1 Introduction
Thyroid dysfunction could be induced by various factors such as ionizing radiations [1], pollution [2–4] malnutrition [5], antithyroid drugs [6,7] dietary goitrigens [8], and iodine-deficiency [9]. Children born in iodine-deficient areas are at risk of neurological disorders and mental retardation because of the combined effects of maternal, foetal, and neonatal hypothyroxinemia [10]. In some parts of African countries like Congo, Uganda, and Kenya, the prevalence of goitre exceeds 81% [11]. Although iodine deficiency is the main factor in the aetiology of endemic goitre, the additional role of goitrogens has been shown or suspected in areas such as Congo and Sudan, where goitre is endemic [12]. In these regions, people are exposed to thiocyanate overload provided by consumption of cassava roots, which are used as a staple food and contain the cyanogenic glucoside linamarine [13]. Linamarine metabolism produces thiocyanate (SCN−), a well-known goitrogen [14]. SCN− ion competes with iodide (I−) trapping, iodide oxidation, and binding to thyroglobulin at the level of the thyroperoxidase enzyme [15]. Indeed, the SCN− and the iodide share some common physiochemical properties, and both are oxidized by the peroxidase enzyme [16].
In Africa, poverty and food scarcity due to drought and war force consumption of incomplete detoxified cassava [17]. The SCN− overload originating from consumption of poorly detoxified cassava is considered a contributing factor responsible for the increase of the goitre rate [18].
Life-style-related factors such as smoking are considered another common source of thiocyanate contamination. In fact, cigarette smoke contains several toxins such as hydrogen cyanide and 2,3-hydroxypyridine [19,20]. In the whole world, tobacco arises as a very serious problem. According to the World Health Organization (WHO) report (2002) dealing with worldwide smoking statistics, about one third of the male adults in global population smoke; smoking is on the rise in the developing world, but is falling in developed nations. Among people of the developing world, tobacco consumption is rising by 3.4% per year [21]. Cigarette smoke containing HCN is rapidly absorbed by the lungs galins, the systemic circulation spreading out in the entire organism by passing the portal circulation [22]. HCN is detoxified by a conversion through an intramitochondrial enzyme, the rhodenase, to the less toxic compound, the thiocyanate [23]. Consequently, smoking increases greatly SCN− levels in the serum [16].
Human thiocyanate contamination can be also the result of pesticide use. For example, aminotriazole, a systemic non-selective herbicide, is often associated with ammonium thiocyanate, which enhances its activity [24].
Manufacturing activities releasing hydrogen cyanide include electroplating, metal mining, metallurgy, and metal cleaning process; these are considered the most important cause of thiocyanate contamination in workers [25,26].
Thiocyanate can be transmitted from dams to pups during the lactating period via breast milk feeding. Indeed, SCN− effects were assessed on different experimental species, such as rats [7], mice [6], and infants [22], during the lactating period. However, there are no previous investigations dealing with thiocyanate consumption and thyroid function of weaned young mice during the developmental period.
The objective of our work was to study the effects of weaning period on the thyroid function of thiocyanate-treated young mice, issued from thiocyanate-treated mothers. Weaning is a very critical period for pups' behaviour and maturity (water and food consumption), especially if we take in consideration the fact that hypothyroidism provokes a delay in nervous system development and behaviour maturity, enhancing more difficulties for adaptation to the new life conditions, in the lack of mother care.
2 Materials and methods
2.1 Animals
According to the European convention for the protection of vertebrate animals used for experimental and other scientific purposes (Council of Europe No. 123, Strasbourg, 1985) and according to the review committee of our institution, mice rearing and experiments of this work were approved. In the present study, adult Swiss strain mice weighing about 30 g were purchased from the Central Pharmacy (SIPHAT, Tunisia). They were housed at with light/dark periods of 12 h and a minimum relative humidity of 40%. They had free access to water and commercial diet (SICO, Sfax, Tunisia). The standard diet contained of iodine by gram of diet. Iodine content of diet was determined, after acid mineralization, using the catalytic method of Sandell and Kolthoff [27]. After one week of acclimatization to the laboratory conditions, female mice were caged overnight with males and the presence of spermatozoa in the vaginal smear indicated day zero of pregnancy.
2.2 Experimental protocol
The initial number of pregnant mice was 18, equally divided into three groups of six individuals (Table 1): controls (group A) and treated with thiocyanate (1 g/l) administered in drinking water of dams from the 15th day of pregnancy, before the beginning of foetal thyroid function, until either day 25 (group B: mice treated during 31 days) or day 15 after parturition (group C: mice treated during 21 days with 10 days of treatment withdrawal before sacrifice). Each pregnant mouse was separated in an individual cage to deliver spontaneously three weeks after coitus. At birth, litters were reduced to eight pups each (four males and four females if possible), because it has been shown that this procedure maximizes lactation performance [28]. Therefore, the total number of pups was 144. The day of birth was considered as postnatal day one.
