1 Introduction
Rooting of microshoots is critical in plant micropropagation systems of woody plants [1–3]. Many researchers have demonstrated that the initiation of adventitious roots in vitro in excised shoots of the apple rootstocks is related to several factors [4–7]. The most important factors in rooting induction and initiation are concentration of auxin and treatment duration [8–11]. To control the rhizogenesis steps, it may be indispensable to know the signals and the specific internal mechanisms [12]. Rooting was at first considered as a single-phase process, but there are several successive reports where adventitious rooting was shown to depend on a series of interdependent phases (induction, initiation and expression) [13–16].
Knowledge of biochemical and anatomical events associated with root induction and expression is useful, as it will permit the improvement of rooting procedures. Various studies on adventitious root formation have shown the role of markers played by peroxidase in rooting of plants cultured in vitro [17–21]. Hausman [22] and Kevers [23] have also reported the role of auxins in relation to the peroxidase activity of various plant species. Auderset et al. [24] suggested a correlation between ontogenic stages and evolution of biochemical markers during in vitro rooting of Malus domestica.
In the present paper, histological events leading to in vitro root formation in apple rootstocks and evolution of peroxidase activity during in vitro rooting of shoots were examined in correlation with the different stages of rooting and the necessary duration of auxin presence.
2 Materials and methods
2.1 Plant material, culture conditions and rooting procedure
Starting material for in vitro culture of rootstocks of apple tree MM106 was supplied by the laboratory of in vitro culture of ‘Mabrouka Company’, located at Mornag city, Tunisia.
The proliferating shoots were subcultured every four weeks on Murashige and Skoog [25] medium supplemented with 0.4 mg l−1 BAP, 0.1 mg l−1 IBA, and 0.2 mg l−1 GA3. These shoots (3–4 cm) were individualized and used later for rooting experiments.
The rooting basal medium (RM) was a half-strength Murashige and Skoog [25] medium supplemented with 1 mg l−1 IBA, 0.4 mg l−1 thiamine–HCl. The same medium without growth regulator (NRM) was used as control for non-rooting. All media were supplemented with 30 g l−1of sucrose and pH adjusted to 5.7–5.8 with KOH or HCl 0.1 M prior to the addition of agar (5 g l−1) and subsequently autoclaved for 30 min at 120 °C. For rooting experiments, shoots were cultured in 500-ml ‘Le Parfait’ glass jars containing 100 ml of medium. Cultures were placed in the dark on RM. Fifteen shoots for in vitro root development were used, and the experiments were repeated six times.
With a view to studying the effect of the duration of dark periods on RM, the shoots were transferred after different times (0 to 10 days) into glass jars with auxin-free basal medium (NRM) in the light and maintained in a growth chamber at 26 °C with a 16-h photoperiod and a light intensity of 40 μE m−2 s−1. Rooting percentages were recorded four weeks after the auxin treatment started.
2.2 Histology
At days 0, 3, 5, 7 and 10 after treatment, bottom 4–7-mm sections of the stem were fixed in formaldehyde/ethanol/acetic acid (FAA) 10:85:5 (v/v/v), dehydrated in alcohol series between 50 and 100%, transferred into gradual alcohol–xylol series and finally embedded in paraffin. Seven-micrometre-thick transverse sections were cut with a rotatory microtome (Leica, RM2125 RT) and stained with haematoxylin and safranin.
2.3 Determination of peroxidase activity
Shoots were collected and their basal part was stored at −80 °C until extraction. Samples (100 mg) were ground in 1 ml of phosphate buffer 0.1 M (pH 7) and centrifuged for 20 min at at 4 °C. The supernatant was used as the crude enzyme extract. 100 μl of each crude enzyme extract were added to the reaction mixture, consisting of 900 μl guaiacol (0.1%) and 100 μl H2O2 (30%) and the optimal density of the reaction was measured at 470 nm. Enzymatic activity was expressed as the absorbance per minute per milligram of protein (ΔDO min−1 mg−1 Prot). Protein concentration was determined by the Coomassie blue method [26].
Three extractions were processed during the ten first days for peroxidase activity measurement. Three replicates were measured for each extract. The results are the mean of at least three values ± standard errors.
