1 Introduction
The gastrointestinal tract is one of the main physiological systems that may be damaged by exposure to ionizing radiation, whether accidental or therapeutic [1], and in general the severity of the gastrointestinal injuries depends on the radiation dose received and the volume implicated. These damages may extend from gastrointestinal dysfunctions such as diarrhoea with cramps and pain [2,3] after sublethal doses to the gastrointestinal syndrome related to acute intestinal death resulting from severe alterations in small intestine morphology and function [4,5] for single total body doses higher than 10 Gy. Mechanisms leading to these injuries are not well known and their understanding is essential for a better therapeutic approach.
Among several factors mentioned classically, bile acids have been proposed to play a major role in gastrointestinal radiation injury in the days following exposure with high doses [6,7] as well as in long-term consequences of irradiation in patients exposed during radiotherapy [8,9]. In both situations, it has been observed that intestinal reabsorption of bile acids was decreased, leading to enhanced bile acid concentrations in the intestine and colon [10–12].
The radio-induced intestinal bile acid malabsorption, classically evoked, may partly explain these changes. However, taking into account the characteristics of the enterohepatic recycling of bile salts, the hypothesis of modified hepatic bile acid biosynthesis must also be taken into consideration. Bile acids are synthesized in the liver and secreted into the gut, where they facilitate lipid digestion and absorption. They are then reabsorbed by the small intestine with high efficiency, since more than 90% of bile acids are returned to the liver [13]. Thus, in the normal physiological state, hepatic biosynthesis of bile acids occurs to balance faecal excretion. In some pathological situations where bile acids are less efficiently reabsorbed by the gut, hepatic biosynthesis is stimulated to compensate for this lack of absorption. Thus, we hypothesized that radio-induced changes of bile acid profiles may result from modified hepatic bile acid biosynthesis in the days following irradiation, even if the liver is described as a radioresistant organ [14].
In most mammals and in humans, hepatic biosynthesis of bile acids from cholesterol is initiated via either the classic (neutral) or the alternative (acidic) pathway. Recently, it was shown in the rat that each pathway contributes 50% to total bile acid biosynthesis [15]. The neutral or classical pathway is initiated in the hepatocyte by the microsomal cholesterol 7α-hydroxylase (EC 1.14.13.17; CYP7A1), a key enzyme that transforms cholesterol into 7α-hydroxycholesterol, which is further metabolised into 3-oxo-7α-hydroxy-4-cholesten, a common intermediate leading to the production of chenodeoxycholic acid or cholic acid after a sequence of multiple enzymatic reactions [16]. The alternative pathway is initiated by the mitochondrial sterol 27-hydroxylase (EC 1.14.13.15; CYP27A1), which transforms cholesterol into 27-hydroxycholesterol [17]. This intermediate is metabolized by oxysterol 7α-hydroxylase (CYP7B1) [18], leading to the production of 7α, 27-dihydroxycholesterol, which is further metabolized to chenodeoxycholic acid or cholic acid. In both pathways, the biosynthesis of cholic acid is dependent on sterol 12α-hydroxylase activity (CYP8B1). In the rat, an additional 6-hydroxylation transforms chenodeoxycholic acid into muricholic acid [19]. To date, the four enzymes CYP7A1, CYP27A1, CYP7B1, and CYP8B1 are considered as the major and rate-limiting enzymes implicated in the biosynthesis of bile acids. Products of these enzymatic cascades lead to bile acids, which are then conjugated with glycine or taurine and constitute the primary bile acids. The major ones in the rat are taurocholic acid (TC), glycocholic acid (GC), taurochenodeoxycholic acid (TCDC), and β-tauromuricholic acid (β-TMC). These primary bile acids are secreted into the gut and are converted by intestinal microflora into the secondary bile acids taurohyodeoxycholate (THDC), tauroursodeoxycholate (TUDC), and taurodeoxycholate (TDC) [13]. Primary and secondary bile acids, which differ in their number and/or position of hydroxyl group, have been classified according to their hydrophobicity [20].