Treatment periods of 25-day-old mice, controls (group A) and treated with thiocyanate [SCN− (1 g/L)] given to mothers' drink from day 15 of pregnancy until either the 25th day (group B) or the 15th day (group C) after delivery
| Biological events | Coitus | Parturition | Weaning | Sacrifice | ||
| Pups age (days) | – | – | 1 | 15 | 21 | 25 |
| Time after coitus (days) | 0 | 15 | 20 | 35 | 40 | 45 |
| Group A | No treatment | |||||
| Group B | ||||||
| Group C |
For each group, all pups (n= 48) were precisely weighed. We have not observed any differences between male and female growth because, for this period of treatment, mice are not pubescent and sexual hormones are not yet secreted by gonads. Only 42 young mice were used for the determination of thyroid weights. The thyroids of the six other pups were taken with a piece of trachea for histological studies.
Mice of the three groups were weaned at the 21st day after parturition (five days before sacrifice). Before the weaning day, mice survived only on the mothers' milk and thus obtained thiocyanate from the breast milk. The daily-consumed food and water by weaned mice was precisely measured during five days before sacrifice (Table 2).
Daily food, water, iodine and thiocyanate intake by weaned mice (during 5 days): controls (group A) and treated with thiocyanate [SCN− (1 g/L)] given to mothers' drink from day 15 of pregnancy until either the 25th day (group B) or the 15th day (group C) after delivery
| Parameters and treatments | A | B | C |
| Food consumption (g/day/mouse) | 4.35±0.25 | 3.98±0.43+++ | |
| Water consumption (ml/day/mouse) | 4.36±0.15 | 3.95±0.47+++ | |
| Ingested iodine (μg/day/mouse) | 3.13±0.18 | 2.86±0.46+++ | |
| Ingested SCN (mg/day/mouse) | 2.03±0.57 | – |
2.3 Determination of morphological and biochemical parameters
After anaesthesia with chloral hydrate by intraabdominal way, body weights of young mice were measured. Femurs (right or left) were carefully dissected out for length and weight determination (). Blood samples were collected from the brachial artery of pups and plasma samples were withdrawn after centrifugation at 2200 g for 15 min and kept at −20 °C. Only eight samples of plasma were randomly chosen for the determination of free T4, free T3 and TSH by radioimmunoassay, using commercial kits from Immunotech, France (refs: 1363 (FT4); 1579 (FT3)), and for that of TSH using a rat-specific TSH kit supplied by IBL-Germany (ref.: AHR001).
2.4 Chemical and histological studies
Thyroid glands () were removed from pups, they were weighed and kept at −20 °C until biochemical analysis. Eight thyroid glands were randomly selected for iodine content determination after alkaline mineralization, according to Sandell and Kolthoff's method [27].
Six thyroid samples were randomly selected for light microscopy. They were taken with a piece of trachea and fixed in a Bouin solution, embedded in paraffin and serially sectioned at 5 μm. Then, the sections were stained with hematoxyline eosin (HE) [29].
2.5 Statistical analysis
Comparisons of mean values between mice treated groups (B and C) and control group (A) or between treated mice (group C and group B) were made using ANOVA followed by Student's t-test. Statistical significance was defined as a P value of less than 0.05. Values were expressed as the means followed by standard deviation.
3 Results
3.1 Growth and feeding
3.1.1 Body weight variation
The dose of SCN− (Table 2), administered to group B, provoked growth retardation of mice comparatively to group A. Indeed, the body weight was decreased by (Fig. 1). When thiocyanate was eliminated from the mothers' drinking water on the 15th day following parturition (mice of group C), a partial recovery was obtained in body weight after ten days () comparatively to animals of group B (Fig. 1). This recovery occurred without reaching control values.