3 Results
3.1 Anatomical changes
Transverse sections of non-treated stems at day 0 showed typical collateral vascular bundles, forming a ring around the pith in the basal end of the microcuttings. There was a cambial zone with 3–4 layers of flat cells between the xylem and phloem of the stem. Epidermis was formed of three cellular layers; a cortex surrounding the vascular cylinder consisted mainly of large parenchymatous cells (Fig. 1A and B).
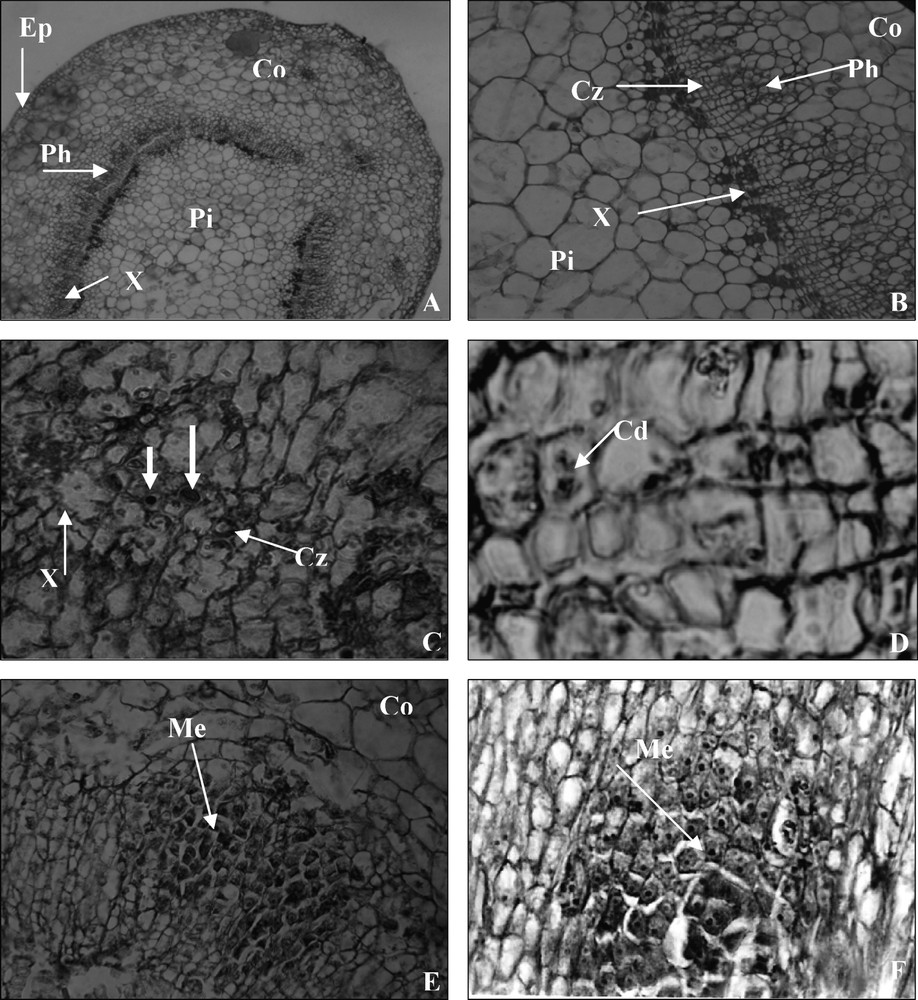
Sections of the stem basal shoot origin 0–5 days after IBA root inductive treatment. (A) Anatomical structures of the stem base at day 0 showing a vascular bundle. (×250); Pi, pith; Co, cortex; Ep, epiderm; Ph, phloem; X, xylem. (B) A cambial zone is bounded externally by phloem and internally by xylem. (×1000); Pi, pith; Co, cortex; Cz, cambial zone; Ph, phloem; X, xylem. (C) Transverse section near the stem base at day 3, cells in the cambium and adjacent to it became activated, cells with big nuclei and nucleoli. (×1000); Cz, cambial zone; X, xylem. (D) The first mitotic divisions (×2500); Cd, cell divisions. (E) Section near the stem bases after 5 days on rooting medium showing the larger region of densely stained meristematic cells make up the organisation of meristemoids (×1000); Me, meristemoids. (F) Longitudinal section showing densely stained cytoplasm with prominent nuclei characteristic of cell divisions and making up of the meristemoids (×1000); Me, meristemoids.