Taking into account our hypothesis that radiation-induced changes of bile acid profiles may result from altered hepatic biosynthesis, the aim of this work was thus to determine the time course of the modifications of the bile acids profiles, with concomitant evaluation of the hepatic activities of the four major enzymes involved in bile acid biosynthesis (CYP7A1, CYP27A1, CYP7B1, and CYP8B1) in the days following irradiation.
2 Materials and methods
2.1 Chemicals and isotopes
[4–14C]Cholesterol and 25-[26,27–3H]hydroxycholesterol were obtained from NEN Products (Les Ulis, France). Cholesterol, 7α-hydroxycholesterol, 25-hydroxycholesterol, 27-hydroxycholesterol and cholesterol oxidase (Cellulomonas species) were obtained from Sigma Chemicals (diagnostics; L'Isle-d'Abeau–Chesnes, France). Hydroxypropyl-β-cyclodextrin (HPβCD) was kindly donated by Roquette Frères (Lestrem, France).
2.2 Animals
Experiments were carried out on male Wistar rats (CERJ, Le Genest-Saint-Isle, France) weighing between 225 and 250 g at the beginning of the experiments. They were housed at a constant temperature (21 °C), maintained on a 12:12 h light: dark cycle with a standard rat chow diet (105 UAR, France) and water ad libitum.
2.3 Irradiation
Animals (280–300 g) were exposed to whole body gamma irradiation (60Co; ICO 4000, CEN-FAR, Fontenay-aux-Roses, France) with a single dose of 8 Gy and a dose rate of 0.75 Gy/min. During the exposure, animals were conscious and restrained in Plexiglas tubes, which were placed perpendicular to the beam axis, in the 90% isodose area, and then turned on the horizontal axis to receive a homogeneous irradiation. Control animals were treated in exactly the same manner, but were not exposed to source (sham-irradiation). All animals were treated at the same time of day. All experiments were conducted according to the French regulations for animal experimentation (Ministry of Agriculture, ‘Décret’ (Act) No. 2001–464, 29 May 2001).
2.4 Total and individual bile acids in bile
2.4.1 Bile collection and samples
Bile was collected in both sham- and irradiated rats, 1, 2, 3, 4 and 7 days after exposure. Bile was collected under anaesthesia (sodium pentobarbital 100 mg/kg; Sanofi, La Ballastrière, France) after catheterisation of the bile duct, which was made 0.5–1 cm below the hilum of the liver, where the bile duct is free of pancreatic tissue. Bile was collected over a period of 1 h and stored (−20 °C) until analysis.
2.4.2 Analysis of total and individual bile acids in bile
Analysis of bile acid composition was made by high-performance liquid chromatography (HPLC), as previously described by Scanff et al. [12] after a first step of purification on Sep-Pak cartridges (Waters, Milford, MA). The total bile acid concentration in bile was obtained by summation of individual bile acids, and the hydrophobicity index was calculated [20].
2.5 Enzyme activities in the liver
2.5.1 Preparation of liver microsomes and mitochondria
Livers were obtained from both sham-irradiated and irradiated rats (three and four days after exposure). Under anaesthesia with sodium pentobarbital (100 mg/kg of body weight i.p.), the liver was rapidly removed and chilled in ice-cold buffer. The livers were sliced and 1 g portions were taken and homogenized in buffer (KH2PO4 50 mM, sucrose 300 mM, dithiothreitol 0.5 mM, EDTA 10 mM, NaCl 50 mM, pH 7.4), according to a procedure already described [21]. The homogenate was centrifuged for 20 min at 20 000 g and the supernatant centrifuged at 100 000 g for 1 h. The 20 000 g pellet was resuspended in buffer and again centrifuged at 100 000 g for 1 h. The microsomal pellet was homogenized in buffer, sampled, and stored at −80 °C until required. The 20 000 g pellet of homogenate was gently resuspended in 7 ml of buffer and homogenized by 10 strokes of a Teflon pestle (motor-driven homogenizer). The homogenate was centrifuged at 2000 g for 10 min. The supernatant was then centrifuged at 9000 g for 10 min, and the mitochondrial pellet finally resuspended in 1.2 ml of buffer, fractionated into 200-μl samples and stored at −80 °C.