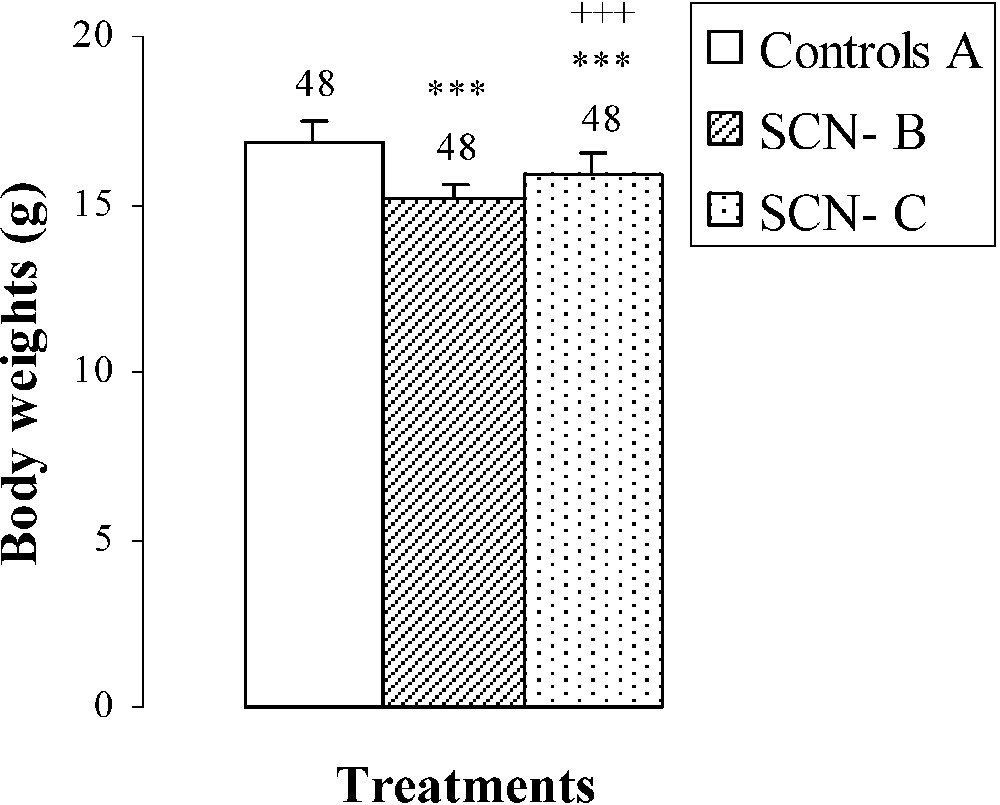
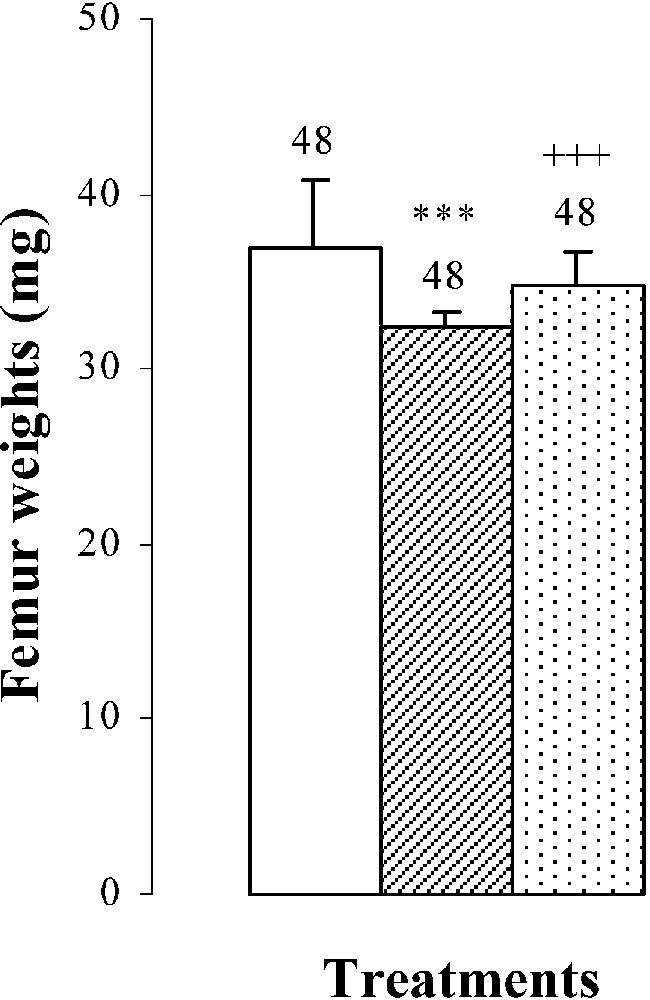
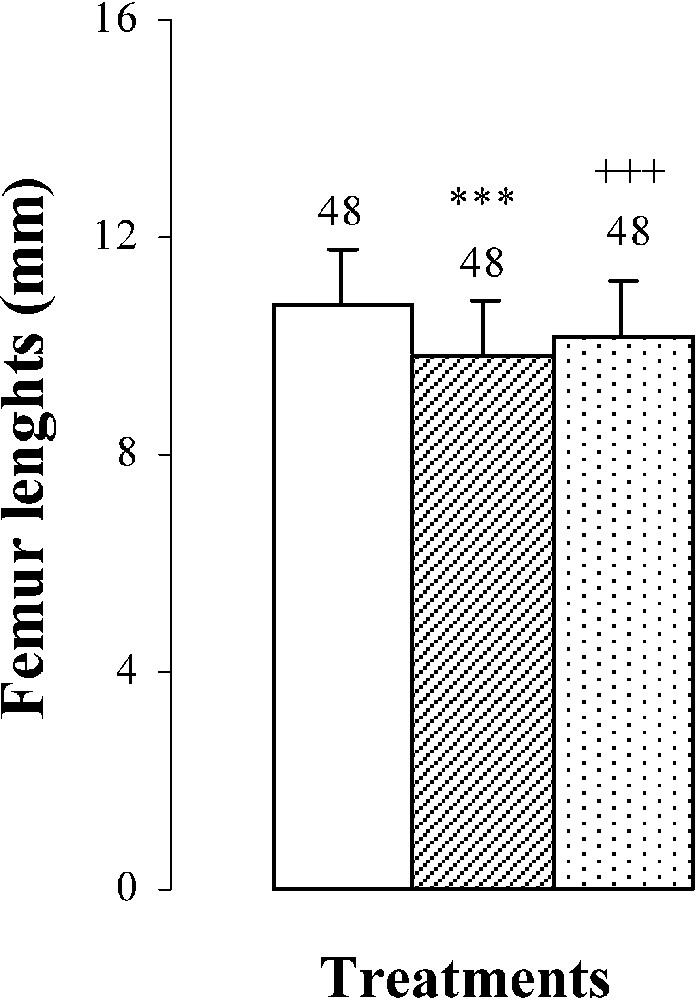
Body weights, femur weights and lengths of 25-day-old mice, controls (group A) and treated with thiocyanate [SCN− (1 g/L)] given to mothers' drink from day 15 of pregnancy until either the 25th day (group B) or the 15th day (group C) after delivery. Treated B or C vs. controls A: *** p⩽0.001. Treated C vs. treated B: +++ p⩽0.001. The number of determinations is represented above the columns.
3.1.2 Femur length and weight variations
The femur weights and lengths of mice of group B were decreased by and , respectively, comparatively to those of controls (Fig. 1). In group C, bone growth was partially recovered in comparison with group B ( in femur lengths and in femur weights). This improvement occurred without reaching control values (group A) (Fig. 1).
3.1.3 Food and water consumption
The decrease in body weight was associated with a reduction in food () and water () consumptions and in ingested iodine quantities () (Table 2). Compared to group B, group C showed an improvement in water and food consumptions by () and (), respectively (Table 2).