After three days of auxin treatment, certain cells in the cambial zone and adjacent phloem became more densely stained in their cytoplasm and nuclei appeared more frequently (Fig. 1C). At the same time, the first mitotic divisions were observed (Fig. 1D).
Five days after the onset of treatment, regions of densely stained cells appeared as a result of active cell division. External to the xylem, most cells in the phloem region near the cambium showed signs of meristematic activity, with a more densely cytoplasm and with nuclei containing large prominent nucleoli. The cells became isodiametric and some of them started to divide and produced meristemoids near the cambium (Fig. 1E and F).
The meristemoids enlarged in volume and cell number increased as a result of their division. The appearance of the meristemoid signalled the initiation of adventitious roots.
At day 7, the meristemoids were individualised and polarisation of the divisions gave birth to the typical pointed shape with the apex extending outward into the cortex of the root primordium. The cells in the curved area were more lightly stained and were presumably those of the root cap (Fig. 2A, B and E).

Sections of the stem basal shoot 7–10 days after IBA root inductive treatment. (A) Seven days after treatment. Transversal section with a well-developed root primordium. (×125); Rp, root primordium. (B) Longitudinal section showing young root primordium apparently having pushed into the cortex tissue. (×500); Rp, root primordium. (C) Detail of young root primordium through organized division cells (×2500). (D) Transverse sections near stem bases, at day 10 on rooting medium, showing root primordia projected beyond the stem surface and two root primordia at various stages of development. (×250); Rp, root primordium; Ar, adventitious root. (E) Protruding root primordium, showing the formation of root caps. (×1000); rc, root cap. (F) Emerging adventitious roots 15 days after treatment, showing complete differentiation of their vascular system, which is now continuous with that of the microcutting stem (×125).
The central cells of the root primordium, which were more densely stained, were presumed to comprise the meristem of the adventitious root [27]. Other cells in the root primordium stained less densely than the central cells and were presumed to be precursors of the future cortical and vascular tissues (Fig. 2C) [27].
At day 10, the root primordia differentiation was achieved; they penetrated the stem cortex and were projected well beyond the stem surface (Fig. 2D).
The processes of initiation and development of adventitious roots were not synchronous, different stages of adventitious root developing in single pieces of stem were observed at the same time (Fig. 2D), and not all meristemoids were developed into adventitious roots. Root primordia had developed a vascular system, which was continuous with that of the stem (Fig. 2F).
3.2 Determination of the duration of the rooting induction–initiation phase
The shoots were cultured on the RM for different times ranging from 0 to 10 days before transferring then into the same medium without auxin (NRM) and examining the percentage of rooting. The results of Fig. 3 indicated that a period of 5 days on RM was sufficient to induce 97% rooting. The exposure to IBA for periods longer than five days in the dark produced undesirable side effects on shoots, such as callus formation and leaf necrosis.
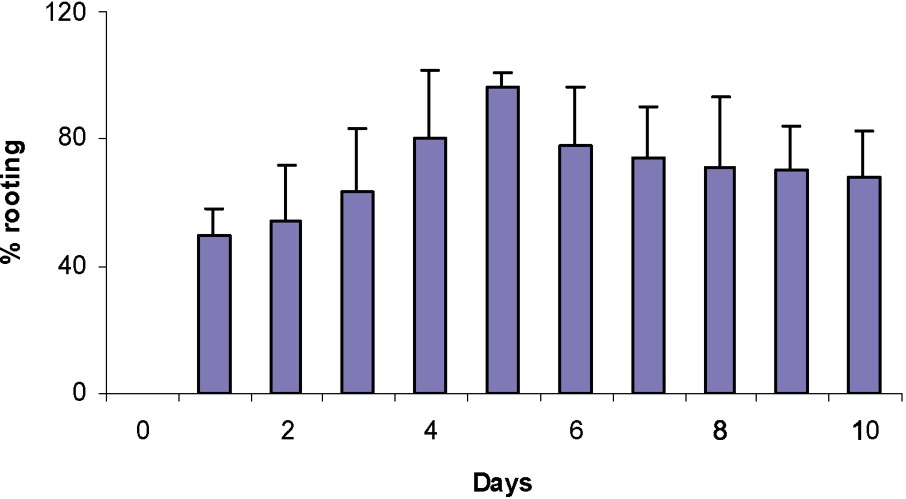
Effect of the period during which the shoot is kept in the dark on the rooting percentage after 4 weeks on the IBA rooting medium.