2.5.2 Determination of liver enzyme activities
Cholesterol 7α-hydroxylase (CYP7A1) was assayed, in the microsomal fractions, according to a radioisotopic method using [4–14C]Cholesterol, solubilized, with hydroxypropyl-β-cyclodextrin as a carrier [22]. Oxysterol 7α-hydroxylase (CYP7B1) microsomal activity was measured using the radioisotope method as previously described [23]. Sterol 27-hydroxylase (CYP27A1) was assayed in the mitochondrial fractions by a radioisotopic method using [4–14C]Cholesterol, solubilized in hydroxypropyl-β-cyclodextrin [21]. The activity of sterol 12α-hydroxylase (CYP8B1) was determined in microsomes using a high-performance liquid chromatography (HPLC) assay described by Parquet et al. [24] and adapted from Vlahcevic et al. [15]. All enzyme activities, obtained in pmol min−1 mg−1 of microsomal or mitochondrial protein, are expressed as pmol min−1 whole liver−1 to take into account the variations of the weight of the liver observed after irradiation.
2.6 Total bile acids in colonic contents
2.6.1 Sample collection
Colonic contents were collected, after collection of bile, in both irradiated and sham-irradiated rats, 1, 2, 3, 4 and 7 day after exposure. The colon was removed from the end of the caecum to the rectum. Total colonic contents were collected by rinsing with 5 ml of a 150 mM NaCl solution at 4 °C. Colonic contents were then homogenised using an Ultraturrax T25 and frozen (−20 °C). Samples were then lyophilised, and dry matter weighed and stored (−20 °C) until analysis.
2.6.2 Analysis of total bile acids in colonic contents
Lyophilisates of colonic contents were resuspended in water (5 mg/ml) at 4 °C for 12 h. An identical volume of a MeOH/H2O (80:20) mixture was then added in order to obtain a final concentration of 2.5 mg/ml MeOH/H2O (40/60). The suspension was stirred for 1 h at 4 °C and was then centrifuged (3000 g, 10 min, 4 °C). Bile acid concentrations were determined in the supernatant by an enzymatic and colorimetric method kit (Sigma, L'Isle-d'Abeau, France), which measures 3α-hydroxy bile acids. Bile acid quantities in the whole colon are then calculated from the concentration values and the quantities of dry matter.
2.7 Other assays
Microsomal and mitochondrial protein contents were determined, as previously described, by the Lowry method, using bovine serum albumin as a standard [25].
2.8 Statistical analysis
All data are expressed as the mean values ± standard error of the mean (SEM) for n animals. Test of significant differences between sham-irradiated and irradiated animals were performed using a Student's t-test.
3 Results
3.1 Animals – general observations
In irradiated animals, both food intake and body weight gain were markedly reduced from the first day after irradiation with the most significant effect occurring at 3 days after (body weight 10% less than in the controls). From the fourth day onwards, irradiated animals gained weight at a rate similar to that of sham-irradiated animals.
3.2 Effect of irradiation on total and individual bile acids in bile
The volume of bile collected over 1 h varied between 0.7 and 1 ml, depending on the rat, without any significant differences between sham-irradiated and irradiated rats, whatever the day post-irradiation. The total bile acid concentration in bile was for control animals, and remained unchanged in the days following exposure, except on the fourth day, when it was slightly increased () (Table 1).
Total bile acid concentration in bile of sham-irradiated (C) and irradiated (D) rats
| Day after exposure | ||||||
| C | D1 | D2 | D3 | D4 | D7 | |
| Total bile acid (mM) | 27.5±1.0 | 32.2±3.2 | 27.8±3.1 | 26.0±2.0 | 24.7±1.6 |
The composition of the total bile acid pool in bile was also determined. In control rats, among the seven forms of bile acids, the two principal ones, β-tauromuricholate (β-TMC) and taurocholate (TC), represent, respectively, 42% and 34% of the total bile acids. Two other primary bile acids are also present: glycocholate (GC, 9%) and taurochenodeoxycholate (TCDC, 6%). In addition, three other bile acids are present, which are secondary bile acids: taurohyodeoxycholate (THDC, 5%), taurodeoxycholate (TDC, 3%), and tauroursodeoxycholate (TUDC, 2%).