3.2 Thyroid gland weight variations
An hypertrophy of the thyroid gland by was noted in pups treated with SCN− (group B) (Fig. 2), probably resulting from the increase () of TSH: plasma thyroid stimulating hormone (Fig. 2). After SCN− withdrawal (group C), thyroid gland weight decreased by () comparatively to animals of group B (Fig. 2). Plasma TSH levels decreased by () and by () when compared to groups B and A, respectively (Fig. 2).
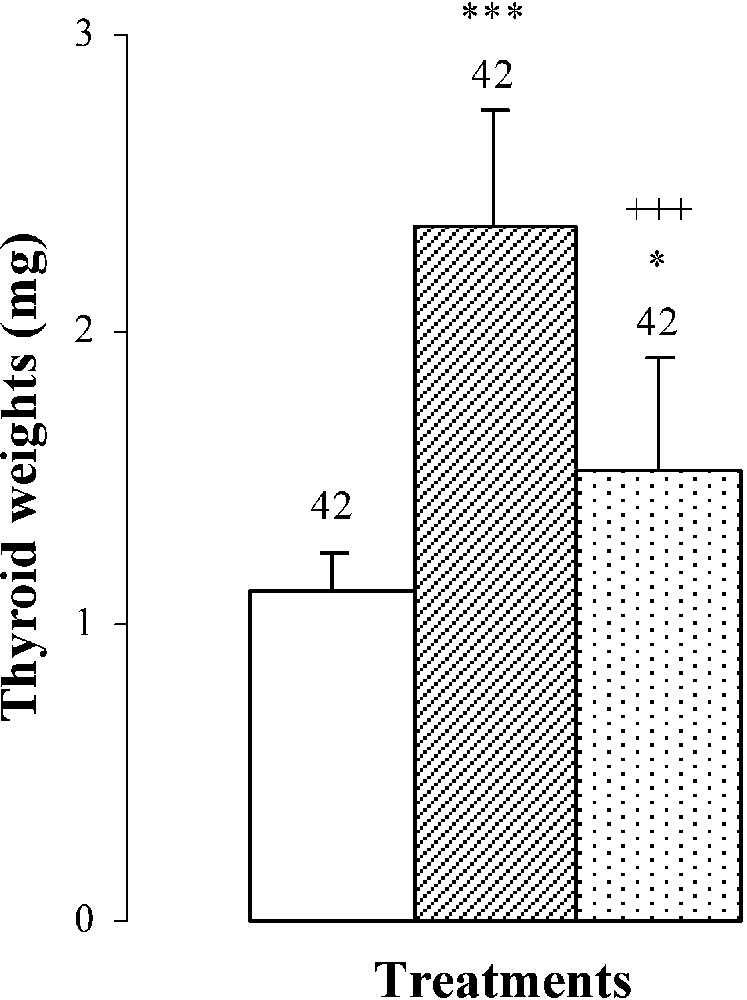
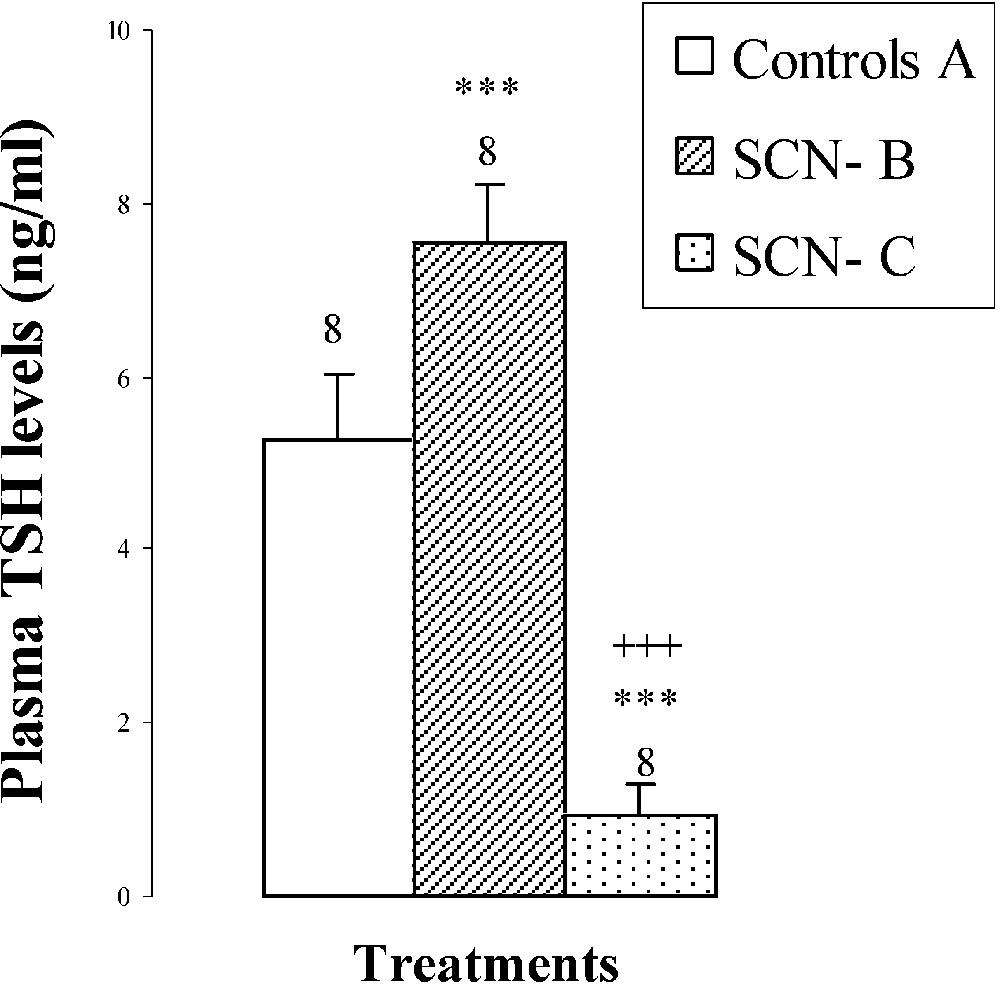
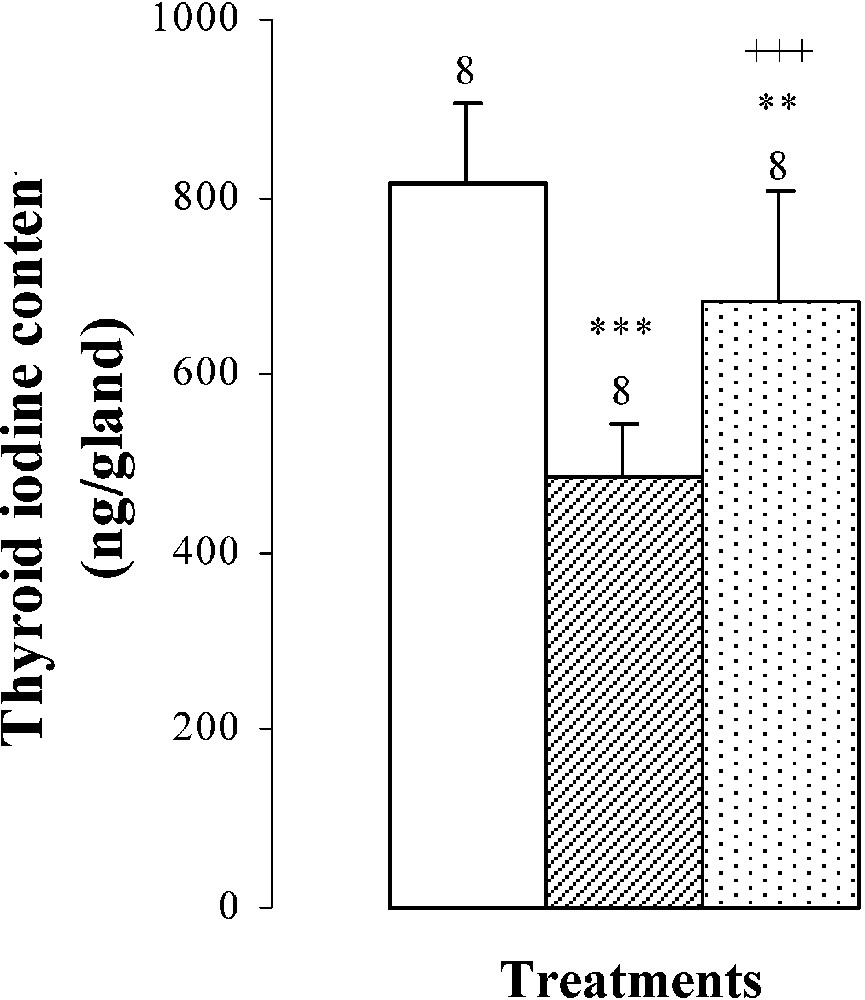


Thyroid weights, plasma TSH levels, thyroid iodine content, and plasma thyroid hormone (FT4, FT3) levels of 25-day-old mice, controls (group A) and treated with thiocyanate [SCN− (1 g/L)] given to mothers' drink from day 15 of pregnancy until either the 25th day (group B) or the 15th day (group C) after delivery. Treated B or C vs. controls A: * p⩽0.05; ** p⩽0.01; *** p⩽0.001. Treated C vs. treated B: ++ p⩽0.01; +++ p⩽0.001. The number of determinations is represented above the columns.