3.3 Peroxidase activity
Peroxidase activities in microshoots cultured over different durations of their rooting process were determined (Fig. 4). In the case of control (on NRM), there was no change in peroxidase activity during the course of the experiment. Auxin treatment induced a sharp increase in peroxidase activity. The peroxidase activity of shoots placed in the rooting medium in the dark decreased on day 1, increased between days 4 and 5 of culture, then declined rapidly, and afterwards stabilized.
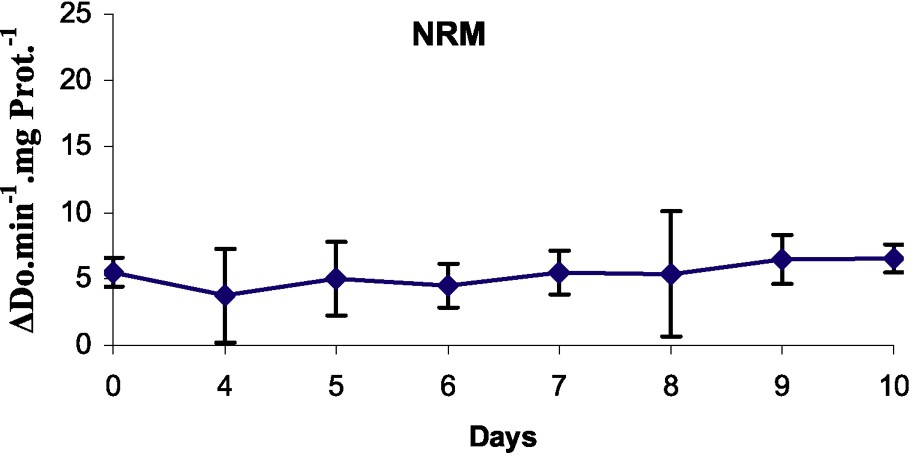
Peroxidase activity in the basal part of rootstocks of MM106 during the first ten days on rooting medium (RM) or on non-rooting medium (NRM).
4 Discussion
This study showed the analysis in the course of time of the rooting phases in microcuttings of rootstocks MM106 apple tree by triggering cell division, leading to the formation of adventitious roots. This was completed by biochemical and physiological analyses with a view to improving the rooting process.
In the present study, shoots of rootstock MM106 did not root on a medium without auxin; there was no spontaneous rooting observed without the application of auxin. This woody plant required a rooting medium with auxin (IBA was excellent). If, for many years, the only evidence of the involvement of auxins in the induction of adventitious came from studies on exogenous applications [28], now variations in the endogenous auxin level were observed [22].
The first histological sign of the formation of adventitious roots was the appearance of larger nuclei and denser cytoplasm (Fig. 1C) in cells of the cambial zone and adjacent phloem. The anatomical changes were similar to these observed in in vitro rooting of chestnut [29,30], apple rootstocks [31,32], oak [33], Rosa multiflora [11], camellia [34], and artichoke [35].
Initiation of cell division by the rooting treatment took place in the phloem region near the cambium (Fig. 1D). In other studies of woody species, the site of origin has been located near to or in the vascular cambium [24,31,33,34,36–39]. In each study, cells leading to root formation could have been phloem parenchyma [40–42] or cambial derivatives leading to phloem parenchyma [30,32,43]. The region of the tissue in which cells become activated is thought to depend in part on physiological gradients of substances entering the shoot from the medium [44], and on the presence of competent cells to respond.
Interestingly, anatomical data indicated that cytological events took place as early as day 3, leading to well visible morphogenetic fields; meristematic activity was seen within 5 days (Fig. 1E). The time required for adventitious root initiation has varied between species. Meristematic activity in response to inductive treatment was observed within: eight days in Camellia japonica L. [34], six days in Cynara scolymus [35], five days in Malus pumila ‘KSC-3’ [31], four days in Rosa hybrida [45], in chestnut [30] and in apple tree [33], three days in Malus domestica Borkh ‘Jork 9’ [24], two days in Prunus avium L. × Prunus pseudocerasus lind [38], and Malus domestica Borkh ‘Gala’ [41]. The auxin concentration in the culture medium can also explain some of these differences.