Table 2 shows the modifications of individual bile acid concentrations in bile after irradiation, which result in fluctuating profiles as early as the first post-irradiation day. Thus, on this very day, a small increase of the concentrations of both TC () and TDC () was observed. A decrease of the concentration of TMC was observed from the second day onwards. The most important changes were observed three and four days after exposure; the profiles were different at these two days. Whereas TC and TMC concentrations were decreased at three days (respectively, and ), only TMC concentration was reduced () four days after irradiation. At this time, TC and GC concentrations were increased by 50% and 140%, respectively. The concentration of TCDC was increased () three days after irradiation and decreased () at the fourth post-irradiation day. TDC concentrations were greatly increased by 6 and 5 fold, respectively, three and four days after exposure. The distribution of individual bile acids into the two classes of primary and secondary bile acids shows that the proportion of secondary bile acids in the total pool was also enhanced three () and four days () after exposure (Fig. 1).
Individual bile acid composition (μmol/ml) in bile in sham-irradiated (C) and irradiated (D) rats
| Day after exposure | ||||||
| C | D1 | D2 | D3 | D4 | D7 | |
| Taurocholate (TC) | 9.3 ± 0.4 | 12.5 ± 1.7∗ | 11.1 ± 2.3 | 6.8 ± 1.1∗ | 13.4 ± 1.3∗ | 6.2 ± 0.7∗ |
| Glycocholate (GC) | 2.4 ± 0.3 | 3.8 ± 0.7 | 3.8 ± 0.7 | 1.5 ± 0.2 | 5.7 ± 1.0∗ | 3.7 ± 0.9 |
| Taurodeoxycholate (TDC) | 0.7 ± 0.04 | 1.0 ± 0.1∗ | 1.2 ± 0.2∗ | 4.2 ± 0.4∗ | 3.3 ± 0.9∗ | 0.6 ± 0.1 |
| Tauromuricholate (TMC) | 11.7 ± 0.6 | 11.6 ± 1.5 | 8.1 ± 0.6∗∗ | 7.3 ± 1.2∗ | 7.1 ± 0.6∗ | 9.9 ± 0.8 |
| Taurochenodeoxycholate (TCDC) | 1.8 ± 0.1 | 2.5 ± 0.4 | 2.1 ± 0.2 | 2.5 ± 0.2∗ | 1.3 ± 0.1∗ | 2.2 ± 0.3 |
| Taurohyodeoxycholate (THDC) | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.3 | 3.2 ± 0.5∗ | 1.0 ± 0.2 | 1.1 ± 0.3 |
| Tauroursodeoxycholate (TUDC) | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.1 | 0.9 ± 0.2∗ | 1.1 ± 0.1∗ |

Proportion of secondary bile acids in the total pool of bile acids in sham-irradiated (C) and irradiated (D) rats. Statistical differences among control (sham) and irradiated groups were assessed using Student's t-test: ∗ p<0.01; ∗∗ p<0.001; n, number of rats per group.
All of these changes of the proportion of individual bile acids in the pool of total bile acids result in a modification of the hydrophobicity index. As shown in Table 3, this was modified as early as the first post-irradiation day and was greatly increased at three and four days ( for controls vs. and for irradiated subjects). Seven days after irradiation, individual bile acid concentrations returned to values very close to those of controls, with a similar proportion of secondary bile acids and hydrophobicity indices.