3.3 Thyroid hormone levels
The thyroid hormone levels FT4 and FT3 in plasma were decreased by and , respectively, in mice of group B comparatively to those of group A (Fig. 2). The reduction of plasma thyroid hormone levels was the result of the decrease in daily-ingested iodine () and thyroid iodine content by () (Table 2 and Fig. 2). After SCN− withdrawal, group C showed an increase in thyroid iodine contents () and in plasma thyroid hormone levels of FT4 by and of FT3 by when compared to those of group B (Fig. 2). Daily-ingested iodine was also improved by () in group C, comparatively to group B (Table 2), while compared to animals of group A, mice of group C presented a total restoration of plasma FT4 levels, although thyroid iodine contents and plasma FT3 levels of group C were lower than those of group A and were respectively increased by () and () (Fig. 2).
3.4 Histological modifications
The morphological and biochemical perturbations were confirmed by the histological study of thyroid glands. Indeed, thyroids of group B presented closed follicles, follicular cell hyperplasia, an increase in follicular number and vascularity as well as a decrease in colloid volume (Fig. 3, photo B). However, in controls (group A), the majority of follicles were larger than those in treated mice (B and C), lined by more flattened epithelium and filled with a deeper staining colloid (Fig. 3, photo A). Ten days after SCN− withdrawal (group C), the histological aspect of the thyroid glands was improved comparatively to animals of group B, with an increase in colloid volume and a decrease of follicular cell hypertrophy (Fig. 3, photo C).
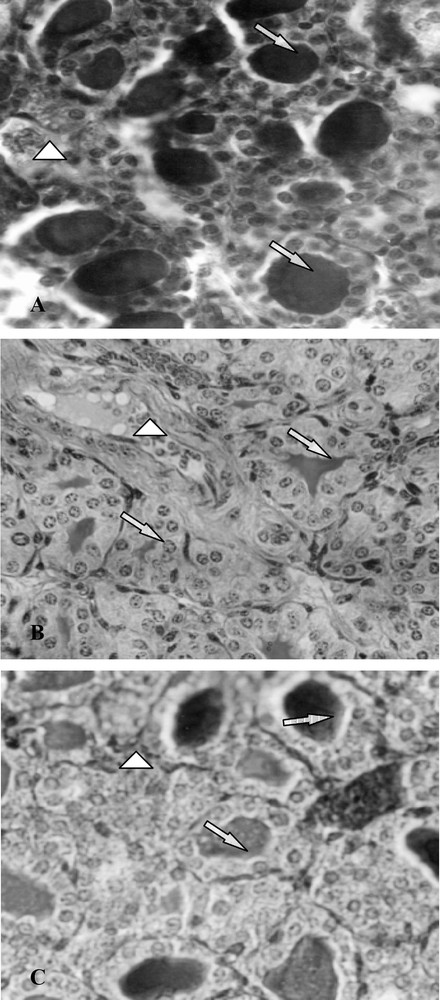
Histological thyroid sections of 25-day-old mice, controls (group A) and treated with thiocyanate [SCN− (1 g/L)] given to mothers' drink from day 15 of pregnancy until either the 25th day (group B) or the 15th day (group C) after delivery. ⟹: Arrows indicate thyroid follicles; ▷: arrows indicate blood vessels.
