic wall: around 200 μm, arrowheads), the gastric-type heteroplasia that protrude into the caecum cavity (asterisks) and Payer's patches that appear as local thickening of the gut wall (arrows). (c) Macroscopic view of the caecum and colon opened longitudinally in the Cdx2+/– mouse analyzed in (b) by μCT. The arrowhead shows the heteroplasia in the caecum and arrows denote Payer's patches at the blunt end of the caecum and in the distal colon. (d) Histological section of the heteroplasia, stained with haematoxylin-eosin. The asterisk shows gastric-type tissue, the arrow denotes stratified squamous epithelium and the arrowheads point to the normal caecal epithelium adjacent to the heteroplasia. Scale bar is 400 μm. B. Colonic tumour in AOM-treated mice injected intraperitoneally with Iomeron 400 and intrarectally with Telebrix, and given BaSO4-containing diet. (a,b) Two examples of colonic tumours visualized by multiple-contrast μCT in AOM-treated mice are illustrated. Upper panels correspond to transverse slices (left and right are low and high magnifications, respectively), and lower panels to coronal (left panel) and sagittal (right panel) slices. The dark endoluminal polyps (arrowheads) are clearly distinct from the stools containing contrasting material (asterisk). Double arrows allow measurement of the polyp size; contrast material concentrates in the bladder (stars). A movie of the polyp shown in (b) is available as supplementary material. (c) Macroscopic view (d) and histological section of the colonic polyp illustrated in (b). Scale bar is 400 μm. Masquer
Visualization of non-cancerous heteroplasia in the caecum and of tumours in the colon by multiple-contrast X-ray μCT in mice. A. Heteroplasic lesions in the caecum of Cdx2+/– mice injected intraperitoneally with Iomeron 400 and intra-rectally with Telebrix. (a,b) Two examples ... Lire la suite
Taking advantage of the gastric-type heterotopia occurring in the caecum region of Cdx2+/– mice [6], we tested whether multiple-contrast μCT is appropriate to detect these morphologically abnormal structures in the soft intestinal tissue. As shown in Fig. 3Aa and b, these lesions appeared as dark grey polyp-like structures protruding into the caecal lumen in each Cdx2+/– mouse (
3.2 Detection of AOM-induced tumours in the colon by μCT
Having demonstrated that multiple-contrast μCT is appropriate to detect genetically determined polyp-like lesions in the soft intestinal tissue, we set out to assess its utility in the investigation of colonic tumours. Wild-type mice received intraperitoneal injections of the carcinogen AOM for five weeks, leading to DNA methylation, point mutations and ultimately colon adenocarcinomas 30 weeks later [7]. Since a number of studies on CRC are focused on the role of nutrients, pre- and/or pro-biotics, we wished to develop a protocol that avoided starvation or dietary changes and thus did not interfere with the experimental regimen. For this purpose, mice were prepared as described above, except that starvation was omitted and replaced by ad libitum feeding with BaSO4-containing chow to contrast the faecal matter. With this method, the stools appeared as contrasted pellets in the colonic lumen. Inversely, the endoluminal polyps induced by AOM formed dark structures connected to the gut wall (Fig. 3Ba, b, and see supplementary material for a dynamic view). A total of 11 tumours were recorded by μCT in the six mice that were analyzed, and macroscopic examination at necropsy demonstrated that none of the tumours induced by AOM escaped μCT detection; histology confirmed the presence and cancerous nature of the polyps (Fig. 2Bc and d). Thus, it is very easy to distinguish the endoluminal tumours from the faecal pellets by feeding with a contrast agent. Tumour development is a long-lasting process that takes several months in mice. Thanks to the careful control of anaesthetized mice environment and follow-up of physiological parameters, repeated anaesthesia and μCT imaging were performed up to five times on the same individual without precocious death, indicating that mouse preparation for the administration of multiple contrast agents can be recurrently performed to survey tumour growth without compromising viability. Although each X-ray examination involves potential hazard linked with ionization, the protocol was close to that described in [12], leading to moderate irradiation. Nevertheless, consequence of such a procedure during long time follow-up of wild-type and transgenic mice needs to be checked.
4 Discussion
The constant research of new therapeutic agents against CRC requires a huge number of preclinical studies in mice, which may be greatly facilitated by imaging living animals to survey tumour growth in situ. Optical fibre colonoscopy has been described in mice [13,14], but this method is invasive, with a risk of mechanical injury of the colonic wall and resident tumours. Mouse intestinal tumour imaging by magnetic resonance has been reported [15], yet without providing a clear visualization of the gut wall. Recently, a method of X-ray μCT was reported based on the shift from normal to a low-density diet and corn oil enema for two days to contrast the gut lumen [5]. The dietary change before imaging lasting several days may represent a methodological problem for nutritional studies related to CRCs. Moreover, without contrast agent in the peritoneal cavity, the intestinal wall itself is not observable, as shown here when contrast agent is only delivered in the colonic lumen, leading to the possible confusion between tumours and regular folds in the gut wall. The method of multiple-contrast μCT described here has several advantages over existing methods of in vivo imaging in mice. It is non-invasive, it can be repeated without compromising viability and it does not require dietary change, thus being appropriate for nutritional studies related to CRC. In addition, the high spatial resolution and the isotropic nature of μCT images allow a clear identification and demarcation of the tumours, giving in situ quantitative measurements amenable to statistical analysis. The observation in vivo and in situ of colonic tumours in mice replicates the radiological procedure of the clinical diagnosis follow-up of spontaneous colonic tumours in human beings, thanks to the use of the same technology.
In conclusion, the imaging method reported here to detect intestinal tumours in situ in living mice is simple, sensitive, and non-invasive. Multiple-contrast X-ray μCT will improve the management and analysis of animal series enrolled in preclinical trials aimed at developing and validating new chemotherapeutic approaches against colorectal cancer.
Acknowledgements
This work was supported by INSERM. A. Calon was a fellow of the INSERM/Région Alsace and of the ‘Ligue régionale contre le cancer’ (Haut-Rhin and Bas-Rhin, France). E. Breton was funded by the Région Alsace and GE Healthcare.