Hydrophobicity index of bile of sham-irradiated (C) and irradiated (D) rats
| Day after exposure | ||||||
| C | D1 | D2 | D3 | D4 | D7 | |
| Index | −0.31±0.01 | −0.21 ± 0.01tsup∗ | −0.18 ± 0.02tsup∗ | −0.10 ± 0.03tsup∗∗ | −0.11 ± 0.02tsup∗∗ | −0.28±0.02 |
3.3 Effect of irradiation on major liver enzyme activities implicated in bile acid synthesis
In order to explore the possibility that change in individual bile acid concentrations in bile may be due to altered activities of the major enzymes implicated in bile acid synthesis, livers were removed and the activity of these enzymes was evaluated in sham-irradiated and irradiated animals, three and four days after exposure, when the changes were the most marked. Liver weight was decreased () three and four days after irradiation. This decrease is more important than the loss of body weight, since liver weight represented 4.5% of the body weight in control rats vs. only 3.6% in three-day post-irradiation ones. Results concerning the activity of enzymes are shown in Fig. 2. The activity of CYP7A1 was unchanged three days after exposure, but decreased at four days by 51% compared to control value (). Among the major enzymes of the alternative pathway, only the activity of CYP7B1 was changed. It was decreased by 50% and 90% three and four days after irradiation, respectively, compared to control value (). The major enzyme activity leading to cholic acid (CYP8B1) was markedly increased three days after exposure () and returned to control values () four days after exposure.
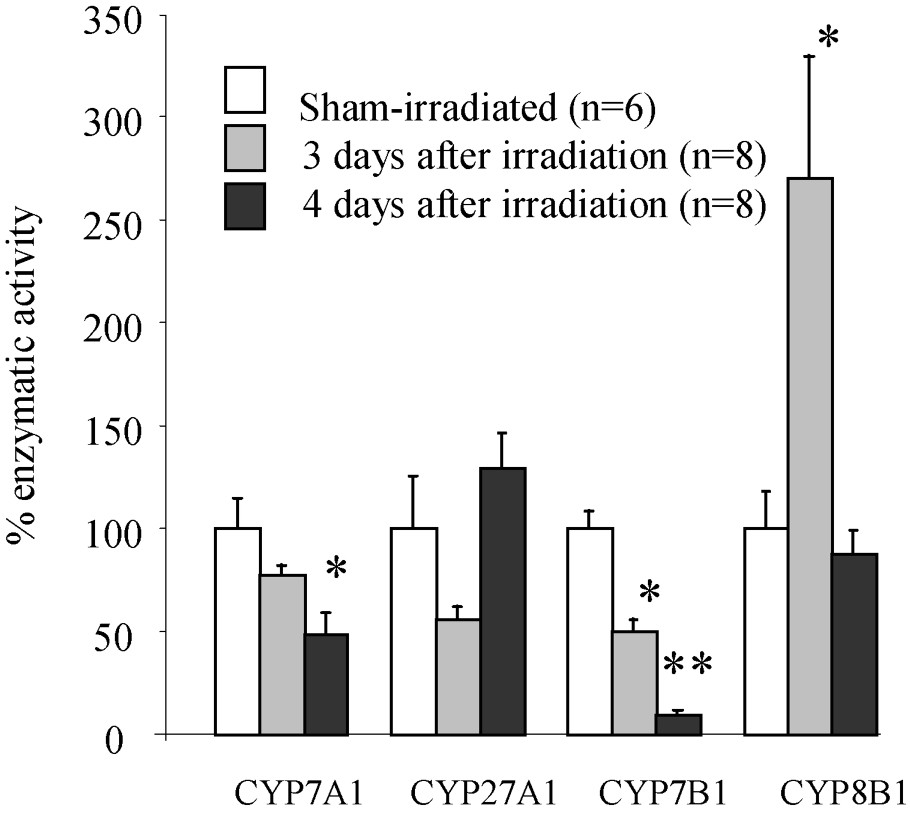
Activity of the major enzymes (CYP7A1; CYP27A1; CYP7B1; CYP8B1) implicated in bile acids synthesis three and four days after irradiation. Statistical differences among control (sham) and irradiated groups were assessed using Student's t-test: ∗ p<0.05; ∗∗ p<0.01; n, number of rats per group.
3.4 Effect of irradiation on total bile acid in colonic contents
Total bile acid concentrations and quantities in colonic contents were determined. Total bile acid concentration was increased from the first post-irradiation day ( of dry matter vs. in controls). A maximum value was observed three days after exposure, when this concentration was 8 fold higher than in controls. Four days after irradiation, this concentration decreased (only 3.5 fold higher than in controls) and thereafter returned progressively to control values. Total bile acid quantities in colon were enhanced at two and three days after exposure by 3.3 and 4.6 fold, respectively (Fig. 3). One, four, and seven days after irradiation, total bile acid quantities are very close to that found in controls.