4 Discussion
Weaning is one of the most important events in the early stage of life. After weaning, pups become nutritionally and behaviourally independent of their dam, so the weaning procedure influences their physiological and neurobehavioural development [30].
In this study, we noted important thyroid hormone changes within the pup, which occurred during the weaning time period. It is well known that two hormones, specifically thyroxin (T4) and corticosterone, show developmental surges prior to weaning initiation [31,32]. These hormones have been shown to induce maturational changes in various organ systems during the third postnatal week [33]. Moreover, it was previously demonstrated that hypothyroidism affects the timing of weaning [34], whereas weaning effects on hypothyroid offspring physiology and behaviour are yet less well understood.
In the present study, thiocyanate treatment of group B mice provoked a decrease in body weight, femur growth, thyroid iodine content, and plasma thyroid hormone levels. Because the iodine is the precursor of thyroid hormones' biosynthesis, the transport and the concentration of iodine within the thyroid gland represent the first step in the production of the iodine content of thyroid hormones [35]. Indeed, iodide uptake occurs across the basolateral membrane of polarized thyroid follicular cells and is an active transport driven by inwardly directed Na+ gradient [36]. In rats, the activity of sodium iodide symporter (NIS), an intrinsic membrane-associated protein cloned and characterized, was shown to be under the control of TSH [37]. Thiocyanate and iodide (I−) have a similar charge and a comparable ionic volume, and the SCN− could be transported by the NIS system [38]. Furthermore, similarly to iodide trapping, the SCN− catch by the thyroid cell is increased in the presence of the TSH [39] and the intrathyroidal metabolism of SCN− also increased after TSH stimulation. Hence, the SCN− would be rapidly oxidized by the thyroperoxidase (TPO) enzyme in the presence of H2O2. Therefore, as it was recently demonstrated by Contempré et al. [16], the SCN− is not accumulated in the thyrocyte, and it ultimately generates an I-deficient thyroid with a decrease in thyroid hormones' synthesis that would induce growth retardation [16]. Actually, many other studies have shown the stimulation of bone growth by thyroid hormones that have a direct effect on longitudinal bone growth in both humans and rats [40,41] in addition to their direct effect on the nervous system's maturation [42].
During the five days of mice weaning, we have noted an important daily decrease in food and water consumptions and in iodine intake. These behavioural perturbations could be the result of the stress caused by weaning when the totally mother-dependent pups become self-autonomous mice. Most of the physiological functions were modified in correlation with an important maturation of organs (increase in intestine size, acceleration of bone ossification, autonomous thermoregulation...). Moreover, young mice have to find their food and respond to external stimuli [43]. Furthermore, Goody and Kitchen [44] have demonstrated that weaning rat pups at day 21 activate the δ-opioid receptor that mediates swim-stress-induced analgesia (swim SIA), while Muhammad and Kitchen [45] have shown that a delay of weaning until the 25th day beyond the normal day of weaning was able to halt the μ- to δ-opioid receptor transition and the mediation of swim SIA by 5 to 10 days. The recent study of Hickey et al. [46] showed that the abrupt weaning of calves is a stressor that also increases the physiological measures of stress hormones. These data correlates with our findings, showing that while adding SCN− in the drinking water, a feeding decrease and behavioural perturbations occurred.
On the sacrifice day of mice treated during the full period (group B), an increase in thyroid gland weights was obtained. This result can be explained by a feedback to the hypothalamo-hypophysis axis, indicating the low levels of FT4 and FT3 in the blood; hence, more thyroid-stimulating hormone (TSH) was released in order to increase the production of these hormones. Consequently, hypothyroidism and hypertrophy of the thyroid gland were evidenced. These states of the thyroid gland, observed in group B, were the result of the continuous SCN− exposure from the neonatal period until the weaning period. Indeed, SCN− is transmitted from dams to foetus during pregnancy by placenta [47,48] and from mothers to pups during the lactating period by breast milk [6,22,49,50]. After weaning, young mice consumed directly SCN− in the drinking water until the sacrifice day. Recent studies showed that hypothyroidism is associated with modest pituitary enlargement in humans, and several case reports of pituitary enlargement sufficient to present visual impairment through comparison of the optic chiasma have been published [51,52]. These data correlate with an important TSH secretion under hypothyroid state, as it was found in our study.