From our histological observations, we can suggest that, in our material, induction of rooting was realized at day 3, where the first cell divisions were observed. The development of meristemoids occurred during the initiation stage, meristemoids were visible after 5 days of culture on an auxin-containing medium. The development of primordia into organized roots occurred during the growth stage, after day 5 of culture in our material.
Changes in peroxidase activity measured after in vitro rooting induction in apple rootstocks MM106 were consistent with the results previously described for various other species [14]. Peroxidase activity of the shoots in rooting condition underwent a typical variation with an increase up to a peak, and then a rapid decline, as it is now observed for many other materials [14,16–18,20,39,46–51]. This various studies have shown the important role of marker played by peroxidase in plants' rooting.
In our material, the peroxidase specific activities decreased at day 1 and increased until day 5. The minimum, observed at days 1 and 2, theoretically corresponded to the induction phase and the maximum, observed at day 5, corresponded to the end of the initiation phase, as shown by Gaspar et al. [14,15]. At this time, meristemoids have been organized [46].
It was clear that auxin treatments affected this sharp increase in peroxidase activity. In fact, in the control, there was no change in peroxidase activity during the course of the experiment. In addition, the level variation of peroxidase activity occurrence was also depending on species and genotype; it was observed within 17 days for Sequoiadendrum giganteum [46], six days for oak [33], five days for Fraxinus angustifolia [50], three days for Psoralea corylifolia L. [16], one day for almond microcuttings [48], Malus domestica Borkh ‘Jork 9’ [24] and Gardenia jasminoides Ellis [52]. From the results obtained with our material, the maximum of peroxidase activity that determines the end of the initiation stage can be situated at day 5.
If root induction in woody plants includes auxin in the medium, there is considerable evidence indicating that auxin is not needed during all the stages of adventitious root production. Indeed, some evidences suggest an inhibitory action at a later stage [28,53]. Better, the transfer of microcuttings to auxin-free medium resulted in the differentiation of root primordium. Most of the woody plant required a sequence of two rooting media, as did Sequoiadendron [46,54].
Shoots of apple rootstocks MM106 did not root without passage on an auxin medium or when auxin was continuously kept on such a medium. A maximum of 97% rooting was obtained when passing from an auxin-containing medium to a medium deprived of it on day 5 (Fig. 3). These results mean that the induction and the initiation stages were finished at day 5 and that the suppression of auxin was necessary to favour the development of the roots. A comparison with other recent data indicates that the length of the auxin phase was dependent on the type and concentration of auxin used, but species and variety differences were also important parameters [9]. For apple tree clones, the optimal length of auxin treatment was generally between five to nine days in the dark [9,55–57].
Anatomical data indicated that very first cytological events took place as early as day 3, leading to well-visible meristemoids at day 5. This means that the inductive period ended before day 3, the end of the initiation stage corresponding to the maximum of peroxidase activity at day 5. During this period, various cytological events took place, from the first cellular divisions to the formation of meristemoids. Five days was also the optimum duration of auxin treatment to obtain a maximum of rooting percentage. At day 5, the root meristemoids were formed and, after that, no auxin was needed to root primordium development. After day 5, auxin must be suppressed. This end of the induction stage also corresponds to the maximum of peroxidase activity.
In conclusion, root formation appeared as the result of very complex reactions. The peroxidase activity variations as biochemical markers could be correlated with observations of the microscopic tissues in the microshoots and follow-up of the rooting percentage as a function of time. These results confirm the work carried out by other researchers [14,18,23,24]. The present study needs to be completed by analyses of other biochemical markers, such as polyamines, auxins, and phenols on our plant material during the different rooting phases.
Acknowledgements
The National Institute of Agronomic Research of Tunisia (INRAT) supported this research. N.S. expresses her sincere gratitude for collaboration with the Laboratory of Plant Molecular Biology and Biotechnology, at the University of Liege, Belgium.