Total bile acid quantities in colonic contents in sham-irradiated (C) and irradiated (D) rats. Statistical differences among control (sham) and irradiated groups were assessed using Student's t-test: ∗ p<0.05; ∗∗ p<0.001; n, number of rats per group.
4 Discussion
This study presents results concerning modifications of bile acid enterohepatic recycling after exposure to ionizing radiation. Radiation-induced modifications of bile acid profiles and related changes of the activity of the major enzymes implicated in hepatic bile acid biosynthesis were characterized in order to understand mechanisms leading to these modified profiles. Quantitative and qualitative changes of the profiles of bile acids were studied mainly by a time-course analysis of the composition of the bile acid pool into the bile (representing the pool reaching the gut) and by a quantitative analysis of total bile salts in colonic contents, in the first week following a whole body gamma exposure. A dose of 8 Gy was chosen according to previous results on biliary bile acid composition [12]. The more severe modifications in the biliary bile acid profiles were observed three and four days after irradiation, with a high proportion of secondary bile acids in the pool at these times. These results suggest that intestinal conversion of primary bile acids (β-TMC, TC, GC, and TCDC) into secondary (THDC, TUDC and TDC) was higher in irradiated than in control rats. This may be explained by altered intestinal transit [26] and/or by bacterial proliferation [27]. The observed changes in the bile acid profiles also led to a more hydrophobic pool of bile acids as early as the first post-irradiation day, with a significant increase of the hydrophobicity three and four days after exposure. This change of the hydrophobicity of the pool of bile salts reaching the intestine may have deleterious consequences in addition to more direct effects of irradiation on the intestine. In contrast to hydrophilic bile acids, which are known for their cytoprotective effects, hydrophobic bile salts are generally considered as having cytotoxic actions [28]. In particular, dihydroxylated bile acids are involved in inflammatory processes, since they are known to enhance the synthesis of inflammatory mediators such as leukotriene B4 [29] or stimulate the release of histamine [30]. A dihydroxylated bile acid such as deoxycholate stimulates also proliferation in colonic mucosa [31,32] and may be a promoter of colon cancer [33]. Depending on the dose of bile acid used, apoptosis may also be induced in colonic epithelial cells [34].
Radiation-induced qualitative and quantitative modifications of the pool of bile salts may be related to intestinal and hepatic alterations of the metabolism of bile acids. In fact, in physiological conditions, the composition of the bile acid pool is mainly the result of the equilibrium between intestinal reabsorption and hepatic synthesis and secretion [13]. Bile acids are effectively reabsorbed in the intestine by two mechanisms: a passive diffusion, which operates along the whole gut [35], and an active transport, which occurs exclusively in the terminal ileum via the ileal Na+/bile acid transporter (IBAT) [36] and the cytosolic Ileal Bile Acid Binding Protein (IBABP) [37]. These transporters are regulated by hydrophobic bile salts themselves at a transcriptional level [38]. Hepatic bile acid biosynthesis is regulated similarly by the quantity of bile acids returning from the gut and by the hydrophobicity of the pool [39,40]. After exposure to ionizing radiation, it has been previously shown that intestinal bile acid reabsorption is decreased [9,12,41], but the effects on hepatic bile acid biosynthesis have not been studied to date, except in our previous study in the hamster, which has noted a decrease of CYP7A1 activity, six days after exposure [42]. To address this question, the activities of the four major enzymes implicated in the bile acid biosynthesis were measured (CYP7A1, CYP27A1, CYP7B1, and CYP8B1). Such an approach is classically used by some laboratories in order to study the change of biosynthesis in pathological conditions [43] or after treatment with sequestrants of bile acids such as cholestyramine [44]. In the present study, these activities were determined at times when the composition of the pool was most markedly changed, i.e. three and four days after irradiation. Our results demonstrate for the first time radiation-induced modifications of the activity of these major enzymes, since the patterns obtained at three and four days after irradiation were very different. Three days after exposure, activity of CYP8B1 was greatly enhanced, whereas the activity of CYP7B1 was decreased. At the fourth post-irradiation day, the activity of the two enzymes leading to cholesterol 7α-hydroxylation (CYP7A1 and CYP7B1) was reduced.