Histological changes observed in thyroid slides of group B were characterized by closed follicles, follicular cell hyperplasia, an increase in follicular number, and vascularity, as well as a decrease in colloid volume. These data confirmed previous results by Soussia and collaborators [7], who had demonstrated that in the case of rat species, thiocyanate treatment provoked follicular cell hyperplasia and a decrease in colloid volume. Moreover, these authors observed, on the periphery of follicular cavities, the presence of empty vacuoles resulting from colloid resorption. This event was not observed on mice histological slides.
When SCN− was removed from the drink of group C mothers at the 15th day after delivery, the young s' body and femur growth rates, the thyroid iodine content and plasma thyroid hormone levels increased in comparison with those of group B, but did not reach control values (group A).
Improvement of all biochemical parameters might be explained, first, by an amelioration of the mothers' milk iodide content before the weaning day (21st day). Indeed, thiocyanate treatment during the lactating period inhibited the two effects of prolactine on iodide and NIS accumulation by the mammary gland [53]. During this period, thiocyanate inhibited iodide transporter activity as well as bearing functions like a general metabolic inhibitor [53]. Furthermore, results of Ben Hamida et al. [6] showed that after 12 days of thiocyanate withdrawal from drinking water during the lactating period, body weight and plasma thyroid hormone levels of both mothers and pups increased. Second, after the weaning day, the young mice of group C did not receive thiocyanate from water and showed an increase in daily food consumption and ingested iodide; these helped in thyroid iodine retention. Consequently, a reversible thiocyanate effect was noted, with a partial coming back of plasma thyroid hormone levels.
In contrast to iodine deficiency, where there is a preferential FT3 rather than FT4 production, we have noted that the FT3 plasma level is significantly lower, whereas FT4 is slightly reduced compared to those of group A. We can explain this result by the fact that thiocyanate has an inhibitory effect on T4 5′-monodeiodination (DI), as demonstrated by Langer et al. [54].
On the other hand, inhibition of DI could be explained by restriction of calorific supply to mice during the critical period of weaning. In fact, we have obtained a decrease in daily food and water consumption and in body weights of 25-day-old mice. Moreover, it is well known that starvation and protein-calorie malnutrition inhibited specifically DI, inducing the reduction of plasma FT3 levels [55,56]. It seems that the content of carbohydrate in diet as well as calorific supply maintained DI activity at the normal level.
The increase in thyroid hormone production was responsible for the decrease in TSH levels and in thyroid weights, as it was found in this investigation.
In group C, we found an important decrease in plasma TSH level comparatively to the treated group B and the control group A. This result would be explained by reversible pituitary gland hypertrophy associated with cells' transdifferentiation impairment [57].
Restoring iodide, by removing SCN− from drinking water, ten days before sacrifice, reduced the hypertrophy of follicle epithelial cells and increased the colloidal volume. Some follicles became also larger than those of group B. In human pathologies, goitre size has been found to be reduced either by application of an iodide treatment [58], which induced a decrease in the size of cells and follicles [59], or by a reduction of plasma TSH levels, as demonstrated by our results. In fact, Van Sande et al. [60] noted that iodide uptake by the thyroid gland is competitively inhibited by SCN−, especially high-goitrogenic substance consumption, and is alleviated with normal iodine supply [50]. This reversibility explains the return of the thyroid gland to its normal aspect.
In group C, values of consumed food and water as well as daily iodide intake were improved when compared to group B, and showed no significant difference with those of group A. This improvement would be explained by the development of their neurobehavioural activities. Indeed, after withdrawing SCN−, the thyroid function was restored and plasma thyroid hormone levels increased, which enhanced cell multiplication in the central nervous system. Indeed, Rabie et al. [61] have demonstrated that T4 treatment of two-week-old hypothyroid rats induced a transient wave of cell multiplication in the external granular layer of the cerebellum. Therefore, according to these authors, the number of mitoses per Purkinje cell was increased by 45% after treatment with T4 for only two days. It seems that a significant number of differentiated cells in cerebellum was increased after T4 treatment of hypothyroid rats. Moreover, we had obtained, in a previous investigation [62], dealing with the effects of thiocyanate on the nervous system's maturation, an improvement of the biochemical parameters and of the histological aspects of cerebellum after 10 days of thiocyanate withdrawal in 25-day-old mice. Therefore, we can postulate that, during the first 15 days of life, transient hypothyroidism can occur without any effect for the neurobehavioural development of mice.
In conclusion, our study showed that, in the case of mice treated with thiocyanate, hypothyroidism effects added to the weaning event affected greatly the thyroid function and behaviour. The 25-day-old mice treated for 31 days (group B) have lower plasma thyroid hormone levels and grow less than those treated for 21 days (group C). These perturbations could be largely reversed by withdrawing thiocyanate treatment for a period of ten days.
Acknowledgements
The authors are indebted to Mrs Nabiha Mezghanni for her skillful technical assistance in radioimmunoassays. This work was supported by the DGRST grant (Appui à la recherche universitaire de base ARUB 99/UR/08-73), Tunisia.