Such changes in the pattern of the hepatic biosynthetic activities of the bile acids may be explained by several mechanisms, out of which two major ones will be discussed: (i) a consequence of radiation-induced intestinal malabsorption and (ii) a consequence of a radiation-induced inflammatory response.
In fact, radiation-induced modifications of the activity of hepatic enzymes implicated in bile acid biosynthesis may originate from reduced intestinal reabsorption after irradiation. This malabsorption has already been described in the rat [12,41] and, in the present study, it is most probably reflected by increased concentration of bile acids in colonic contents. However, the passive permeability properties of the intestine to each bile acid vary at different intestinal sites and these sites may adapt differently in response to irradiation [41]. Moreover, ileal active transport of bile acids might be decreased, since ileal Na/K-ATPase activity is markedly reduced in irradiated rats [45]. These alterations may result in a change in the ratio of bile acids returning to the liver and this modified pool may thus modulate the activity of hepatic enzymes implicated in bile acid biosynthesis. Thus, pathophysiological mechanisms may be proposed to explain the observed changes three and four days after irradiation. Three days after exposure, small intestinal damage and consequent bile acid malabsorption are maximal, as supported by the measurement of the total bile acids in colonic contents. This is concomitant with an increased production of secondary bile acids, which contribute to an increased hydrophobicity of the intestinal pool. The major hepatic modifications observed consist of a marked increase in the CYP8B1 activity and a slight decrease in the CYP7B1 activity. It may be hypothesized that intestinal bile acid malabsorption probably decreases the bile acid quantity returning to the liver. This quantitative defect of bile acids may induce compensation by the liver and, in particular, a stimulation of the activity of CYP8B1, which may be the more ‘sensitive’ enzyme. Our results suggest that, in addition to its sensitivity to hydrophobic bile acids [15], the CYP8B1 activity may be also sensitive to the quantity of bile acids arriving into the liver. At the fourth post-irradiation day, return of intestinal bile acids to liver seems to be greater than at the third day, as indicated by the level of bile acids in colonic contents. Concomitantly, the proportion of secondary bile acids is also decreased compared to that observed at the third post-irradiation day, but the hydrophobicity index remains very high. All activities varying at day 3 are decreased at the fourth post-irradiation day, but activity leading to chenodeoxycholate (CYP7B1) is more reduced. At this time, modulation of hepatic activities seems to respond to both quantity and quality of bile acids returning to the liver, as already shown in vitro as well as in vivo [46,47]. The modulation of these activities in the liver has been shown by several authors, who have demonstrated that they are regulated by the quantity and quality (hydrophobicity) of bile acids arriving into the liver at a transcriptional level by orphan nuclear receptors [48].
Concerning the hypothesis of inflammation, several studies have shown that cytokines such as TNFα and IL1β are potent inhibitors of the activity of enzymes like CYP7A1 [49]. These cytokines may stimulate hepatocyte transcriptional factors, which are able to suppress gene transcription [50]. It is usually accepted that exposure to ionizing radiation induces an inflammatory process associated with an increase of cytokines in blood as well as in tissues [51]. Thus, the hypothesis that radiation-induced increase of cytokine levels might contribute to decrease the activities of cholesterol 7α-hydroxylation has to be considered.
Our results show for the first time that whole body irradiation leads to alterations of the hepatic metabolism of bile salts by modifying some of the major activities implicated in their biosynthesis. These hepatic alterations are probably related directly to intestinal malabsorption because of the characteristics of the enterohepatic circulation of bile acids. However, a possible role of radiation-induced inflammatory mediators released after radiation exposure should also be considered.
Acknowledgements
The authors wish to thank C. Baudelin, O. Combes, and J. Wysocki for their technical assistance, and Dr N. Dudoignon for his kind assistance in animal management.


